Translate this page into:
Effect of drying methods on the free radicals scavenging activity of Vernonia amygdalina growing in Malaysia
⁎Corresponding author. ruthoalao@gmail.com (O.R. Alara)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The extracts from Vernonia amygdalina leaves dried using different modes of drying were examined for their antioxidant activities. The component identification was carried out on extract with the highest antioxidant property using GC–MS analysis. Using DPPH, ABTS+• and H2O2 radical scavenging assays, the shade, the sun, oven at 40, 50, and 60 °C dried sample extracts were screened. The obtained scavenging activities were in the order: shade drying > sun drying > oven drying at 40 °C > oven drying at 50 °C > oven drying at 60 °C. The extract from shade-dried sample exhibited the highest activity by scavenging 63.21–92.25% of DPPH, 75.32–93.15% of ABTS+•, and 82.53–95.78% of H2O2 radicals, which were higher than those of ascorbic and gallic acid scavenging activities. In addition, the effects of drying methods and extract concentrations on the antioxidant activity of V. amygdalina leaf were significant (p < 0.05). A total number of 11 major compounds were identified from the shade-dried extract viz: phytol (43.69%), (z,z,z)-methyl ester-9,12,15-octadecatrienoic acid (17.60%), 2-methyl-3-hexanol (6.83%), ethyl ester-9,12-octadecadienoic acid (6.07%), ethyl ester linoleic acid (5.19%), ethyl ester hexadecanoic acid (5.06), heneicosane (4.15%), heptacosane (3.25%), and others are less than 3%. Therefore, it can be deduced that an increase in drying temperature may deteriorate the antioxidant activity of Vernonia amygdalina leaf.
Keywords
Antioxidant
Vernonia amygdalina
DPPH
ABTS
1 Introduction
Free radicals had been reported to be responsible for the pathophysiological of several diseases like cancer, diabetes, and cardiovascular diseases (Ramkumar et al., 2007). Antioxidant has capacity to dissolve the free radicals cells in human bodies (Erasto et al., 2007; Thaipong et al., 2006). In recent years, several plants had been studied to contain phytochemicals that can protect a human system against different diseases. However, the structures of these phytochemicals made them scavenge free radical cells (Alara et al., 2017, 2016; Ayoola et al., 2008; Erasto et al., 2007; Farombi and Owoeye, 2011; Igile et al., 1994; Olalere et al., 2017). The World Health Organization had highlighted that many plants in the world possesses high medicinal values and recommended the use of natural products in place of synthetic drugs because of their adverse effects (Qi, 2015). Therefore, studies are being done on novel types of antioxidants from different plant matrices.
Vernonia amygdalina, known as “bitter leaf” in English is one of the commonly consumed plants in Africa (West Africa) as food and medicine (Egharevba et al., 2014; Igile et al., 1994; Nwaoguikpe, 2010; Oduah, 2012). It is a shrub that grows up to 3 m above the sea level. This plant had been studied to possess several pharmacological properties such as antioxidant, anti-diabetes, anti-inflammatory, anti-cancer, anti-malaria, anti-leukaemia, anti-fungi, anti-microbial, and among others (Alam et al., 2013; Atangwho et al., 2013; Erasto et al., 2007; Ilondu, 2010; Iwalokun, 2008; Jisaka et al., 1993; Ngatu et al., 2012; Owoeye et al., 2010). Many bioactive compounds like flavonoids, sesquiterpenes, terpenes, edotides, xanthones and alkaloids had been isolated from the extracts (Farombi and Owoeye, 2011; Igile et al., 1994; Jisaka et al., 1993; Luo et al., 2017).
Studies had revealed that phenolic compounds are responsible for the antioxidative activities of edible and non-edible plant products by providing protection against harmful free radicals and minimize the risk of diseases associated with oxidative stress (Vashisth et al., 2011). Different assays have been frequently used to determine the antioxidant activities in vegetables and fruits including 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-Azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS) and hydrogen peroxide (H2O2) assays. Thaipong et al. (2006) observed the high correlation between ABTS, DPPH, FRAP, and ORAC for guava fruit extracts with ascorbic acid.
The antioxidant activity of V. amygdalina L. growing in different part of the world had been explored but to the best of our knowledge, the effect of drying methods on this activity has yet to be studied (Atangwho et al., 2013; Erasto et al., 2007; Farombi and Owoeye, 2011; Shon et al., 2003; Qing et al., 2014). Therefore, the antioxidant property of Vernonia amygdalina leaf growing in Malaysia as affected by different modes of drying (shade, sun, oven at 40, 50, and 60 °C) using three radical scavenging assays (DPPH, ABTS+•, and H2O2) and the component identifications of the selected drying method extract were studied.
2 Materials and methods
2.1 Plant samples and reagents
The fresh leaves of Vernonia amygdalina were harvested from a garden at Gambang located in Malaysia (Latitude: 3° 42′ 25.183″N and Longitude: 103°6′ 8.982″ E). Methanol, ethanol, 2,2-diphenyl-picryhydrazyl (DPPH), ascorbic acid, 2,2′-Azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS+•), sodium hydrogen phosphate monobasic anhydrous (NaH2PO4), potassium hydrogen phosphate dibasic anhydrous (K2HPO4), and potassium persulfate were purchased from Sigma–Aldrich (M) Sdn Bhd. The distilled water and 30% v/v hydrogen peroxide were obtained from Faculty of Chemical Engineering and Natural Resources Engineering laboratory. All the chemicals used were of analytical grade.
2.2 Soxhlet extraction
Fresh leaves of Vernonia amygdalina were washed and pre-treated using different methods of drying (open sun, shade, oven at 40, 50, and 60 °C) until no significant loss in weight were recorded. The dried samples were blended using a grinder (Panasonic Blender PANA-MX-801S HG, Malaysia). The crushed samples were sieved to 0.105 mm particles size. Thereafter, 100 g dried samples were subjected to soxhlet extraction for 2 h using 60% v/v ethanol solution. After the extraction process, the mixture was allowed to cool to room temperature, filtered through Whatman qualitative filter paper No. 1, and concentrated using rotary evaporator (Buchi Rotavapor R-200 coupled with Buchi Vac V-500 pump, Switzerland). The extracts were stored in the air-tight vial bottles and refrigerated at 4 °C until the time of analysis. The experiments were carried out in triplicate.
2.3 Free radical assays
2.3.1 DPPH free radical scavenging
The free radical scavenging capacities of Vernonia amygdalina leaf ethanolic extract were determined using DPPH assay according to the methods described by Ayoola et al. (2008) and Alam et al. (2013) with some modifications. Briefly, the stock solution of 1 mM DPPH was prepared in analytical grade methanol (99.95% purity) and stored at −20 °C until analysis. Fresh working DPPH solution (0.1 mM) was prepared by diluting 10 ml stock solution with 90 ml methanol. 0.2 ml of extract (or standard) at different concentrations (0.01–0.1 mg/ml) was dissolving in 2 ml of analytical grade methanol and 2 ml of DPPH solution was added. After 30 min of incubation in the dark, the absorbance at 517 nm using a UV–VIS Spectrophotometer (Hitachi U-1800, Japan) was recorded. Methanol was used as the blank. The analysis was done in triplicate. Ascorbic and gallic acid were used as the standards. The percentage of DPPH radical inhibition was determined using Eq. (1).
2.3.2 ABTS radical scavenging assay
This assay was performed according to the procedure described by Thaipong et al. (2006) with some modifications. The ABTS+• stock solution was prepared by mixing 7 mM ABTS solution and 2.45 mM potassium persulfate solution in the ratio 1:1. Then, the mixture was incubated in the dark for 12 h at room temperature. The fresh working solution of ABTS radical cation was prepared by diluting 2 ml of ABTS+• solution with 120 ml methanol to obtain an absorbance of 1.1 ± 0.02 at 734 nm using UV–VIS Spectrophotometer (Hitachi U-1800, Japan). 0.15 ml of Vernonia amygdalina leaves extract (or standard) was mixed with 2.85 ml of ABTS+• solution and allowed to stand in the dark for 2 h at room temperature. Thereafter, the absorbance was measured at 734 nm using UV–VIS Spectrophotometer (Hitachi U-1800, Japan). Methanol was used as the blank. The percentage of ABTS+• was determined using Eq. (2). Gallic acid was used as the standard. The analyses were carried out in triplicate.
2.3.3 Hydrogen peroxide scavenging assay
This assay was carried out followed the procedure described by Keser et al. (2012). Briefly, 50 mM of phosphate buffer (pH 7.4) was prepared. 40 mM hydrogen peroxide solution was prepared from 30% concentration of hydrogen peroxide solution. 0.1 mg/ml extract (or standard) was mixed with 0.6 ml of hydrogen peroxide solution (Asample) and 3.6 ml of phosphate buffer was mixed with 0.6 ml of hydrogen peroxide solution (Acontrol). The mixture was incubated in the dark for 10 min and the absorbance was measured at 230 nm using UV–VIS Spectrophotometer (Hitachi U-1800, Japan). Phosphate buffer (pH 7.4) was used as the blank. Gallic acid was used as the standard. The analyses were repeated in triplicate. The percentage of hydrogen peroxide inhibition was calculated using Eq. (3).
2.4 Gas chromatography–mass spectrometry analysis
The components identification and their percentage of abundance in Vernonia amygdalina leaf extracts were evaluated using gas chromatography-mass spectrometric analysis. Atangwho et al. (2013) procedure with some modifications was employed. An Agilent GC 7890A (USA) coupled with MS 597C, equipped with a DB-1MS capillary column (30 m tubular column length × 0.25 mm i.d. × 0.25 µm film thickness) and autosampler was used. The operating conditions for the analysis were as follows: initial temperature of 35 °C was raised to 95 °C at the rate of 3 °C/min for 10 min, then raised to 270 °C at the rate of 10 °C/min and finally to 300 °C at the rate of 3 °C/min which was maintained for 3 min; column flow rate of 0.8 ml/min; carrier helium at a flow rate of 1.0 ml/min; split ratio 10:1; the ionization voltage of 70 eV; run time of 67.5 min; and sample injection volume was 1 µl solution of extract (5 mg/ml). The components were identified by relating the spectra obtained with those on NIST 05a library database, and the percentages of abundance were calculated with the total ion chromatogram. 1 ml sample was prepared by diluting the extract with analytical absolute ethanol at a ratio of 1:20 (w/v).
2.5 Statistical analysis
The experiments and analyses were carried out in triplicate and expressed as mean ± SD. The calculations and curves were done in MS Office Excel®, 2013. The effects of drying methods and extract concentrations on the antioxidant assays were carried out using two-way ANOVA (p < 0.05).
3 Results and discussion
3.1 Antioxidant activity of Vernonia amygdalina leaf extracts using different drying methods
The free radical scavenging activities of Vernonia amygdalina leaf extracts from different drying methods were evaluated using the DPPH, ABTS+•, and H2O2 assays at 519, 734, 230 nm absorbance, respectively. Variations in the percentage of inhibitions at concentrations 0.01, 0.02, 0.05, and 0.1 mg/ml in comparison with ascorbic and gallic acid are shown in Figs. 1–3. DPPH radical is a stable free radical that can donate hydrogen when reacts with antioxidant compounds and reduced to diphenyl picrylhydrazine (Thaipong et al., 2006). These showed the ability of extracts to neutralize free radicals which possess unpaired electrons (Atangwho et al., 2013; Erasto et al., 2007; Thaipong et al., 2006). The DPPH scavenging activities of the extracts were in the order: shade drying > sun drying > oven drying at 40 °C > oven drying at 50 °C > oven drying at 60 °C (Fig. 2). At different concentrations (0.01, 0.02, 0.04, 0.05, and 0.1 mg/ml), extracts obtained from shade-dried sample showed the highest activities of 63.21 ± 2.11%, 69.70 ± 3.27%, 87.99 ± 1.10%, and 92.25 ± 2.43% of the DPPH radical scavenging, respectively (Fig. 2). An increase in the percentage of DPPH inhibition caused by antioxidant might be due to the scavenging ability of radicals by hydrogen donation. It can also be seen that the extracts were more active than that of ascorbic and gallic acid at the same concentrations. This showed that Vernonia amygdalina leaf extract possesses a stronger antioxidant activity. The DPPH radical scavenging for ascorbic and gallic acid ranged from 40.21 to 66.55% and 41.12 to 59.54%, respectively. Therefore, it can be deduced that drying methods have significant effect on the antioxidant activity of the plant extracts resulting in different values of antioxidant inhibitions.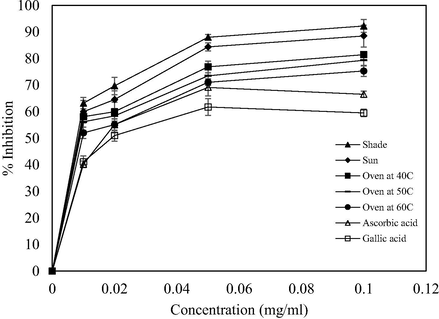
DPPH radical scavenging activities at different drying methods.
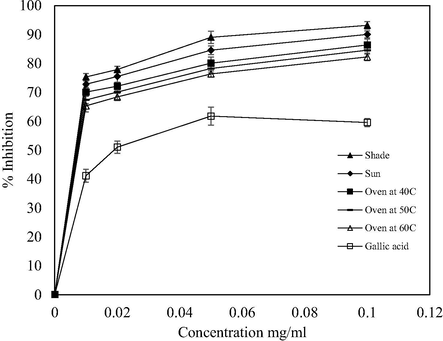
ABTS scavenging activities at different drying methods.
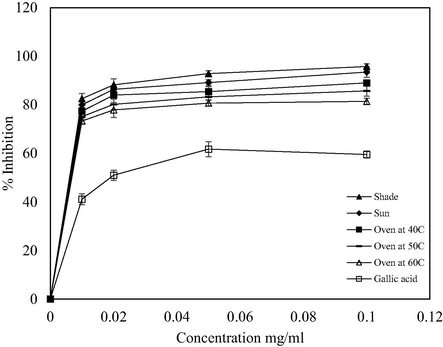
H2O2 scavenging activities at different drying methods.
The antioxidant activity of the extract using ABTS radical assay was generated by potassium persulfate in order to determine its hydrogen donating ability. Likewise, hydrogen peroxide is a weak oxidizing agent that can inactivate a few enzymes directly, usually by oxidation of essential thiol (-SH) groups (Keser et al., 2012). In the ABTS+• and H2O2 assays, similar trends of DPPH assay were obtained. Extract from the shade-dried sample exhibited the highest antioxidant activity in the ranges 75.32–93.15% and 82.53–95.45%, respectively (Figs. 3 and 4). Higher percentage inhibition might be due to its ability in donating hydrogen and terminating the oxidation process by converting free radicals into their stable form (Thaipong et al., 2006; Xiong et al., 2012). The oven-dried samples at 60 °C showed the lowest antioxidant activity but slightly higher than gallic acid. The scavenging activity at different concentrations followed the trend: 0.01 < 0.02 < 0.05 < 0.1 mg/ml (Figs. 3 and 4). The ability of V. amygdalina leaf to scavenge H2O2 may be attributed to the presence of phenols and tannins which could donate electrons, thereby neutralizing it into water (Babu et al., 2013). The obtained results confirmed the antioxidant activity of the V. amygdalina leaf.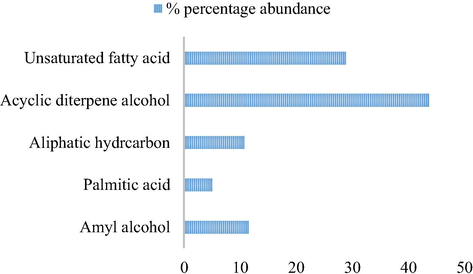
Percentages of different classes of compounds extracted from shade-dried Vernonia amygdalina leaves.
The extracts from Vernonia amygdalina leaves dried at different conditions have demonstrated concentration-dependent radical scavenging activities. In general, the extracts were found to possess higher antioxidant activities even at different drying conditions whereby shade drying showed the best method of obtaining the highest antioxidant from Vernonia amygdalina leaf. The obtained results were similar to those in the literature for Vitex negundo and Vitex trifolia leaves (Chong and Lim, 2012), ginger species leaves (Chan et al., 2009), and Lamiaceae herbs (Adámková et al., 2015). However, drying process may cause changes in the percentage of antioxidant inhibition (Adámková et al., 2015; Chan et al., 2009; Vashisth et al., 2011). Therefore, it can be deduced that increase in drying temperature may lead to deterioration of antioxidant activity of the extracts.
Furthermore, two-way analysis of variance was conducted on the effect of drying methods and extract concentrations on the antioxidant activities of V. amygdalina L. extracts (Table 1). Drying methods included five levels (shade, sun, oven drying at 40, 50, and 60 °C) and extract concentrations consisted of five levels (0, 0.01, 0.02, 0.05 and 0.1 mg/ml). All the effects were statistically significant at 0.05 significance level. The main effect for drying methods yielded an F ratio of 383.18 and p < 0.0001, indicating a significant difference between shade, sun, oven drying at 40, 50 and 60 °C. Likewise, the main effect for extract concentrations yielded an F ratio of 4.81, p = 0.002, indicating a significant difference between 0, 0.01, 0.02, 0.05, and 0.01 mg/ml of the extracts. In contrary, the interaction effect was insignificant with the F ratio of 0.34 and p = 0.989. Therefore, it can be deduced that drying methods and extract concentrations have a significant influence on the antioxidant activity of V. amygdalina leaves Table 2. df: degree of freedom, SS: sum of square, MS: mean square.
Source of Variation
SS
df
MS
F
P-value
F critical
Drying methods
75165.99
4
18791.50
383.18
<0.0001
2.56
Extract concentrations
943.6985
4
235.92
4.81
0.002
2.56
Interaction
267.191
16
16.70
0.34
0.989
1.85
Within
2452.025
50
49.04
Total
78828.9
74
S/N
Compound name
Retention time (min)
Molecular weight
Abundance (%)
1
2-Pentanol
7.617
C5H120
2.28
2
2-Methyl-3-hexanol
8.029
C8H16O
6.83
3
2,4-Dimethyl-3-pentanol
8.029
C7H16O
2.42
4
Ethyl ester hexadecanoic acid
41.998
C18H36O2
5.06
5
Heptacosane
42.212
C27H56
3.25
6
Phytol
43.140
C20H40O
43.69
7
Heneicosane
43.164
C21H44
4.15
8
Ethyl ester-9,12-octadecadienoic acid
43.498
C20H36O2
6.07
9
Ethyl ester linoleic acid
43.501
C20H36O2
5.19
10
(z,z,z)-methyl ester-9,12,15-octadecatrienoic acid
43.523
C19H32O2
17.60
11
Eicosene
45.716
C20H40
3.36
3.2 GC–MS chemical compositions of shade-dried ethanolic Vernonia amygdalina leaf extract
The quantitative composition and percentage abundance of compounds in the shade-dried ethanolic extracts of Vernonia amygdalina leaf are shown in Table 1. A total number of 11 major compounds were identified viz: phytol (43.69%), (z,z,z)-methyl ester-9,12,15-octadecatrienoic acid (17.60%), 2-methyl-3-hexanol (6.83%), ethyl ester-9,12-octadecadienoic acid (6.07%), ethyl ester linoleic acid (5.19%), ethyl ester hexadecanoic acid (5.06), heneicosane (4.15%), heptacosane (3.25%), and others (less than 3%). Previous studies had reported that V. amygdalina leaf possesses several phytochemical including diterpenes, flavonoids, alkaloids, saponins and tannins (Farombi and Owoeye, 2011; Igile et al., 1994; Jisaka et al., 1993; Luo et al., 2017). Thus, the results obtained therein were in agreement with the observations from previous studies. However, the phytochemicals identified from the GC–MS analysis of the shade-dried extract were acyclic diterpene alcohol (43.69%), unsaturated fatty acid (28.86%), amyl alcohol (11.53%), aliphatic hydrocarbon (10.76%), and palmitic acid (5.06%) as illustrated in Fig. 4. The presence of the higher percentage of acyclic diterpene alcohol and unsaturated fatty acids reflected the antioxidant and anti-inflammatory potentials of V. amygdalina leaf (Akinmoladun et al., 2007; Atangwho et al., 2013; Erasto et al., 2007; Saravanan and Parimelazhagan, 2014). Moreover, unsaturated fatty acids had been reported as one of the predominant fatty acids that play important role in the infant brain development, reduction of coronary heart diseases and treatment of rheumatic symptoms in the traditional medical system (Erasto et al., 2007; Oliver, 1997). Therefore, this study showed that the crude extracts of V. amygdalina leaf have a higher potential in alternative medicine.
4 Conclusion
The obtained results showed that shade drying is more effective to other drying methods in preserving the antioxidant activity of Vernonia amygdalina leaf. Thermal drying (sun and oven) resulted in the deterioration of the antioxidant activity as confirmed using DPPH, ABTS+•, and H2O2 radical scavengers. More so, the extracts showed promising antioxidant potential from the in vitro analysis. In addition, the GC–MS analysis of the shade-dried extract showed the presences of unsaturated fatty acids indicating that Vernonia amygdalina leaves could be utilized in pharmaceutical as well as food industries. It is therefore recommended that in vivo antioxidant activity of V. amygdalina L. be considered for further studies.
Conflict of interest
We declare no conflict of interest.
References
- The effect of drying on antioxidant activity of selected Lamiaceae herbs. Potravinarstvo. 2015;9:252-257.
- [Google Scholar]
- Chemical constituents and antioxidant activity of Alstonia boonei. African J. Biotechnol.. 2007;6:1197-1201.
- [Google Scholar]
- Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J.. 2013;21:143-152.
- [Google Scholar]
- Optimization of mangiferin extracted from Phaleria macrocarpa fruits using response surface methodology. J. Appl. Res. Med. Aromat. Plants.. 2017;5:82-87.
- [Google Scholar]
- Review on Phaleria macrocarpa pharmacological and phytochemical properties. Drug Des.. 2016;5:1-5.
- [Google Scholar]
- Antioxidant versus anti-diabetic properties of leaves from Vernonia amygdalina del. growing in Malaysia. Food Chem.. 2013;141:3428-3434.
- [Google Scholar]
- Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop. J. Pharm. Res.. 2008;7:1019-1024.
- [Google Scholar]
- Antioxidant and free radical scavenging activity of triphala determined by using different in vitro models. J. Med. Plants Res.. 2013;7:2898-2905.
- [Google Scholar]
- Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem.. 2009;113:166-172.
- [Google Scholar]
- Effects of drying on the antioxidant properties of herbal tea from selected Vitex species. J. Food Qual.. 2012;35:51-59.
- [Google Scholar]
- Significance of bitter leaf (Vernonia amagdalina) in tropical diseases and beyond: A review. Malar. Chemother. Control Elimin.. 2014;3:1-10.
- [Google Scholar]
- Evaluation of antioxidant activity and the fatty acid profile of the leaves of Vernonia amygdalina growing in South Africa. Food Chem.. 2007;104:636-642.
- [Google Scholar]
- Antioxidative and chemopreventive properties of Vernonia amygdalina and Garcinia biflavonoid. Int. J. Environ. Res. Public Heal.. 2011;8:2533-2555.
- [Google Scholar]
- Flavonoids from Vernonia amygdalina and their antioxidant activities. J. Agric. Food Chem.. 1994;42:2445-2448.
- [Google Scholar]
- Antifungal activities of three Vernonia spp. phytochemical composition and efficacy of ethanolic leaf extracts of some. J. Biopestic.. 2010;6:165-172.
- [Google Scholar]
- Enhanced antimalarial effects of chloroquine by aqueous Vernonia amygdalina leaf extract in mice infected with chloroquine resistant and sensitive Plasmodium berghei strains. Afr. Health Sci.. 2008;8:25-35.
- [Google Scholar]
- Antitumoral and antimicrobial activities of bitter sesquiterpene lactones of Vernonia amygdalina, a possible medicinal plant used by wild chimpanzees. Biosci. Biotechnol. Biochem.. 1993;57:833-834.
- [Google Scholar]
- Hydrogen peroxide radical scavenging and total antioxidant activity of hawthorn. Chem. J.. 2012;2:912.
- [Google Scholar]
- Isolation and structure determination of a sesquiterpene lactone (vernodalinol) from Vernonia amygdalina extracts. Pharm. Biol.. 2017;49:464-470.
- [Google Scholar]
- Anti-allergic effects of Vernonia amygdalina leaf extracts in hapten-induced atopic dermatitis-like disease in mice. Allergol. Int.. 2012;61:597-607.
- [Google Scholar]
- The effect of extract of bitter leaf (Vernonia amygdalina) on blood glucose levels of diabetic rats. Int. J. Biol. Chem. Sci.. 2010;4:721-729.
- [Google Scholar]
- Oduah, I., 2012. Numerous Uses of Bitter Leaf. Lulu.com. 1–8.
- Parametric optimization of microwave reflux extraction of spice oleoresin from white pepper (Piper nigrum) J. Anal. Sci. Technol.. 2017;8:8.
- [Google Scholar]
- It is more important to increase the intake of unsaturated fats than to decrease the intake of saturated fats: Evidence from clinical trials relating to ischemic heart disease. Am. J. Clin. Nutr.. 1997;66:980S-986S.
- [Google Scholar]
- Another anticancer elemanolide from Vernonia amygdalina del. Int. J. Biol. Chem. Sci. 2010;4:226-234.
- [Google Scholar]
- WHO traditional medicine strategy 2014–2023: Background and progress in the last decade. Glob. Heal. Hist. Semin. Tradit. Med. Ayurveda 2015:1-28.
- [Google Scholar]
- Antimicrobial and antioxidant studies of Vernonia amygdalina. J. Appl. Pharm.. 2014;6:360-371.
- [Google Scholar]
- Antimicrobial properties and phytochemical constituents of an antidiabetic plant Gymnema montanum. Food Chem.. 2007;1:67-71.
- [Google Scholar]
- In vitro antioxidant, antimicrobial and anti-diabetic properties of polyphenols of Passiflora ligularis juss. fruit pulp. Food Sci. Hum. Wellness. 2014;3:56-64.
- [Google Scholar]
- Antioxidants and free radical scavenging activity of Phellinus baumii (phellinus of hymenochaetaceae) extracts. Food Chem.. 2003;82:593-597.
- [Google Scholar]
- Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal.. 2006;19:669-675.
- [Google Scholar]
- Effects of drying on the phenolics content and antioxidant activity of Muscadine pomace. LWT - Food Sci. Technol.. 2011;44:1649-1657.
- [Google Scholar]
- Xiong, Y., Rao, L., Xiong, L., Ai, Q., Wu, X., 2012. Effects of extraction solvent on polyphenolic contents and antioxidant activities of Osmanthus fragrans’ seed. In: Proc. – 2012 Int. Conf. Biomed. Eng. Biotechnol. iCBEB 2012, pp. 217–220.







