Translate this page into:
Does water salinity affect the level of 17β-estradiol and ovarian physiology of orange mud crab, Scylla olivacea (Herbst, 1796) in captivity?
⁎Corresponding author. ikhwanuddin@umt.edu.my (Mhd Ikhwanuddin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study determined the effect of water salinity on the level of 17β-estradiol and the ovarian physiology (histological changes) of Scylla olivacea in captivity. Sixty newly mature crabs with immature ovaries (carapace width > 90.0 mm, Stage 1 ovary) were exposed to three salinity levels (10, 20 and 30 ppt) for 60 days. The level of 17β-estradiol peaked in the early days of each treatment for all salinity levels but decreased as the crabs matured. Stage 4 ovaries were most produced in the 20 ppt treatment, followed by the 10 ppt treatment, and then the 30 ppt treatment. No significant difference was found for the level of 17β-estradiol between salinity levels, but the oocyte size was observed to be significantly different between salinity levels. Meanwhile, there was a significant negative correlation between oocyte diameter size and the 17β-estradiol level. The study concludes that salinity can affect ovarian maturation but not 17β-estradiol levels.
Keywords
Mud crab
Scylla olivacea
Salinity
17β-estradiol
Histological assessment
1 Introduction
The reproductive biology of crustaceans is influenced by many factors such as temperature (Gilman, 2006), pH (Allan and Maguire, 1992), dissolved oxygen (Miller Neilan and Rose, 2014) and nutrients (Varadharajan et al., 2013), with salinity being the most influential parameter (Kumlu et al., 2001; Romano and Zeng, 2006; Talpur and Ikhwanuddin, 2012). Several studies explored the effect of salinity on the physiological regulation of mud crabs (Ikhwanuddin et al., 2014a; Parado-Estepa et al., 1987; Romano and Zeng, 2006), affecting their habitat preferences, reproduction and survival (Talpur and Ikhwanuddin, 2012). Until now, few studies have evaluated the optimal salinity levels for survival, growth, maturation and reproduction of crustaceans such as shrimp (Penaeus monodon, Metapenaeus monoceros), crayfish (Cherax tenuimanus) and crabs (Portunus pelagicus, Scylla paramamosain) (Jie et al., 2015; Kumlu et al., 2001; Parado-Estepa et al., 1987; Rouse and Kartamulia, 1992; Romano and Zeng, 2006; Ye et al., 2009); most studies have focused on larval stages, but relatively few considered the broodstock.
The relationship between steroid hormones and crustacean reproduction has become a focus among researchers (Muhd-Farouk et al., 2016; Sheng li et al., 2001). Vertebrate-like hormones have been detected in various marine invertebrate species (Muhd-Farouk et al., 2016; Ollevier et al., 1986; Vooget et al., 1984), and many steroid hormones have been recognized as gonad-stimulating hormones (Subramoniam, 2000; Tsukimura, 2001; Wilder et al., 2002). Based on previous studies by Menuet et al. (2005) and Thomas et al. (2007), 17β-estradiol plays an important role in the physiological process of reproduction through the application of the estrogen receptors signaling pathway including vitellogenin synthesis. A correlation was observed between 17β-estradiol levels and gonadal development in the freshwater crayfish, Cherax albidus, by Coccia et al. (2010), and a correlation was also proven in P. monodon between vitellogenin fluctuation levels and 17β-estradiol levels during ovarian maturation processes. However, there are still no in-depth studies regarding the level of 17β-estradiol in female mud crabs or the effect of salinity on this hormone.
Orange mud crab, Scylla olivacea, is a commercially important mud crab species (Ikhwanuddin et al., 2011). Mud crabs inhabit mangrove and tidal areas (Keenan et al., 1998; Keenan, 1999), and their constant exposure to salinity fluctuations may influence their reproductive hormone levels, thus affecting ovarian maturation. Hormone regulations and ovarian maturation may be supported by an optimal water salinity level. Therefore, this study was conducted to determine whether water salinity affects 17β-estradiol levels and the ovarian histology of orange mud crab, S. olivacea, in captivity. These results may support the development and improvement of broodstock management practices in hatcheries based on optimal water salinity for ovarian maturation.
2 Methodology
2.1 Animals
A total of 95 newly mature S. olivacea females (carapace width, CW > 90.0 mm) with immature ovaries (Stage 1) were sampled from Setiu Wetlands Mangrove Forest, Terengganu, Malaysia (5°31′23.1″N, 102°55′56.1″E), from July to September 2015. Setiu Wetlands is an open and free fishing area; thus, no licensing was required for the acquisition of the mud crabs (Waiho et al., 2017). We adhered to the Association for the Study of Animal Behaviour (ASAB) (2012) “Guidelines for the treatment of animals in behavioural research and teaching” published in Animal Behaviour 83: 301–309. None of the work involved endangered or protected species. All experimental procedures and crab handling were approved by the Ethics Committee of Institute of Tropical Aquaculture, Universiti Malaysia Terengganu, according to the “Malaysian code of practice for the care and use of animals for scientific purposes” outlined by the Laboratory Animal Science Association of Malaysia.
S. olivacea identification was based on the morphological description by Keenan et al. (1998). Previous studies by Ikhwanuddin et al. (2010) and Waiho et al. (2016) stated that the sizes at maturity (CW50) recorded for female S. olivacea from the same sampling site used in this study were 90.6 mm and 90.7 mm, respectively. Therefore, newly mature females (Fig. 1b) ranging in size of 90.0–100.0 mm CW (mean: 91.3 ± 4.80 mm CW) with a body weight (BW) of 85.0–95.0 g (mean: 89.98 ± 20.79 g BW) were selected for this study. Samples were then transferred to the Institute of Tropical Aquaculture (AKUATROP) Hatchery, Universiti Malaysia Terengganu, for further study.
Abdominal flap of different maturation stages of mud crab sampled from Setiu Wetlands, Terengganu, Malaysia. (a) Immature female showing a small and pale abdominal flap, (b) Newly mature female with immature ovary showing an intermediate (less globular) and slightly darkened abdominal flap, (c) Mature female crab commonly shows a widened, globular and darkened abdominal flap.
The size of the crab was measured as the external CW, the distance between the tips of the 9th anterolateral spine of the crab carapace, using a six-inch liquid crystal display (LCD) digital Vernier caliper (accuracy: 0.01 mm; Kingsmart brand, Hong Kong), whereas the BW was measured using a digital balance (accuracy: 0.01 g; Shimadzu model, Japan). Each crab was labeled with a cable tie tag (Nylon brand; width and length: 3 × 150 mm) on its pleopods (swimming legs).
2.2 Treatments
Initially, 30 crabs were randomly chosen and dissected for the confirmation of Stage 1 ovaries. Five crabs from the remaining 65 crabs were randomly chosen as the control treatment. Hemolymph extraction was performed using a sterile syringe with a needle (TERUMO, 1.0 cc/ml), and dissection was conducted for the control treatment, referred to as Day 0 (C0). The remaining 60 crabs were transferred into treatment tanks to investigate the salinity effect on the level of 17β-estradiol and ovarian physiology. Individual crabs were kept in separate aquariums (36 cm × 22 cm × 21 cm) to avoid cannibalism and injury that could induce stress. Crabs were reared for a period of 15, 30, 45 and 60 days.
Twenty newly mature female crabs with an ovary development of Stage 1 were reared in 10 ppt (Treatment 1; T1), 20 ppt (Treatment 2; T2) and 30 ppt (Treatment 3; T3) water salinity. The salinity of the collected seawater was in the range of 28 to 32 ppt. First, crabs were acclimated to lower salinity accordingly before each treatment. For T1, the crabs were acclimated at 30 ppt for 5 days and continued at lower salinities of 25 ppt, 20 ppt, 15 ppt and 10 ppt for 5 days before being transferred into the 10 ppt treatment. For T2, the crabs were acclimated at 30 ppt, 25 ppt and 20 ppt for 5 days each before being introduced to the 20 ppt treatment, and for T3, the crabs were acclimated at 30 ppt for 5 days before starting day 1 of the treatment. Seawater was filtered and treated with chlorine, then dechlorinated using anti-chlorine before being diluted with filtered fresh water until the appropriate salinity level for each treatment was achieved (10, 20, and 30 ppt). The diluted seawater was then vigorously aerated overnight. Water parameters, such as dissolved oxygen, temperature, pH, conductivity, oxidation–reduction potential (ORP) and salinity, were determined using a YSI 556 MPS multi-probe meter (YSI Incorporated, Ohio) and a refractometer (ATAGO, Japan). Every two days, 100% water changes were performed. The crabs were cultured under treatment with different salinities (10, 20, and 30 ppt), in ambient temperature (27–29 °C) and in a natural photoperiod (12H:12D) in the hatchery. The crabs were fed with chopped yellow stripe scad fish, Selaroides leptolepis, at 10% body weight twice daily (0900 and 1700 h).
Five crabs were randomly selected every 15 days throughout the 60-day study period (Day 15, 30, 45, and 60) to extract the hemolymph and assess 17β-estradiol levels. These crabs were then dissected to evaluate ovarian maturation stages. After the confirmation of ovarian maturation stages through coloration, ovaries were fixed in Davidson’s solution for the confirmation of stages through histological analysis to determine oocyte diameter and structure.
2.3 Ovarian maturation stages
2.3.1 Coloration
The ovarian maturation stages were distinguished by the coloration of the ovary based on observations from previous studies by Ikhwanuddin et al. (2014b) and Muhd-Farouk et al. (2016), where Stage-1 = translucent to creamy white, Stage-2 = yellowish, Stage-3 = pale to dark orange, and Stage-4 = dark orange to red–orange.
2.3.2 Histological assessment of the ovary
Samples of the ovarian lobes obtained after the external morphology determination were then fixed in Davidson’s solution for 24 h, followed by dehydration in 70% alcohol overnight. They were then placed in a tissue processor for 18 ± 1 h at 60 °C. After the completion of tissue processing, the samples were mounted onto their cassette using paraffin wax and labeled. Next, the samples were sectioned into 5-µm thin films using a microtome (Leica, RM835). Samples were then incubated in a water bath (temperature between 40 and 45 °C) for expansion before mounting them onto glass slides. The slides were then dried using a hot plate (40 °C) overnight. Staining was based on the standard hematoxylin and eosin staining method of Muhd-Farouk et al. (2016). Oocyte diameter size and structure (n = 100 oocytes for each sample; x-axis) were measured using an advanced microscope (Leica Microsystem, Wetzlar GmbH, DM LB 2, Germany). The final reading of the oocyte diameter size was averaged according to respective treatments.
2.4 Extraction of 17β-estradiol in hemolymph
Hemolymph was extracted (on Day 15, 30, 45, and 60) from the crab's third right walking leg using a sterile syringe with a needle (TERUMO, 1.0 cc/ml). Extraction were prepared as described in the manual of the ELISA kit (NEOGEN, USA/CANADA) for estradiol. Approximately 100 µL of hemolymph was pipetted into a centrifuge tube (1.5 mL), and 1 mL of ethyl ether was added. After the extraction of the hemolymph, the fluid was directly subjected to enzyme-linked immunosorbent assay (ELISA) analysis. The tube was vortexed for 30 s to allow the phases to separate. The organic phase was then transferred to a clean centrifuge tube, and the solvent was evaporated under a stream of nitrogen gas (N2). Next, the residues were dissolved in 500 µL of diluted extraction buffer, the solution was vortexed, and duplicate 50 µL assays were conducted. The resulting values were multiplied by five to get the actual concentrations. If the level was higher than the high range of the standard curve, the samples were further diluted and reassayed. If additional dilution was necessary, the values were multiplied by the dilution factor to calculate the final concentration (ng/mL).
2.5 ELISA analysis
Samples were prepared as described in the manual of the ELISA kit (NEOGEN, USA/CANADA) for estradiol. Each well contained 50 µL of sample, 1 µL of estradiol enzyme conjugate and 50 µL total volume of EIA buffer. Then, 110 µL of the enzyme conjugate was added to a 5.5 mL EIA buffer for the whole plate, and the solution was mixed thoroughly. The plate was then shaken and incubated at room temperature for one hour. After incubation, the contents of the plate were deposited onto a clean lint-free towel. Each well was washed three times with 300 µL of the diluted wash buffer. Next, 150 µL of the substrate was added to each well, shaken gently, and incubated at room temperature for 30 min. After incubation, 50 µL of 1 N HCl was added to each well to stop the reaction. The plate was then shaken gently before taking a reading to ensure uniform color throughout each well. The plate was read at 450 nm using a microplate photometer (Multiskan TM FC, Thermo Fisher Scientific Inc.). The final reading of the estradiol level was averaged according to respective treatments.
2.6 Data analysis
The color of the ovary was recorded according to different ovarian maturation stages. The hormone levels and oocyte sizes were analyzed using the Kruskal-Wallis test and Pearson’s correlation with the application of Social Sciences (SPSS) software (version 22.0 for Windows; SPSS Inc., Armonk, NY: IBM Corp.). The data are presented as the mean ± SD.
3 Results
3.1 Confirmation of mature size with immature ovary of S. Olivacea
Morphologically, all 30 newly mature size female crabs dissected had Stage 1 ovaries (Fig. 2a). The ovaries were small, thin and difficult to differentiate from the digestive gland. Ovaries were strand- and ribbon-like tissue structures. For histological assessment, the mean oocyte diameter (n = 100) was 25.67 ± 4.38 µm (Fig. 2b).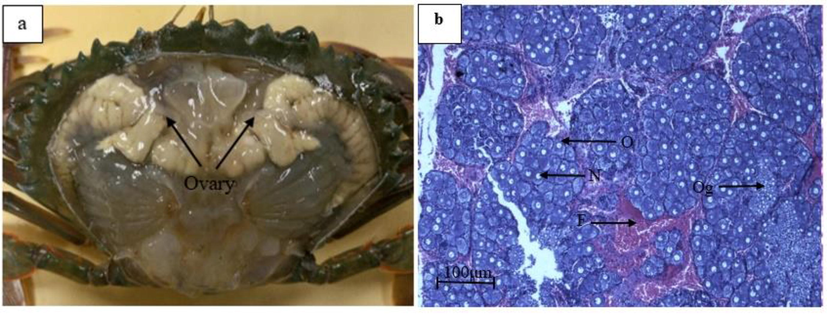
Confirmation of Stage 1 ovary of S. olivacea. (a) External morphology of Stage 1 ovary and (b) Histology assessment of Stage 1 ovary (n = 30), O: oocyte, F: follicle cell, N: nucleus, Og: oogonia.
3.2 Ovarian maturation stages
3.2.1 Coloration
Four ovarian maturation stages were used to differentiate the stages based on ovarian coloration (Fig. 3). The control (C0) appeared to have 100% Stage 1 ovaries that were translucent and creamy white in color with a ribbon-like structure. By Day 15, all crabs in the 10 ppt (T1), 20 ppt (T2) and 30 ppt (T3) treatments appeared to have 100% Stage 1 ovaries with the same characteristics as those of the control at Day 0 (C0). By Day 30, 100% Stage 1 ovaries were observed in T1; 40% Stage 1 and 60% Stage 3 ovaries (light-orange coloration) were observed in T2; and 60% Stage 1, 20% Stage 2 (yellow coloration) and 20% Stage 3 ovaries were observed in T3. By Day 45, T1 started to show development with 40% Stage 1 and 60% Stage 3 ovaries, T2 had 40% Stage 1 and 60% Stage 3 ovaries, and T3 had 80% Stage 1 and 20% Stage 3 ovaries. By Day 60, all treatments had Stage 4 ovaries (orange to dark orange coloration). T2 had the highest percentage of Stage 4 ovaries (80%) compared to the other treatments. This result showed that T2 produced the highest number of Stage 4 crabs compared to T1 and T3 (Fig. 4).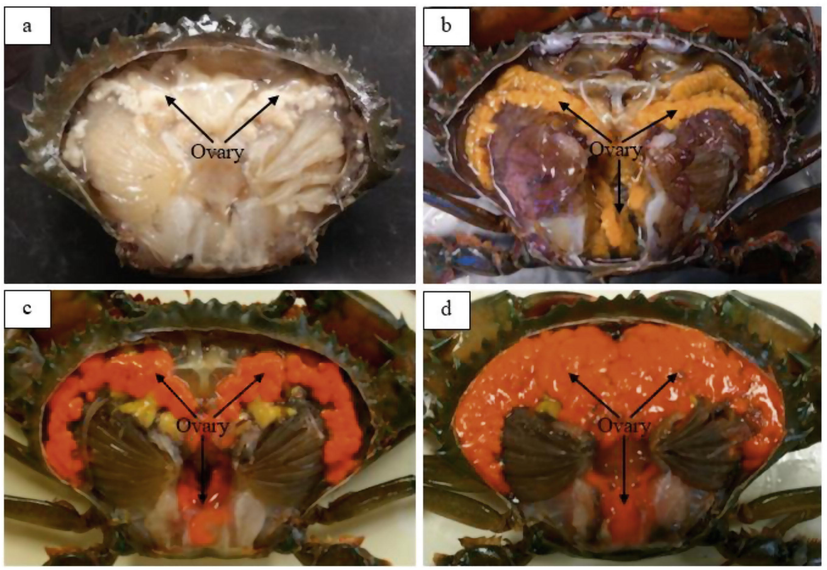
External morphological view of ovaries at various ovarian maturation stages of S. olivacea. (a) Stage 1 ovary (whitish to creamy white), (b) Stage 2 ovary (yellow), (c) Stage 3 ovary (orange) and (d) Stage 4 ovary (dark orange).
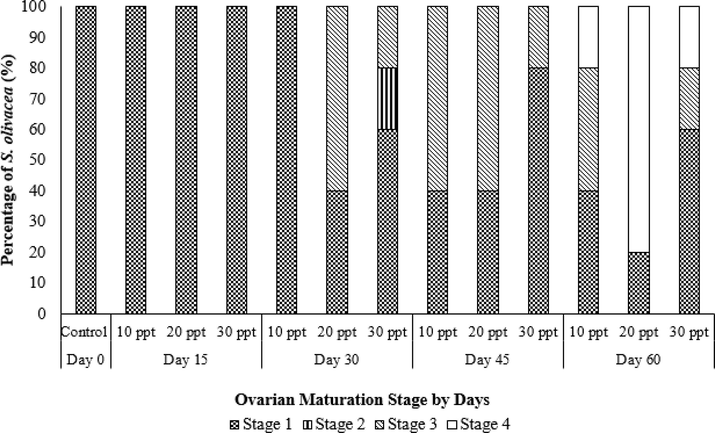
Percentage of ovarian maturation stages of S. olivacea based on coloration for each salinity treatment regime (10 ppt, 20 ppt and 30 ppt) sampled every 15 days within a 60-day culture period (n = 5).
3.3 Ovary histology assessment
3.3.1 Oocyte morphological structure
The crabs from the control group (C0) possessed Stage 1 ovaries. The ovaries of crabs from this group were filled with oogonia, abundant in follicle cells, and had no yolk globules. Moreover, the primary oocytes possessed large nuclei. After 60 days in the T1 (10 ppt), T2 (20 ppt) and T3 (30 ppt) salinity treatments, various ovarian maturation stages were present. The T1 and T2 treatments included ovaries of all development stages except Stage 2, whereas the T3 treatment possessed ovaries with all maturation stages. Overall, the oocyte morphological characteristics were similar for all salinity treatments. Table 1 shows the oocyte structural characteristics, and Fig. 5 shows the S. olivacea oocyte morphological structures for all of ovarian maturation stages (Stage 1, Stage 2, Stage 3 and Stage 4).
Ovarian maturation stages
Microscopic observations after histology assessment
1
Present of primary oocytes, follicle cells and ovary filled with oogonia. Primary oocyte possessed the large nuclei. Follicle cells can be seen surrounding the large nuclei.
2
Primary oocyte and follicle cells were visible. Small yolk globules develop in the cytoplasm of larger oocytes, follicle cells surrounded the oocytes.
3
Large yolk globule formation in the cytoplasm with visible oocyte nucleus. Follicle cells hardly visible and flattened.
4
Cytoplasm filled with very large yolk globules and oocytes. Yolk globules appear attached to each other. Follicle cells hardly seen and poor visibility of the nucleus.
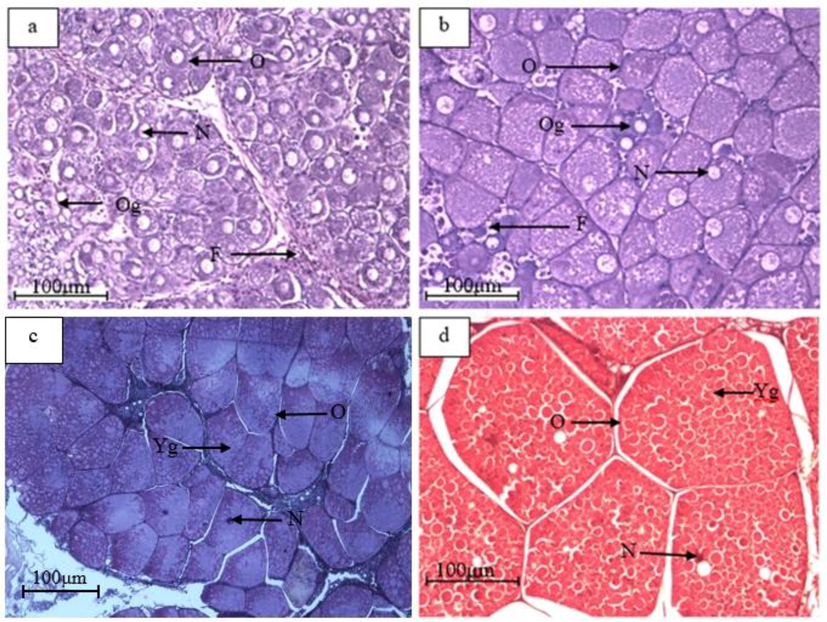
Oocyte morphological structures of S. olivacea, for all of the ovarian maturation stages. (a) Stage 1, (b) Stage 2, (c) Stage 3, (d) Stage 4, O: oocyte, F: follicle cell, N: nucleus, Og: oogonia, Yg: yolk globule.
3.3.2 Oocyte diameter size
Fig. 6 shows the mean oocyte diameter of females subjected to different treatments in a 60-day culture period. The average oocyte diameter in all treatments increased significantly after 60 days (Kruskal-Wallis, ρ = 0.001). The mean oocyte diameter for the control (Day 0) was 34.76 ± 12.13 µm. By Day 15, there was no significant increment in mean oocyte diameter for all three treatments. From Day 30 until Day 60, T2 recorded the highest mean oocyte diameter compared to other treatments. Starting from Day 45, females in T1 exhibited significantly larger mean oocyte diameter compared to those in T3. Overall, T2 showed the highest mean oocyte diameter, followed by T1 and T3.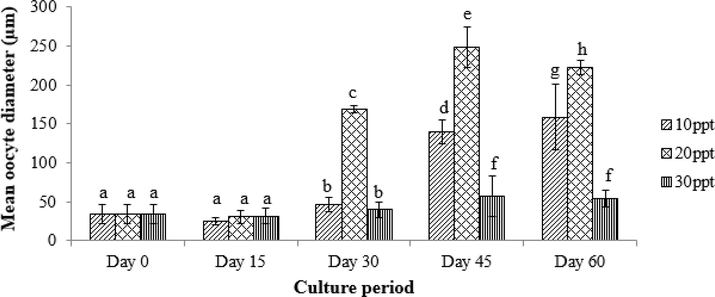
Mean oocyte diameter size of S. olivacea from the treatment salinities of T1 (10 ppt), T2 (20 ppt) and T3 (30 ppt) sampled every 15 days over a 60-day culture period (n = 5); a, b, c, d, e, f, g and h indicate significant differences (Kruskal-Wallis, ρ < 0.05) among salinities for the whole culture period.
3.4 Hormone (17β-estradiol) levels in hemolymph
The average hormone levels were as follows: 1.56 ± 0.29 ng/ml for the control (C0); 3.23 ± 0.08 ng/ml for T1, 3.52 ± 0.29 ng/ml for T2 and 3.17 ± 0 .23 ng/ml for T3 (Day 15); 1.44 ± 0.22 ng/ml for T1, 1.61 ± 0.06 ng/ml for T2 and 0.97 ± 0.08 ng/ml for T3 (Day 30); 0.68 ± 0.15 ng/ml for T1, 1.10 ± 0.37 ng/ml for T2 and 0.71 ± 0.17 ng/ml for T3 (Day 45); and 1.35 ± 0.53 ng/ml for T1, 0.41 ± 0.22 ng/ml for T2 and 1.50 ± 0.28 ng/ml for T3 (Day 60). There were no significant differences (Kruskal-Wallis, p = 0.571) (Fig. 7).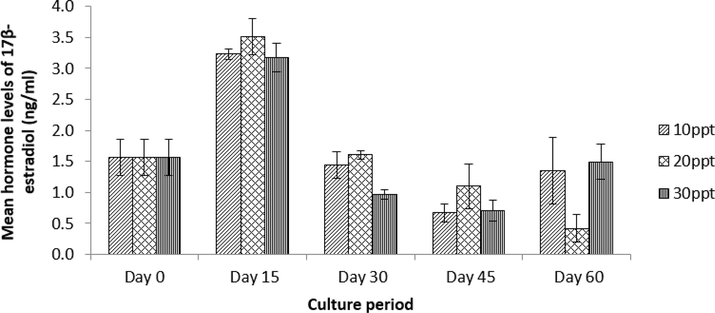
Mean hormone levels of 17β-estradiol for S. olivacea in various salinities of T1 (10 ppt), T2 (20 ppt) and T3 (30 ppt) sampled every 15 days over a 60-day culture period (n = 5). No significant different in mean hormone levels of 17β-estradiol was observed among treatments and culture period.
3.5 Relationship between 17β-estradiol levels and oocyte diameter
The results showed a significant negative correlation (Pearson correlation, ρ = 0.001) between the 17β-estradiol levels and the oocyte diameter. This result indicates that as hormone levels decrease, oocyte diameter increases.
4 Discussion
Madlen et al. (2012) stated that histological assessment is needed to give accurate results for the ovarian maturation stages, even though external morphological characteristics, such as coloration, can be used as a factor to determine the stages. Through histology, oocyte structure and size can be determined precisely due to developmental changes evident in advanced maturation. The present study showed that immature ovaries were translucent to yellow in color but became orange and reddish-orange as maturation advanced, similar to the ovarian coloration results observed previously by Muhd-Farouk et al. (2016) and Ikhwanuddin et al. (2014b) for S. olivacea and Quinitio et al. (2007) for S. serrata. According to Quinitio et al. (2007), this variability in color may be due to the diet intake of the crab; however, according to Ikhwanuddin et al. (2014b), lipid accumulation in the form of yolk in the oocytes changes ovary coloration. Differences in the ovarian maturation stages were observed, thus demonstrating that the manipulation of water salinity altered the ovarian maturation of the mud crab.
Based on ovarian coloration in the present study, there was no Stage 4 (dark orange/reddish orange color) on Day 45 for T2. However, after the confirmation through histological assessment, we concluded that T2 actually were in Stage 4 based on oocyte size and structure of ovary. Ovarian coloration can be taken as one of the ovarian staging parameter, however, histological assessment determine the precise information in order to determine the stages of ovary. This was supported by previous studies such as Muhd-Farouk et al. (2016), Ikhwanuddin et al. (2014b), Madlen et al. (2012) and Quinitio et al. (2007). The coloration may be influenced by pigmentation, carotenoid, surrounding environment and also their diet. Therefore, histology was agreed as the most precise in order for staging conformation.
No significant difference in the mean oocyte diameter was observed in all three salinity treatments during the first 15 days of culture (Fig. 6). This might be due to the acclimatization period experienced by the crabs after subjecting to different salinity treatments before any obvious internal physiological changes in terms of ovarian maturation could be detected, as also supported by the all-Stage 1 ovarian maturation stage crabs during the first 15 days (Fig. 4). Previous study by Long et al. (2018) also reported no significant difference on survival rate of female Chinese mitten crab, Eriocheir sinensis subjected to different salinities for the first 20 days, meanwhile Gong et al. (2015) recorded no significant difference for percentage of molting success and molting interval for the first stage crabs (C1) of S. paramamosain subjected to different water salinities (10, 20 and 30 ppt).
There were differences between mean S. olivacea oocyte diameters from this study and previous studies by Azmie et al. (2012) and Muhd-Farouk et al. (2016). Bigger mean oocyte size with 64.60 ± 8.99 μm (Stage 1), 70.99 ± 9.52 µm (Stage 2), 206.16 ± 29.77 μm (Stage 3) and 282.38 ± 55.52 μm (Stage 4) was reported by Azmie et al. (2012). However, a previous study by Muhd-Farouk et al. (2016) showed smaller mean oocyte sizes (31.56 ± 12.65 μm (Stage 1), 69.50 ± 3.71 μm (Stage 2), 156.3 ± 18.39 μm (Stage 3), 167.39 ± 37.72 µm (Stage 4)) compared to this study. These results might be due to the maturity of samples, diet intake of the crabs and the environmental factors practiced by Azmie et al. (2012). Nevertheless, the present study already involved mature size S. olivacea females, whereas the study done by Muhd-Farouk et al. (2016) used immature crabs (size less than 90.0 mm) that were administered with 17α-hydroxyprogesterone and 17α-hydroxypregnenolone to induce growth and maturation.
This study showed that T2 (20 ppt) was the best treatment, followed by T1 (10 ppt) and finally T3 (30 ppt) based on the higher percentage of Stage 4 ovarian maturation adults produced in 60 days. This result differed in comparison with previous studies where the optimal salinity for the mud crab was in the range of 25 ppt to 32 ppt (Shelley and Lovatelli, 2011). Additionally, based on the results of Shelley and Lovatelli (2011), mud crabs are known to tolerate low water salinity for a limited period but higher salinity for an extended period. The present study proved that the mud crab, S. olivacea, can tolerate both low and high salinity well over a prolonged period up to 60 days. In this study, T3 produced the fewest Stage 4 ovaries, demonstrating that it was the least suitable condition for mud crab broodstock maturation in captivity, similar to what has been observed in wild environments; typically, adult mud crabs have been associated with the mangrove ecosystem, where salinity is frequently less than 30 ppt (Webley et al., 2009). The osmotic stress from T3 also made the crabs work harder for survival compared with their behavior in the other two salinities, which ultimately produced the fewest Stage 4 ovaries.
These findings were supported by Ikhwanuddin et al. (2015) for another crustacean, mud spiny lobster, P. polyphagus; a lower salinity (20 ppt) clearly showed higher oocyte diameter and the best ovarian development compared to high salinity. Kagwade (1988) and Romano and Zeng (2006) reported that high salinity conditions might contribute to reduce food consumption or assimilation, thus reducing the growth of crabs. La Sara (2001) stated that S. olivacea lives near mangrove areas at low salinity, thus verifying that lower salinity was preferred by this species.
This study demonstrates that 17β-estradiol is involved in oocyte formation and ovarian development as decreasing hormone levels indicate hormone intake and utilization in ovary development. Based on the study by Muhd-Farouk et al. (2016), hormone levels activate the reproductive physiology of the ovary, thus triggering the vitellogenesis process. However, the hormone levels vary among species depending on their involvement in different pathways. These results are similar to those of Quinitio et al. (1994) on tiger prawn, P. monodon, in which 17β-estradiol significantly induced ovarian development. Moreover, positive correlations between vitellogenin circulating levels and hemolymph levels of progesterone and 17β-estradiol have been reported in crabs (Shih, 1997; Zapata et al., 2003) and shrimp (Quinitio et al., 1994; Yano, 2000). However, a negative correlation between 17β-estradiol with oocyte diameter sizes has been reported herein. These findings show that the levels of 17β-estradiol decrease due to the intake of this hormone for the vitellogenesis process in mud crabs; this conclusion is supported by the previous study on various aquatic invertebrates conducted by Lafont and Mathieu (2007).
Scarce information exists regarding the effect of water salinity on hormone levels and on crustacean ovarian maturation, especially for S. olivacea. Here, 20 ppt water salinity showed the highest peak of the hormone level between Day 15 and Day 45, thus signifying the acceleration of the ovarian development of the mud crab under the T2 salinity treatment compared with the other two treatments. The paucity of studies that use various salinity levels to induce crustacean reproduction and gonad maturation or that demonstrate the correlation between salinity and 17β-estradiol hormone levels make these findings valuable to the aquaculture field. There are no studies on the association of water salinity with 17β-estradiol hormone levels regarding the ovarian maturation of S. olivacea broodstock except for a study that involved the mating attempt of mud crabs in different salinities by Ikhwanuddin et al. (2014a) and several studies that involved larvae growth and survival in different salinities (Jantrarotai et al., 2002; Ong, 1964; Parado-Estepa and Quinitio, 1997).
In this study, 17β-estradiol played a major role in ovary development and maturation of female S. olivacea, which were influenced by salinity treatments. However, these findings showed that there was no significant difference (Kruskal-Wallis, p = 0.571) between the levels of 17β-estradiol among salinity treatments. Statistical analysis showed that the salinities did not affect the 17β-estradiol levels of S. olivacea in captivity. Therefore, further study regarding the effect of water salinity on hormone regulation is needed.
Salinity could be an environmental factor that negatively regulates the inhibitory hormones in the X-organ (also known as gonad inhibiting hormone – GIH or vitellogenesis inhibiting hormone – VIH), thereby stimulating vitellogenesis and speed up the ovarian maturation of S. olivacea in the present study. Further research on the relationship between salinity and GIH or VIH is needed to validate this postulate. However, the exact regulation mechanisms relating to the vitellogenin process in crustaceans remain unclear due to the species-specific nature of effector molecules (Subramoniam, 2011). Information from this study can be helpful for culturing mud crabs in captivity because of the difficulty in obtaining mature gametes in captivity, where most crustacean species are incapable of spontaneous maturation. Based on the present study, the vertebrate-like steroid, 17β-estradiol, is not the right factor to directly correlate with various salinity levels. However, salinity can affect maturation. The levels of 17β-estradiol may also vary depending on the gonadal maturation of the crab but not vary directly based on the salinity. Therefore, this study provides information regarding the optimum water salinity for ovarian maturation and development of S. olivacea in captivity. The optimum salinity level was 20 ppt, followed by 10 ppt and lastly 30 ppt.
5 Conclusion
Based on these findings, there was no significant correlation between 17β-estradiol hormone levels and salinity. However, there was a significant difference among oocyte diameter size and salinity. T2 (20 ppt) was the best salinity to stimulate S. olivacea ovarian maturation and development compared with the other two treatments (10 ppt in T1 and 30 ppt in T3). As 17β-estradiol hormone levels reached their highest peak, the utilization of reproductive hormones quickened, accelerating the vitellogenesis process. As a recommendation, both the manipulation of salinity (20 ppt) and the injection of hormone (17β-estradiol) may be essential methods to induce maturation of S. olivacea in captivity and to produce berried female crabs. Additionally, more extreme salinity treatments (e.g., 5 ppt and 35 ppt) should be evaluated to determine a more precise salinity effect. A longer study period is suggested to observe a clearer pattern among these parameters in order to understand why the salinity mechanism did not affect the hormone. Therefore, we suggest 20 ppt as the best water salinity to induce ovary maturation and development for female S. olivacea reared in captivity.
Acknowledgements
This study was funded by the Malaysia’s Ministry of Higher Education under the Niche Research Grant Scheme (NRGS) of Vote No. 53131. Our great appreciation goes to the Institute of Tropical Aquaculture, Universiti Malaysia Terengganu, and everyone involved directly or indirectly in this study.
Conflict of interest
There is no conflict of interest among the authors, and the submission of this manuscript is agreed upon by all authors for publication.
References
- Effects of pH and salinity on survival, growth and osmoregulation in Penaeus monodon Fabricius. Aquaculture. 1992;107:33-47.
- [CrossRef] [Google Scholar]
- Azmie, G., Abol-Munafi, A.B., Faizal, M., Ikhwanuddin, M., 2012. Ovarian maturation stages of orange mud crab, Scylla olivacea. In: Proceeding UMT 11th International Annual Symposium of Sustainable Science and Management, Terengganu, Malaysia, pp. 58–64.
- Effects of estradiol and progesterone on the reproduction of the freshwater crayfish Cherax albidus. Biol. Bull.. 2010;218:36-47. Retrieved from http://www.jstor.org/stable/25622857
- [Google Scholar]
- The northern geographic range limit of the intertidal limpet Collisella scabra: a test of performance, recruitment, and temperature hypotheses. Ecography. 2006;29:709-720. https://www.jstor.org/stable/pdf/30243161.pdf
- [Google Scholar]
- Evaluating the effects of temperature, salinity, starvation and autotomy on molting interval and expression of ecdysone receptor in early juvenile mud crabs, Scylla paramamosain. J. Exp. Mar. Biol. Ecol.. 2015;464:11-17.
- [CrossRef] [Google Scholar]
- Biological information and population features of mud crab, genus Scylla from mangrove areas of Sarawak. Malaysia Fish. Res.. 2011;108:299-306.
- [CrossRef] [Google Scholar]
- Species diversity, carapace width-body weight relationship, size distribution and sex ratio of mud crab, genus Scylla from Setiu Wetlands of Terengganu coastal waters. Malaysia J. Sust. Sci. Manage.. 2010;5:97-109. Retrieved from http://agris.fao.org/agris-search/search.do?recordID=AV2012083143
- [Google Scholar]
- Effect of water salinity on ovarian maturation stages and embryonic development of mud spiny lobster, Panulirus polyphagus. J. Fish. Aquat. Sci.. 2015;10(4):244-254.
- [CrossRef] [Google Scholar]
- Effect of water salinity on mating success of orange mud crab, Scylla olivacea (Herbst, 1796) in captivity. J. Fish. Aquat. Sci.. 2014;9(3):134-140.
- [CrossRef] [Google Scholar]
- Reproductive biology on the gonad of female orange mud crab, Scylla olivacea (Herbst, 1796) from the west coastal water of peninsular Malaysia. Asian J. Cell Biol.. 2014;9:14-22.
- [CrossRef] [Google Scholar]
- Salinity levels on survival rate and development of mud crab (Scylla olivacea) from zoea to megalopa and from megalopa to crab stage. Kasetsart J. Nat. Sci.. 2002;36:278-284.
- [Google Scholar]
- Evaluating the effects of temperature, salinity, starvation and autotomy on molting success, molting interval and expression of ecdysone receptor in early juvenile mud crabs, Scylla paramamosain. J. Exp. Mar. Biol. Eco.. 2015;464:11-17.
- [Google Scholar]
- Reproduction in the spiny lobster Panulirus polyphagus (Herbst) J. Mar. Biol. Assoc. India. 1988;30:37-46. URIhttp://eprints.cmfri.org.in/id/eprint/1081
- [Google Scholar]
- Keenan, C.P., 1999. Culture of the Mud Crab, Genus Scylla – Past, Present and Future. In: Keenan, C.P., Blackshaw, A.A. (Eds.) Mud Crab Aquaculture and Biology. Proceeding of International Scientific Forum ACIAR, Darwin, Australia, vol. 78, pp. 216.
- A revision of the genus Scylla De Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae) Raff. Bull. Zool.. 1998;46:217-245.
- [Google Scholar]
- The effect of salinity and added substrates on growth and survival of Metapenaeus monoceros post-larvae (Decapoda: Penaeidae) Aquaculture. 2001;196:177-188. Retrieved fromhttps://www.academia.edu/5064912/The_effects_of_salinity_and_added_substrates_on_growth_and_survival_of_Metapenaeus_monoceros_Decapoda_Penaeidae_post-larvae.
- [Google Scholar]
- Habitat and some biological parameters of two species of mud crab Scylla in Southeast Sulawesi, Indonesia. In: Carman O., Sulistiono A., Purbayanto T., Suzuki S., Watanabe, Arimoto T., eds. TUF International JSPS Project Vol. 10. Proceedings JSPS-DGHE International Symposium of Fisheries Science in Tropical Area, Konan Minato-ku, Tokyo, Japan. 2001. p. :341-346.
- [Google Scholar]
- Physiological responses and ovarian development of female Chinese mitten crab Eriocheir sinensis subjected to different salinity conditions. Front. Physiol.. 2018;8:1072.
- [CrossRef] [Google Scholar]
- Morphological and histological studies on the embryonic development of the freshwater prawn, Macrobrachium rosenbergii (Crustacea, Decapoda) J. Basic App. Zool.. 2012;65(3):157-165.
- [Google Scholar]
- Regulation and function of estrogen receptors: comparative aspects. In: Melamed P., Sherwood N., eds. Hormones and their Receptors in Fish Reproduction. Singapore: World Scientific Publishing Co., Pte. Ltd.,; 2005. p. :224-253.
- [Google Scholar]
- Simulating the effects of fluctuating dissolved oxygen on growth, reproduction, and survival of fish and shrimp. J. Theor. Biol.. 2014;21:54-68.
- [CrossRef] [Google Scholar]
- Effect of vertebrate steroid hormones on the ovarian maturation stages of orange mud crab, Scylla olivacea (Herbst, 1796) Aquaculture. 2016;451:78-86.
- [CrossRef] [Google Scholar]
- Identification of nonecdysteroid steroids in hemolymph of both male and female Astacus leptodactylus (Crustacea) by gas chromatography-mass spectrometry. Gen. Comp. Endocrinol.. 1986;61(2):214-228.
- [CrossRef] [Google Scholar]
- The early developmental stages of Scylla serrata Forskal (Crustacea: Portunidae), reared in the laboratory. Proceeding Indo-Pacific Fisheries Council. 1964;11:135-246.
- [Google Scholar]
- Responses of intermolt Penaeus indicus to large fluctuations in environmental salinity. Aquaculture. 1987;64:175-184.
- [CrossRef] [Google Scholar]
- Parado-Estepa, F.D., Quinitio, E.T., 1997. Larval survival and megalopa production of Scylla sp. at different salinities. In: Proceeding of International Science Forum, Mud crab aquaculture and biology, pp. 175–177.
- Changes in the steroid hormone and vitellogenin levels during the gametogenic cycle of the giant tiger shrimp, Penaeus monodon. Comp. Biochem. Physiol.. 1994;109:21-26.
- [CrossRef] [Google Scholar]
- Ovarian maturation stages of the mud crab Scylla serrata. Aquacult. Res.. 2007;38:1434-1441.
- [CrossRef] [Google Scholar]
- The effects of salinity on the survival, growth and haemolymph osmolality of early juvenile blue swimmer crabs, Portunus pelagicus. Aquaculture. 2006;260:51-162.
- [CrossRef] [Google Scholar]
- Influence of salinity and temperature on molting and survival of the Australia freshwater crayfish (Cherax tenuimanus) Aquaculture. 1992;105:47-52.
- [CrossRef] [Google Scholar]
- Shelley, C., Lovatelli, A., 2011. Mud crab aquaculture: A practical manual FAO, Fisheries and Aquaculture Technical Paper No. 567, FAO, Rome, Italy, pp. 78.
- Profile of progesterone in hepatopancreas, ovary, and hemolymph of shrimp Penaeus chinensis during reproduction cycle. J. Fish. Sci. China.. 2001;25(4):304-310.
- [Google Scholar]
- Sex steroid-like substances in the ovaries, hepatopancreas and body fluid of female Mictyris brevidactylus. Zool. Stud.. 1997;36:136-145.
- [Google Scholar]
- Crustacean ecdysteroids in reproduction and embryogenesis. Comp. Biochem. Physiol. C.. 2000;125:135-156.
- [CrossRef] [Google Scholar]
- Mechanism and control of vitellogenesis in crustaceans. Fish. Sci.. 2011;77:1-21.
- [CrossRef] [Google Scholar]
- Effects of stress tests on larvae of blue swimming crab, Portunus pelagicus (Linnaeus, 1758) Adv. Environment. Biol.. 2012;6:1909-1915.
- [Google Scholar]
- Sex steroid hormone receptors in fish ovaries. In: Babin P.J., Cerda J., Lubzens E., eds. The Fish Oocyte: From Basic Studies to Biotechnological Applications. Netherlands: Springer; 2007. Retrieved from https://link.springer.com/content/pdf/10.1007%2F978-1-4020-6235-3.pdf
- [Google Scholar]
- Crustacean vitellogenesis: its role in oocyte development. Amer. Zool.. 2001;41:465-476.
- [CrossRef] [Google Scholar]
- Effect of physico-chemical parameters on crabs biodiversity. J. Marine Sci. Res. Dev.. 2013;3(1):116.
- [CrossRef] [Google Scholar]
- Steroids and reproduction in starfish. In: Engels, ed. Advances in Invertebrate Reproduction. Netherland: Elsevier Science publishers Amsterdam; 1984. p. :151-161.
- [Google Scholar]
- Size distribution, length-weight relationship and size at the onset of sexual maturity of the orange mud crab, Scylla olivacea. Malaysia waters. Mar. Biol. Res.. 2016;12(7):726-738.
- [CrossRef] [Google Scholar]
- Transcriptome analysis and differential gene expression on the testis of orange mud crab, Scylla olivacea, during sexual maturation. Plos One. 2017;12(1) e0171095
- [CrossRef] [Google Scholar]
- Habitat selectivity of megalopae and juvenile mud crabs (Scylla serrata): implications for recruitment mechanism. Mar. Biol.. 2009;156:891-899.
- [CrossRef] [Google Scholar]
- Progress in vitellogenesis. In: Raikhel A.S., Sappington W., eds. Reproductive Biology of Invertebrates. Plymouth, UK: Science Publishers Inc.,; 2002. p. :131-174.
- [Google Scholar]
- Endocrine control of reproductive maturation in economically important crustacea for aquaculture. In: Adiyodi K.G., Adiyodi R.G., eds. Reproductive Biology of Invertebrates. New York: Wiley; 2000. p. :161-194.
- [Google Scholar]
- Effects of salinity on growth and energy budget of juvenile Penaeus monodon. Aquaculture. 2009;290:140-144.
- [CrossRef] [Google Scholar]
- Ovarian growth in the crab Chasmagnathus granulata induced by hormones and neuroregulators throughout the year. In vivo and in vitro studies. Aquaculture. 2003;224(1-4):339-353.
- [CrossRef] [Google Scholar]







