Translate this page into:
Distribution of APOE gene variations in the Jordanian population: Association with longevity
⁎Corresponding author at: Faculty of Applied Medical Sciences, Jordan University of Science and Technology, P.O. Box 3030, Irbid 22110, Jordan. khabour@just.edu.jo (Omar F. Khabour)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
APOE gene common variants, known as ε2, ε3 and ε4, can modulate the risk of several age- associated diseases in human. The aim of the current investigation was to investigate the distribution of APOE gene variations and their contribution to human longevity in Jordan. The genotypes of the APOE gene were identified in 203 subjects (101 young and 102 older adults) using polymerase chain reaction. Allele frequencies of APOE variants were: 0.03, 0.925 and 0.045 for ε2, ε3 and ε4 respectively. The ε4 frequency was more abundant in young (0.045) than in older adults (0.005, p < 0.01). The APOE gene polymorphism might be associated with longevity phenotype in Jordanian population.
Keywords
Longevity
Allele
ApoE gene
Variant
Isoform
Lipid metabolism
1 Introduction
Apolipoprotein E (apoE) is a member of apolipoprotein family that mediates several functions inside the body. It exists as a constituent of chylomicrons, chylomicron remnants, and high- very low- and intermediate-density lipoproteins (HDL, VLDL, and IDL respectively). ApoE is involved in lipid metabolism (Rasmussen, 2016) via the regulation of uptake of remnant lipoproteins by the liver and facilitation of cholesterol efflux from foam cells (Greenow et al., 2005). In addition, apoE contributes to the inflammation (Gonzalez et al., 2017) by regulation of macrophages and suppression of T cell proliferation (Liu et al., 2016). High expression of apoE has been reported in the brain, liver and retina (Elshourbagy et al., 1985). In the brain, apoE is among the players that mediate the central nervous system response to injury and oxidative stress pathways (Handattu et al., 2013; Verghese et al., 2011).
The gene that encode apoE (APOE) contains 4 exons and 3 introns and is located 19q (Smith et al., 1988). Three different variations (ε2, ε3 and ε4) have been reported in the APOE gene that code for three isoforms (Schachter et al., 1994). The three isoforms of APOE differ at amino acids of the protein sequence (at position 112 and position 158). ε3 isoform has cystein and arginine at these positions, whereas ε4 has arginine at both sites, and ε2 has cysteines at both sites. Compared with ε3, individuals with ε4 isoform have high levels of LDL, whereas ε2 individuals have low levels of LDL (Mahley, 2016). Several diseases have been shown to be associated with APOE isoforms. For example, APOE ε4 variant has been shown to play a role in the pathogenesis of Alzheimer’s disease (Limon-Sztencel et al., 2016; Liu et al., 2013), head trauma (Olivecrona and Koskinen, 2017) ischemic stroke (Chauhan and Debette, 2016), coronary artery disease (Ciftdogan et al., 2012), PD (Li et al., 2004; Lopez et al., 2007) and diabetic neuropathy (Ng et al., 2006). In addition, a strong association has been reported between APOE ε4 variant and decline in cognition in multiple sclerosis patients, particularly in the domains of learning and memory (Mazurek and Shi, 2008). Previous studies showed differences in the APOE ε4 allele among different ethnic populations and to be varied with latitude as higher frequencies of ε4 allele were reported in countries of the equator and in the northern polar regions (Hu et al., 2011; Kern et al., 2015; Lucotte et al., 1997).
Due to the involvement of apoE in the body metabolism, immune response and oxidative stress, APOE gene has been suggested to be a good candidate that might impact human longevity (Raichlen and Alexander, 2014; Tindale et al., 2017). In fact, genome-wide association studies (GWAS) identified the APOE allele ε4 as a strong determinant of human mortality before age 90 (Deelen et al., 2011; Fortney et al., 2015; Nebel et al., 2011). In this study, we aimed at examining the distribution of APOE gene variants in the Jordanian population. In addition, the association between APOE variants and longevity was examined.
2 Materials and methods
2.1 Study population
Two hundred and three unrelated subjects were recruited from Northern Jordan to participate in the study. Participants were recruited into two groups based on their age: older adults group (>85 yrs, mean age 91.4 yrs, n = 102: 67 male and 35 female) and young group (20–50 yrs, mean age 31.8 yrs, n = 101: 64 male 37 female). Since specialized aging centers are absent in Jordan, recruitment was performed by advertising through the university e-mail directory and via asking in the neighborhoods in the Northern part of Jordan. Since men are usually more known in the neighborhoods than women, this could explain the relatively higher number of male participants recruited in the study. Thus, the sample might not reflect the actual distribution of both genders in older adult population in Jordan. The study design and selection criteria were according to previous longevity investigations reviewed in (Glatt et al., 2007). In addition, the age range of the young group was selected based on the reported Jordanians mortality rate that starts gradually increasing from the early fifties and reach a maximum in the late seventies (Khoury et al., 1999). According to statistics, life expectancy in Jordan is 74.1 years and death rate is 3.4 deaths/1000 population (Alloubani et al., 2016). Accordingly, persons who are >85 years are rare in the country (Khabour et al., 2010) with an estimated percentage of less than 0.3% of the population distribution of the year 2016 (Jordan in Figures, department of statistics, http://dosweb.dos.gov.jo/product/jordan-in-figures-2016/) All participants gave written informed consent as required by the IRB (Institutional Review Boards) of King Abdulla University Hospital/ Jordan University of Science and Technology.
2.2 DNA isolation
Blood (3 ml) from each participant was obtained from cubital vein in EDTA collection tubes. Genomic DNA was extracted from white blood cells using the Promega DNA extraction kit as shown in the kit manual (Promega, Madison, USA). After extraction, concentration of DNA was determined using Nanodrop Spectrophotometer (2000c, Thermo Scientific, Wilmington, DE, USA) and then DNA was stored at −35 °C.
2.3 APOE genotyping
The APOE variations were determined using polymerase chain reaction followed by restriction digestion of amplified fragment (Wenham et al., 1991). The template DNA fragment (227 bp) was amplified using the following primers (Forward: 5′CCAAGGAGCTGCAGGCGGCGCA3′) and a reverse primer (Reverse: 5′ACAGAATTCGCCCCGGCCTGGTACAC-3′) (Alpha DNA). The reaction volume of 20 µl contained 50 ng of DNA, 0.30 mM of each dNTP, 0.30 µM of each primer, 5% dimethyl sulfoxide, 1X Gotaq green buffer, and 0.5 u of DNA polymerase (Promega, Madison, USA). The reaction was denatured at 95 °C for 6 min, followed by 31 cycles of 94 °C for 35 s, 60.5 °C for 30 s, and 72 °C for 90 s. Then the reaction was subjected to 7 min final extension at 72 °C. Amplification was carried out using a BioRad thermocycler model C1000 (Philadelphia, PA, USA). The amplified 227 bp gene fragment was restricted with 4 u of HhaI (Fermentas, Germany) at 37 °C for 4 h. The restricted DNA fragment was then separated using 10% polyacrylamide gel and vertical electrophoresis tank obtained from Bio Rad (Philadelphia, PA, USA). DNA bands were detected by ethidium bromide staining. The PCR product with the ε3 allele was digested to six fragments (16, 18, 21, 33, 48 and 91 bp), ε4 allele to seven fragments (16, 18, 19, 21, 33, 48 and 72 bp) and ε2 allele to five fragments (16, 18, 21, 81 and 91 bp). DNA ladder of 10–150 bp was used as a marker to estimate the sizes of the restricted DNA fragments (Fig. 1).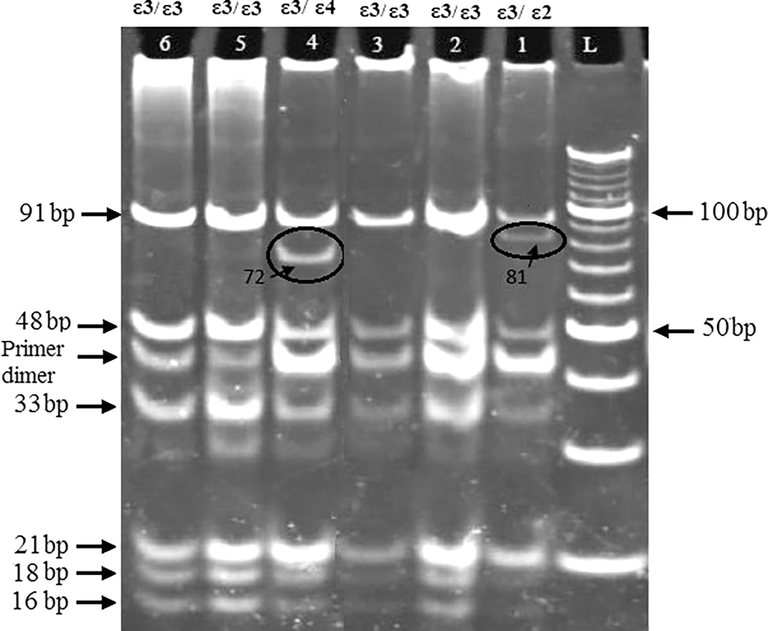
Gel electrophoresis of APOE isoforms. Lanes 1–6 are treated samples with HhaI enzyme. Sample in lane 1 represents ε3/ε2 genotype. Sample in lane 4 represents ε3/ε4 genotype. Samples in lanes 2, 3, 5, 6 represent ε3/ε3 genotype. L represents 10 bp DNA ladder. Digestion of the PCR product with the ε3 gives six fragments (16, 18, 21, 33, 48 and 91 bp) whereas ε4 allele gives seven fragments (16, 18, 19, 21, 33, 48 and 72 bp) and ε2 allele gives five fragments (16, 18, 21, 81 and 91 bp). The prominent band between 33 and 48 bp appeared in all PCR reactions and from its size is expected to be for primer dimers.
2.4 Statistical analysis
The APOE allelic and genotype frequencies were analyzed using Chi square/ Fisher's exact tests as appropriate. A P value of less than 5% was considered significant.
3 Results
Demographic characteristics of participants are shown in Table 1. The average age of the young group was 31.8 years and of the older adults was 91.4 years. The distribution of the sample with respect to gender and smoking status between older adults and young was similar (P > 0.05).
Older adults
N = 102Controls
N = 101P value
Age (mean ± SEM)
90.06 ± 1.2
31.8 ± 5.4
<0.001
Gender
Male
67 (66%)
64 (63%)
0.405
Female
35 (34%)
37 (37%)
% of smokers
30.5%
28.2%
0.775
Presence of first/second degree relatives >85 years
70.3%
59.8%
0.013
The allelic and genotypic frequencies of the APOE variations in the studied sample are shown in Table 2. Three genotypes were detected and their frequencies were ε3/ε3: 85.1%, ε3/ε2: 5.6%, ε3/ε4: 8.5%. Thus, allele ε3 is very common in the Jordanian population with a frequency that reaches 92.5%. On the other hand, allele ε2 and ε4 occur in very low frequency (3% and 4.5% respectively). This distribution of APOE alleles was within the range observed in other populations (Table 3).
Genotypes and Alleles
Older adults N (percentage)
Young N (percentage)
P value a
ε3/ε3
89 (87.3)
86 (85.1)
0.013
ε3/ε2
12 (11.8)
6 (5.9)
ε3/ε4
1 (1)
9 (8.9)
Allele ε3
191 (93.6)
187 (92.5)
0.012
Allele ε2
12 (5.9)
6 (3.0)
Allele ε4
1 (0.5)
9 (4.5)
Region/country
Sample size
ε2 allele
ε3 allele
ε4 allele
Middle East
Jordan (this study)
203
0.030
0.925
0.045
Omani Al-Yahyaee et al. (2005)
162
0.052
0.886
0.062
Lebanese Almawi et al. (1999)
155
0.071
0.881
0.048
Saudis Al-Muhanna et al. (2008)
62
0.030
0.840
0.130
Asia
Chinese Guan et al. (2011)
746
0.162
0.698
0.150
Europe
Serbian Topic et al. (2008)
326
0.083
0.766
0.152
Turkey Ilhan et al. (2007))
108
0.043
0.935
0.022
Spanish Haddy et al. (2002)
1009
0.077
0.812
0.111
Greek Sklavounou et al. (1997)
216
0.053
0.882
0.065
South America
Brazilian Alvim et al. (2010)
1493
0.123
0.661
0.266
Argentinean Morelli et al. (1996)
101
0.059
0.787
0.153
The genotypes of APOE were different between the young and older adults (P < 0.05). Similarly, the allelic distribution between the two groups were significantly different (P < 0.012). Thus, APOE polymorphism might be related to life span in the studied population.
4 Discussion
The aim of this case-control study was to examine the distribution of APOE variations and their contribution to human longevity in Jordan. Strong association was found between APOE variations and longevity phenotype in the studied population.
The clinical importance of APOE variations promotes the researcher to investigate their distribution among different populations. In this study, the results showed that the frequency of ε3 allele in Jordan is 92.5%, which considered among the highest globally (Table 3). Similar frequencies were also detected in neighboring countries like Lebanon, Oman and Turkey (Al-Yahyaee et al., 2005; Almawi et al., 1999; Ilhan et al., 2007). However, a slightly lower abundance of the ε3 was reported in countries like Brazil, Serbia, Spain and China with a range: 66–81% (Alvim et al., 2010; Guan et al., 2011; Haddy et al., 2002; Topic et al., 2008). On the other hand, the prevalence of ε2 and ε4 is very low among Jordanians with frequencies of 3% and 4.5% respectively. These frequencies are within the range of neighboring countries, but are slightly lower than that in countries far from Jordan (Table 3). Thus, Jordan belongs to the countries with the lowest APOE ε4 allele frequency, which seems characteristic for the countries from the Middle East (Al-Muhanna et al., 2008; Alloubani et al., 2016). The present distribution of APOE isoforms in Jordan is also in concordance with global pattern that shows relatively high frequency of ε4 allele in countries of the equator and in the northern polar regions (Hu et al., 2011; Kern et al., 2015; Lucotte et al., 1997; Morelli et al., 1996; Sklavounou et al., 1997). Despite these population variance in the distribution of APOE isoforms, in the majority ε3 is the common allele followed by ε4 and then ε2.
The findings of the current study revealed a strong association between APOE and longevity in Jordanian population. Data showed that APOE ε4 is associated with reduction in life span. This result is in agreement with several studies done in many populations such as Finland, Ireland, Denmark, Japan, China, Sweden and USA (Reviewed in (Ang et al., 2008). In addition, GWAS identified the APOE allele ε4 as a strong determinant of human mortality before age 90 (Deelen et al., 2011; Fortney et al., 2015; Nebel et al., 2011). In contrast, few reports were unable to show such association between APOE and longevity (Galinsky et al., 1997; Liu et al., 2017).
The APOE ε4 might affect lifespan in different ways. Many studies showed that ε4 contributes to the risk of many age related conditions such as Alzheimer’s disease, head trauma, stroke; PD; diabetic neuropathy and cognitive impairment (Ciftdogan et al., 2012; Crawford et al., 2002; Li et al., 2004; Lopez et al., 2007; Mazurek and Shi, 2008; Olivecrona and Koskinen, 2012; Rassas et al., 2012; Takei et al., 2009; Wang et al., 2009). Other reports found that presence of APOE ε4 might affect the oxidative stress status inside the body (Stephens et al., 2008). In addition, the antioxidant effectiveness of apoE lipoproteins against cytotoxicity of H2O2 was ε2 > ε3 > ε4 (Jolivalt et al., 2000). Moreover, levels of circulatory lipid peroxides are higher in ε4 isoform carriers compared to ε2 ones (Fernandes et al., 1999; Smith et al., 1998). Furthermore, lipoproteins in the plasma from mice deficient in APOE gene are more prone to oxidative stress than the ones from normal animals (Stephens et al., 2008). The last pathway by which ε4 might impact longevity is via its effect on promoting inflammation by causing macrophage dysfunction, and on enhancing apoptosis via endoplasmic reticulum stress induction (Dose et al., 2016).
It is worth to mention that longevity is a multifactorial trait that can be affected by genetic as well as environmental factors. Among the other loci that have been shown to affect longevity are adiponectin, tyrosine hydroxylase, IL-6 and, haemochromatosis, interferon-gamma and APOC-I (Glatt et al., 2007; Khabour et al., 2010). None genetic (environmental) factors might include diet, pollution and life styles (Christen, 2003). The contribution of genetic factors to human longevity is estimated to be close to 25%, while 75% is attributed to environmental factors (Snejdrlova et al., 2011).
In this study, we assumed that the initial APOE allelic distribution in the young and older adults are not different, and the mortality risk due to the different genotypes of APOE is not affected by the year of birth. However, a review study that was conducted on fifteen investigations of APOE has shown variation in APOE allele frequencies between geographically proximate populations and changes in APOE related causes of death over time (Lewis and Brunner, 2004). The current study was conducted in Jordan, which is a small country in the Middle East. About 98% of the current population are Arab (Khabour et al., 2010). In addition, the distribution of the common APOE alleles among Jordanians is similar to that of neighboring countries. Moreover, older adults and young were from the same neighborhood and thus the sample is considered homogeneous. However, the finding that APOE related causes of death changes over the years (Lewis and Brunner, 2004) is considered as a confounding factor that might affect the conclusion drawn from the study. Another possible confound factor could be the high frequency of consanguineous marriage in Jordan.
In conclusion, the results of the current investigation indicate that APOE gene might be associated with longevity in Jordanian population.
Acknowledgements
The authors thank Deanship at JUST University for providing financial support to conduct the study (grant number 182/2007 to OK). The authors thank Mr. J. Barnawi for his technical help.
References
- Polymorphism in methylenetetrahydrofolate reductase, plasminogen activator inhibitor-1, and apolipoprotein E in hemodialysis patients. Saudi J. Kidney Dis. Transpl.. 2008;19:937-941.
- [Google Scholar]
- Distribution of apolipoprotein E alleles in the Omani population. Med. Princ. Pract.. 2005;14:73-78.
- [Google Scholar]
- Relative and global health: a comparative study between healthcare systems of jordan and france. World Health Popul.. 2016;16:9-19.
- [Google Scholar]
- Apolipoprotein E polymorphism in a healthy Lebanese population. Med. Principles Pract.. 1999;8:6.
- [Google Scholar]
- APOE polymorphism is associated with lipid profile, but not with arterial stiffness in the general population. Lipids Health Dis.. 2010;9:128.
- [Google Scholar]
- Apolipoprotein E, an important player in longevity and age-related diseases. Exp. Gerontol.. 2008;43:615-622.
- [Google Scholar]
- Genetic risk factors for ischemic and hemorrhagic stroke. Curr. Cardiol. Rep.. 2016;18:124.
- [Google Scholar]
- The association of apolipoprotein E polymorphism and lipid levels in children with a family history of premature coronary artery disease. J. Clin. Lipidol.. 2012;6:81-87.
- [Google Scholar]
- APOE genotype influences acquisition and recall following traumatic brain injury. Neurology. 2002;58:1115-1118.
- [Google Scholar]
- Genome-wide association study identifies a single major locus contributing to survival into old age; the APOE locus revisited. Aging Cell. 2011;10:686-698.
- [Google Scholar]
- APOE genotype and stress response – A mini review. Lipids Health Dis.. 2016;15:121.
- [Google Scholar]
- Apolipoprotein E mRNA is abundant in the brain and adrenals, as well as in the liver, and is present in other peripheral tissues of rats and marmosets. Proc. Natl. Acad. Sci. U.S.A.. 1985;82:203-207.
- [Google Scholar]
- Effects of apolipoprotein E genotype on blood lipid composition and membrane platelet fluidity in Alzheimer's disease. Biochim. Biophys. Acta. 1999;1454:89-96.
- [Google Scholar]
- Genome-Wide Scan Informed by Age-Related Disease Identifies Loci for Exceptional Human Longevity. PLoS Genet.. 2015;11:e1005728.
- [Google Scholar]
- Analysis of the apo E/apo C-I, angiotensin converting enzyme and methylenetetrahydrofolate reductase genes as candidates affecting human longevity. Atherosclerosis. 1997;129:177-183.
- [Google Scholar]
- Tau Spread, apolipoprotein E, inflammation, and more: rapidly evolving basic science in alzheimer disease. Neurol. Clin.. 2017;35:175-190.
- [Google Scholar]
- The key role of apolipoprotein E in atherosclerosis. J. Mol. Med. (Berl.). 2005;83:329-342.
- [Google Scholar]
- The relationship between apolipoprotein (apo) E polymorphism and lipid changes: an 8-year cohort study in Beijing elderly persons. Arch. Gerontol. Geriatr.. 2011;55:713-717.
- [Google Scholar]
- The importance of plasma apolipoprotein E concentration in addition to its common polymorphism on inter-individual variation in lipid levels: results from Apo Europe. Eur. J. Hum. Genet.. 2002;10:841-850.
- [Google Scholar]
- In vivo and in vitro effects of an apolipoprotein e mimetic peptide on amyloid-beta pathology. J. Alzheimer's Dis.. 2013;36:335-347.
- [Google Scholar]
- Does the geographical gradient of ApoE4 allele exist in China? A systemic comparison among multiple Chinese populations. Mol. Biol. Rep.. 2011;38:489-494.
- [Google Scholar]
- Apo E gene polymorphism on development of diabetic nephropathy. Cell Biochem. Funct.. 2007;25:527-532.
- [Google Scholar]
- Differential oxidation of apolipoprotein E isoforms and interaction with phospholipids. Free Radic Biol. Med.. 2000;28:129-140.
- [Google Scholar]
- The distribution of apolipoprotein E genotype over the adult lifespan and in relation to country of birth. Am. J. Epidemiol.. 2015;181:214-217.
- [Google Scholar]
- Associations of polymorphisms in adiponectin and leptin genes with men's longevity. Aging Male. 2010;13:188-193.
- [Google Scholar]
- Mortality and causes of death in Jordan 1995–96: assessment by verbal autopsy. Bull. World Health Organ.. 1999;77:641-650.
- [Google Scholar]
- Methodological problems in genetic association studies of longevity-the apolipoprotein E gene as an example. Int. J. Epidemiol.. 2004;33:962-970.
- [Google Scholar]
- Apolipoprotein E controls the risk and age at onset of Parkinson disease. Neurology. 2004;62:2005-2009.
- [Google Scholar]
- The algorithm for Alzheimer risk assessment based on APOE promoter polymorphisms. Alzheimer's Res. Ther.. 2016;8:19.
- [Google Scholar]
- Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol.. 2013;9:106-118.
- [Google Scholar]
- Allergic lung inflammation promotes atherosclerosis in apolipoprotein E-deficient mice. Transl. Res.: J. Lab. Clin. Med.. 2016;171:1-16.
- [Google Scholar]
- APOE gene polymorphism in long-lived individuals from a central China population. Sci. Rep.. 2017;7:3292.
- [Google Scholar]
- Apolipoprotein E epsilon4 allele is associated with Parkinson disease risk in a Mexican Mestizo population. Mov. Disord.. 2007;22:417-420.
- [Google Scholar]
- Pattern of gradient of apolipoprotein E allele *4 frequencies in western Europe. Hum. Biol.. 1997;69:253-262.
- [Google Scholar]
- Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J. Mol. Med.. 2016;94:739-746.
- [Google Scholar]
- APOE epsilon4 allele is associated with cognitive impairment in patients with multiple sclerosis. Neurol.. 2008;71:1203. author reply 1203
- [Google Scholar]
- Apolipoprotein E polymorphism and late onset Alzheimer's disease in Argentina. J. Neurol. Neurosurg. Psychiatry. 1996;61:426-427.
- [Google Scholar]
- A genome-wide association study confirms APOE as the major gene influencing survival in long-lived individuals. Mech. Ageing Dev.. 2011;132:324-330.
- [Google Scholar]
- Association of lipoprotein lipase S447X, apolipoprotein E exon 4, and apoC3 -455T>C polymorphisms on the susceptibility to diabetic nephropathy. Clin. Genet.. 2006;70:20-28.
- [Google Scholar]
- APOE epsilon4 positive patients suffering severe traumatic head injury are more prone to undergo decompressive hemicraniectomy. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas.. 2017;42:139-142.
- [Google Scholar]
- The release of S-100B and NSE in severe traumatic head injury is associated with APOE epsilon4. Acta Neurochir. (Wien). 2012;154:675-680. discussion 680
- [Google Scholar]
- Exercise, APOE genotype, and the evolution of the human lifespan. Trends Neurosci.. 2014;37:247-255.
- [Google Scholar]
- Plasma levels of apolipoprotein E, APOE genotype and risk of dementia and ischemic heart disease: a review. Atherosclerosis. 2016;255:145-155.
- [Google Scholar]
- High APOE epsilon 4 allele frequencies associated with Alzheimer disease in a Tunisian population. Neurol. Sci.. 2012;33:33-37.
- [Google Scholar]
- Genetic associations with human longevity at the APOE and ACE loci. Nat. Genet.. 1994;6:29-32.
- [Google Scholar]
- Apolipoprotein E polymorphism in the Greek population. Clin. Genet.. 1997;52:216-218.
- [Google Scholar]
- Expression of the human apolipoprotein E gene is regulated by multiple positive and negative elements. J. Biol. Chem.. 1988;263:8300-8308.
- [Google Scholar]
- The relationship between apolipoprotein E and serum oxidation-related variables is apolipoprotein E phenotype dependent. Int. J. Clin. Lab. Res.. 1998;28:116-121.
- [Google Scholar]
- APOE polymorphism as a potential determinant of functional fitness in the elderly regardless of nutritional status. Neuro. Endocrinol. Lett.. 2011;32(Suppl 2):51-54.
- [Google Scholar]
- Dendritic cell differentiation induced by a self-peptide derived from apolipoprotein E. J. Immunol.. 2008;181:6859-6871.
- [Google Scholar]
- Genetic association study on in and around the APOE in late-onset Alzheimer disease in Japanese. Genomics. 2009;93:441-448.
- [Google Scholar]
- Lipid and Alzheimer's disease genes associated with healthy aging and longevity in healthy oldest-old. Oncotarget. 2017;8:20612-20621.
- [Google Scholar]
- Gender-related effect of apo E polymorphism on lipoprotein particle sizes in the middle-aged subjects. Clin. Biochem.. 2008;41:361-367.
- [Google Scholar]
- Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol.. 2011;10:241-252.
- [Google Scholar]
- Association of genetic variation in apolipoprotein E and low density lipoprotein receptor with ischemic stroke in Northern Han Chinese. J. Neurol. Sci.. 2009;276:118-122.
- [Google Scholar]







