Diastereoselective synthesis and anticancer potential of a small library of cage-like heterocyclic hybrids
⁎Corresponding author. sraju@ksu.edu.sa (Raju Suresh Kumar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Polycyclic cage-like heterocyclic hybrids comprising structurally diverse heterocyclic units have been synthesized in good yields. Anticancer evaluation of these compounds against MCF-7 and NCI-H460 cell lines revealed dose dependent reduction with significant anticancer activity. Compound 4b inhibited these cell lines respectively with IC50 of 10.86 ± 0.94 and 9.17 ± 0.63 µM. Apoptosis and cell cycle analysis in MCF-7 revealed that this compound was able to induce early apoptosis.
Abstract
With an aim to construct novel anticancer drugs, a series of polycyclic heterocycles comprising diverse structural sub-units based on molecular hybridization strategy have been designed and synthesized through a three-component [3 + 2]-cycloaddition/annulation strategy. Anticancer evaluation of these compounds against MCF-7 and NCI-H460 cell lines revealed dose dependent reduction with noteworthy anticancer activity. Compound 4b inhibited MCF-7 and NCI-H460 cell lines with IC50 values 10.86 ± 0.94 and 9.17 ± 0.63 µM respectively. Further, apoptosis and cell cycle analysis revealed that this compound was able to prompt apoptosis at an early stage in MCF-7 cell line besides increasing the threshold of MMP and % of cells expressing FITC-dUTP. These results suggest that compound 4b is a potential molecule for the further exploration.
Keywords
Cage-like heterocyclic hybrids
Stereoselective synthesis
MCF-7
Flow cytometry
Apoptosis
JC1
Cell cycle
TUNEL assay
Caspase 3
1 Introduction
Cancer is defined as an abnormal cell division that may have a potential to invade (metastasis) the other body parts. There are various types of cancer classified based on the site of tissue or origin viz., breast cancer, non-small lung cancer, ovarian cancer, melanoma and brain gliomas. The incidence of global cancer was estimated as 18 million cases in 2018 which by 2040 presumed to further rise of 25 million cancer cases especially in low- or middle-income nations (Seyfried and Huysentruyt, 2013; Carbone, 2020). The global cancer data of the International Agency for Research on Cancer (IARC) revealed higher mortality rate in men than women i.e., 196.8 cases of men per 10,000 cases versus 139.6/10000 of women cancer (Lyon, 2018). The major types of cancer commonly noticed worldwide are lung, breast, bowel, and prostate cancers (Parkin, 2001). The cell line has been proved as an appropriate in vitro model to screen the molecules for anti-breast cancer activity worldwide. MCF-7 cell line is Estrogen Receptor (ER) and Progesterone Receptor (PR) – positive and that belongs to the luminal a molecular subtype. MCF-7 is a less aggressive and low malignant cell line (Comşa et al., 2015). Among the lung cancer, 80–85% cancers are contributed by non-small lung cancer which is a rare subtype of lung cancer originated in alveoli known as adenocarcinoma in situ (AIS). AIS is non-aggressive and non-invasive tumor needs immediate treatment (Ten Haaf et al., 2017). For in vitro cytotoxicity investigation of molecules lung cancer cell lines NCI-H460 was found to be a suitable in vitro model (Love, 2015; Smith et al., 1984).
Chemical synthesis of isoquinoline-based heterocyclic hybrids is one of the central themes for synthetic organic chemists. Pyrroloisoquinolines is a highly biologically active nucleus and the central core of many drugs and natural products. Their importance has been further enriched by their usefulness as intermediates for alkaloids synthesis (Mikhailovskii and Shklyaev, 1997). In particular, the pyrrolo[1,2-b]isoquinoline nucleus is prevalent in some natural products such as lycorine (Hoshino and The, 1998) and the phenanthroindolizidine alkaloids (Li et al., 2001; Michael, 2003). Besides, isoquinoline and pyrroloisoquinoline structural moieties possess important pharmocological properties such as antimicrobial, antitumor, antibiotic, antidepressant, muscarinic agonist, antiplatelet, and anticancer activity (Bentley, 1998; Dyke and Quessy, 1981).
4-Piperidone derivatives with one or more α,β-unsaturated unit were found to be highly active against the cancer cell lines (Dimmock et al., 2005; Das et al., 2008, 2007). The major interests to have these α,β-unsaturated keto pharmacophore in the heterocyclic hybrid is for two important reasons. Firstly, heterocyclic hybrids possessing α,β-unsaturated ketone units react better or entirely with thiols when compared to amino and hydroxy groups (Mutus et al., 1989; Dimmock et al., 1983). Therefore, the reactions with nucleic acids may be absent and hence these α,β-unsaturated ketone embedded molecules would be devoid of the genotoxic effects related with a number of anticancer drugs (Chen and Moore, 2007). Secondly, the existence of two thiol-alkylating groups viz. the olefinic carbon atoms in these molecules enables successive attacks of cellular thiols to occur which may be more detrimental to neoplasms than normal cells (Das et al., 2009). This phenomenon happens when an early chemical interaction in malignant cells creates greater chemosensitivity to a subsequent chemical insult in tumors rather than with normal cells (Chen and Waxman, 1994; Tsutsui et al., 1986). Besides, palladium cage compounds have been evaluated as drug delivery systems for cisplatin in vitro in cancer cells and ex vivo in healthy rat liver tissue (Schmidt et al., 2016a, 2016b; Ubaidullah et al., 2020). The rationale for the compound design is based on the molecular hybridization strategy as the molecules holding various structural motifs, in which each active motif exploits diverse means of action, could be of support in the treatment of difficult and multifactorial diseases such as cancers. We presume the combination of structural sub units viz. 4-piperidone derivatives with a α,β-unsaturated group, pyrroloquinoline and acenaphthene units with preselected biological activities in a cage-like framework would enhance biological profile of the molecule. In this context, we have explored the anticancer potential of few of the cage-like heterocycles of this kind (Almansour et al., US 10357485 B1; Kumar et al., 2020). The promising activity obtained by these heterocycles encouraged us to investigate the anticancer potential of library of similar cage-like compounds and report the results in this article.
2 Materials and methods
2.1 General procedure for the synthesis of cage-like heterocyclic hybrids 4(a–l)
An equimolar mixture of bisarylmethylidenetetrahydropyridinone 1, acenaphthenequinone 2 and Isoquinoline-3-carboxylic acid 3 in 100 mg of ionic liquid, 1-Butyl-3-methylimidazolium bromide ([bmim]Br) was subjected to microwave irradiation in a CEM microwave synthesizer at 100 °C for 9–12 min. When the reaction was complete, as judged by TLC analysis, 10 mL of ethyl acetate was added, followed by stirring for 15 min at ambient temperature. The residue obtained after removal of ethyl acetate was purified by column chromatography.
Materials, methods and complete experimental details can be found in the “Supplementary data” section.
3 Results and discussion
3.1 Chemistry
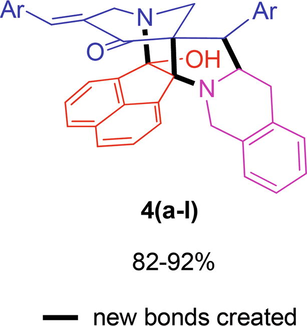
In the present work, we decided to construct a series of cage-like heterocycles, the methodology employed for their synthesis was constructed by a three-component reaction strategy comprising cycloaddition reaction of pyridinone 1, acenaphthenequinone 2 and 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid 3 followed by an annulation step. It is noteworthy to mention that these compounds were obtained in an excellent yield (82–92%) in [bmim]Br under microwave irradiation conditions. In an illustrative reaction, compounds 1, 2 and 3 in 100 mg of [bmim]Br was exposed to microwave irradiation at 100 °C (Scheme 1). TLC analysis of this reaction revealed completion of the reaction in 9–12 min. [bmim]Br besides serving as the reaction medium, also serves as catalyst assisting the reaction for completion comparatively in a shorter reaction time when compared to the conventional methods. All the reactions proceeded smoothly irrespective of the substituents on the aryl ring of pyridinones 1.
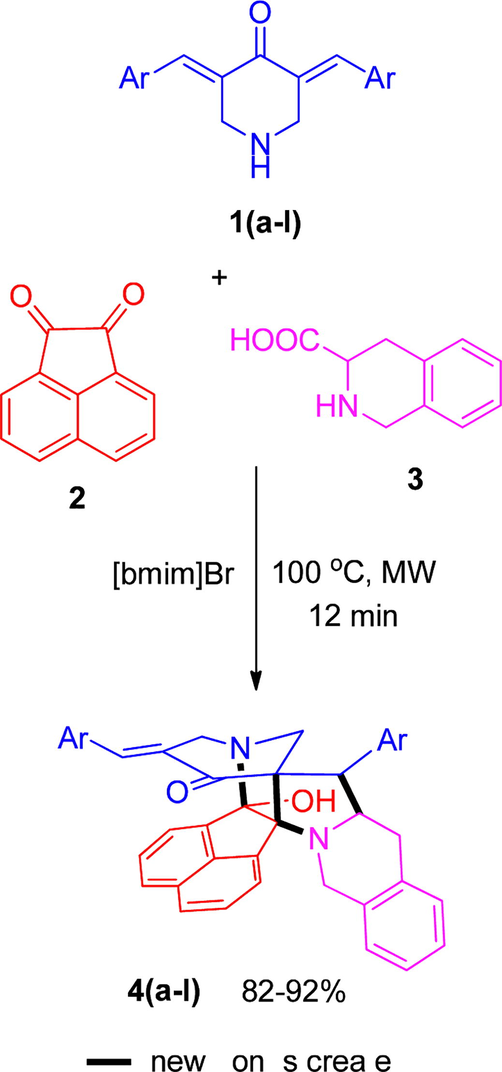
- Synthesis of polycyclic cage-like heterocycles 4(a–l).
FT-IR and NMR spectroscopic techniques were employed to arrive at the structures of 4(a-l) and the probable mechanism for their formation is shown in Scheme 2 and the pathway is similar to our previous reports (Almansour et al., 2019, US 10357485 B1; Kumar et al., 2019).
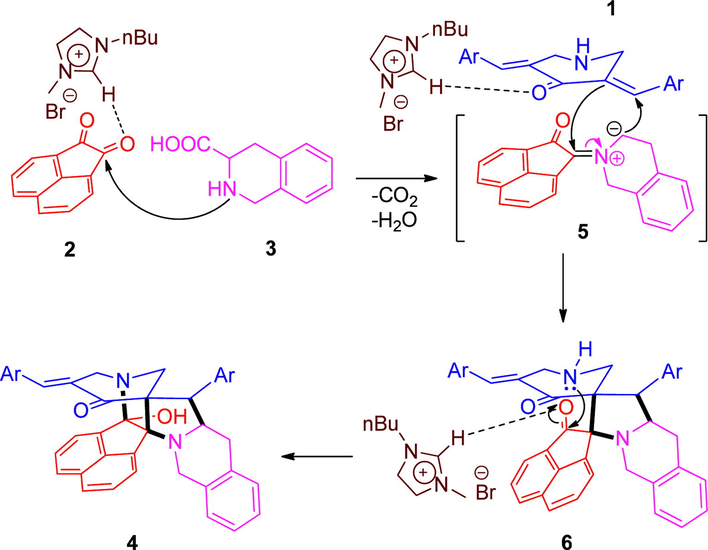
- Proposed mechanism for the construction of 4(a–l).
3.2 Biology
3.2.1 MTT antiproliferative assay
The presence of diverse heterocyclic units in single structural framework of cage-like compounds 4(a-l) might be extraordinary approach to explore them as anticancer agents. MTT assay (Mosmann, 1983; Yadav et al., 2014) was performed to analyze the cytotoxic effect of synthesized compounds and the IC50 value of test compounds have been identified. In the present investigation, compounds 4(a-l) were initially screened at 25, 50, 75, 100, 125 µM concentration, revealed a significant activity against breast cancer cell lines i.e., MCF-7 and non-small lung cancer cell lines viz., NCI-H460 which was the basis to carry out further assays at lower concentration. Based on potent activity, we further screened these compounds at 1, 5, 10, 15, and 20 µM concentrations. The assay conducted at 1, 5, 10, 15, and 20 µM of compounds showed concentration dependent reduction in the cell viability. Among the twelve analogs, compound 4k shows potent activity against MCF-7 cell line with an IC50 value of 10.52 µM, which was higher than standard drug camptothecin (14 µM) upon 48 h of the incubation period Fig. 1. Moreover, Compound 4b showed potent activity on both MCF-7 and NCI-H460 cell lines with IC50 values 10.86 and 9.17 µM, respectively. The IC50 of these cage-like heterocycles against MCF-7 and NCI-H460 cell lines are depicted in Table 1.
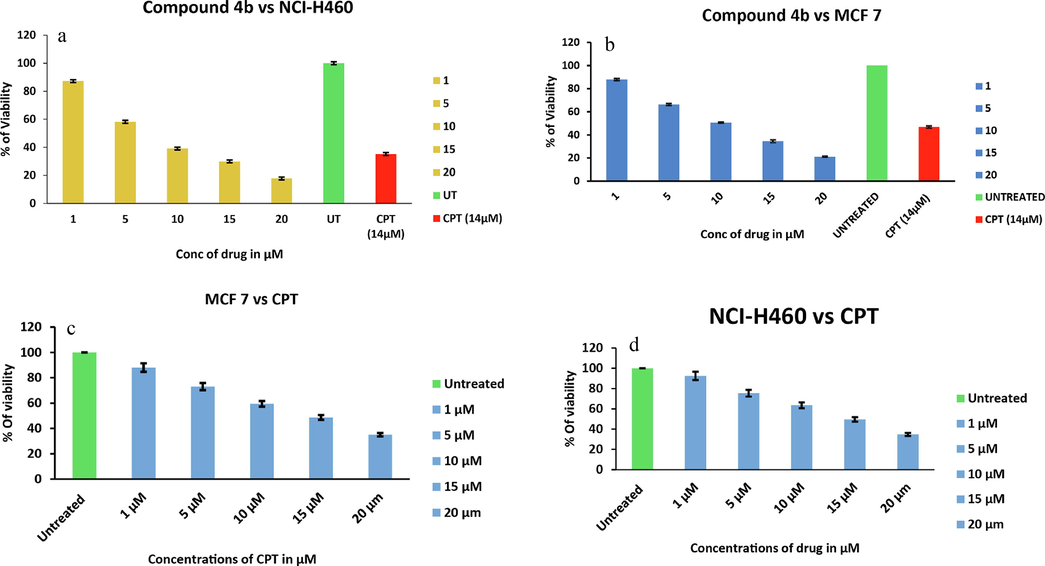
- Bar graphs representing % of cell viability after exposing to increasing concentrations of compound 4b and CPT for 48 hrs. (a) % of NCIH-460 cell viability vs 4b; (b) % of MCF7 cell viability vs 4b; (c) % of MCF7 cell viability vs CPT; (d) % of NCIH-460 cell viability vs CPT.
| Entry | Comp | Ar | 24 hrs. treatment IC50 values | 48 hrs. treatment IC50 values | ||
|---|---|---|---|---|---|---|
| MCF7 | NCI-H460 | MCF7 | NCI-H460 | |||
| 1 | 4a |
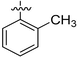
|
102.37 ± 0.98 | NA | 24.67 ± 0.732 | 42.5 ± 0.49 |
| 2 | 4b |
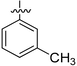
|
34.9 ± 0.13 | NA | 10.86 ± 0.94 | 9.17 ± 0.63 |
| 3 | 4c |
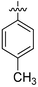
|
NA | 15.94 ± 0.37 | 10.74 ± 0.69 | 9.50 ± 0.72 |
| 4 | 4d |
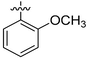
|
NA | NA | 14.07 ± 1.13 | 8.60 ± 0.28 |
| 5 | 4e |
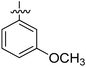
|
NA | NA | 11.87 ± 0.68 | 74.44 ± 0.91 |
| 6 | 4f |
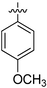
|
NA | NA | NA | NA |
| 7 | 4g |
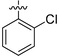
|
26.27 ± 1.08 | 114.57 ± 1.043 | 11.12 ± 0.81 | 9.727 ± 0.79 |
| 8 | 4h |
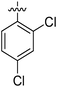
|
NA | NA | 57.94 ± 0.66 | NA |
| 9 | 4i |
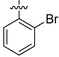
|
NA | NA | 39.44 ± 1.02 | NA |
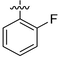
| 10 | 4j |
|
57.98 ± 0.58 | 68.25 ± 0.92 | 11.38 ± 0.69 | 10.32 ± 0.23 |
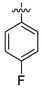
| 11 | 4k |
|
15.6 ± 1.14 | 58.14 ± 0.16 | 10.52 ± 0.96 | 10.46 ± 0.91 |
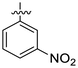
| 12 | 4l |
|
16.05 ± 1.45 | 15.66 ± 0.69 | 11.67 ± 0.77 | 10.37 ± 0.82 |
| 13 | CPT | – | 24.55 ± 0.12 | 21.32 ± 0.34 | 14.00 ± 0.73 | 14.00 ± 0.17 |
3.2.2 Apoptosis assay
To identify the apoptosis induction capabilities of compound 4b, we carried out apoptosis assay using Annexin V/FITC. The fluorescence intensities of FITC-conjugated annexin-V and PI in cells were analyzed using flow cytometry. Upon treatment with compound 4b at IC50 value, 15.22% cells showing early apoptosis and 25.55% cells showing late apoptosis. These results revealed that the heterocyclic compound 4b induces apoptosis in the MCF-7 cell lines Figs. 2 and 3.
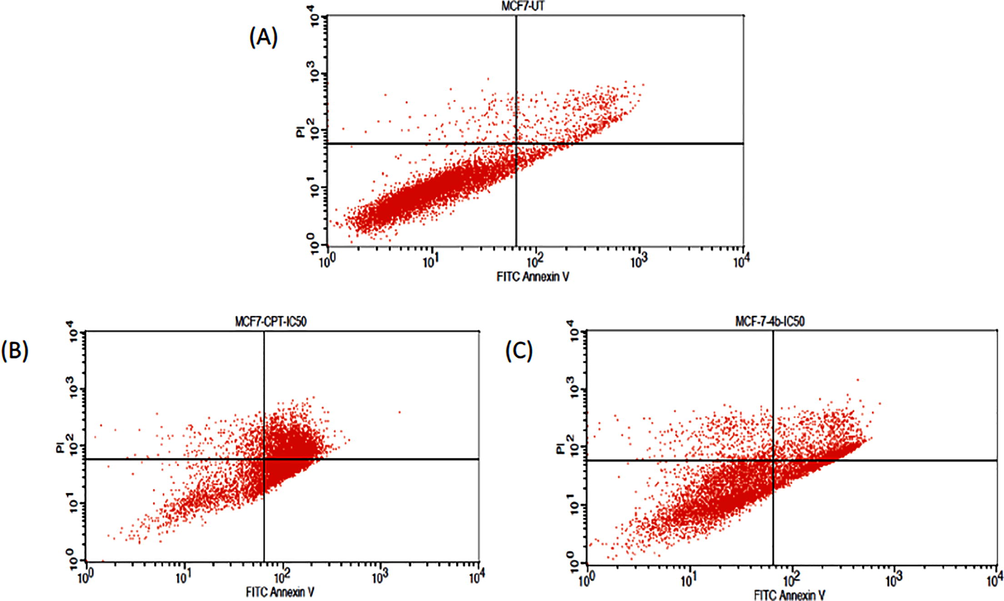
- Graph of FITC-Annexin V Vs PI staining against MCF-7 cell lines upon treatment with compound 4b and CPT at IC50 value; (A) Control untreated MCF-7 cells; (B) MCF-7 Cells exposed to standard drug CPT at IC50 value; (C) MCF-7 Cells incubated with 4b at IC50 value.
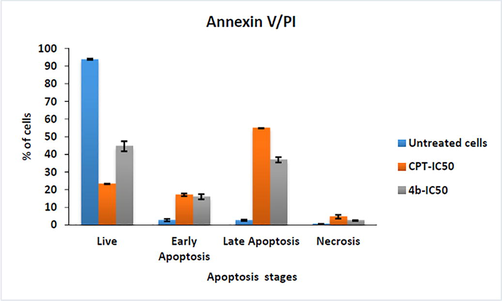
- Graph % of cells in each quadrant Vs compounds upon treatment with standard drug and Compound 4b.
3.2.3 Cell cycle analysis
Cell cycle analysis of MCF-7 cells revealed that the cells treated with IC50 concentrations of 4b has high cells at the G2/M phase. The positive control, CPT (14 μM) showed the lowest number of cells at the sub G0/G1 phase and the highest number of cells at the G0/G1 phase. There is no substantial change observed amongst all the groups for the G2/M phase and S phase Figs. 4 and 5.
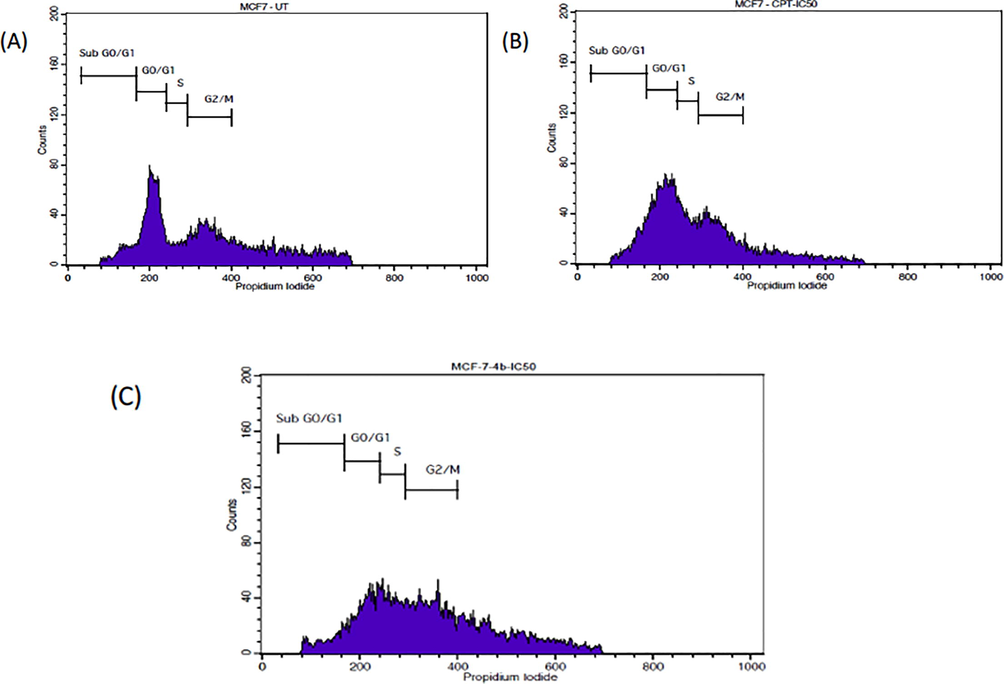
- FACS Histograms showing the phases of cell cycle distribution in the MCF-7 cell line treated with Compound 4b and standard drug CPT at IC50 value.
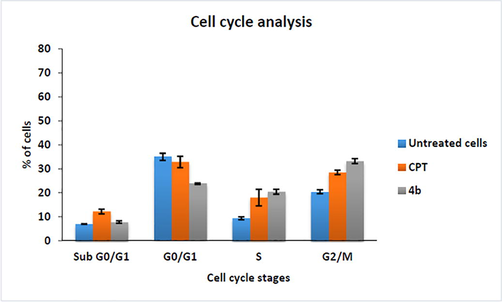
- Bar Plots of cell cycle phases of cells treated with compound 4b at its IC50 value.
3.2.4 Caspase 3 induction
Caspase 3 was up-regulated, as shown in Figs. 6 and 7. Camptothecin treated cells (positive control) showed the highest induction while there was the lowest expression in untreated cells (negative control). The upregulation of caspase 3 was observed in MCF-7 cell line when treated with compound 4b intermediate molecule. The percentage of breast cancer cell line expressing caspase 3 was found 19% versus 68.69% cell lines treated with of standard drug CPT.
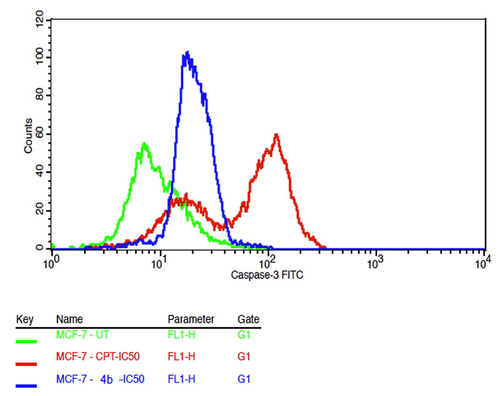
- Overlay of fluorescence intensities of Caspase 3 – FITC.
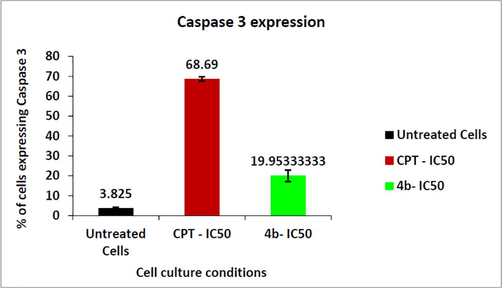
- Graph depicting the Caspase 3 quantification in MCF-7 cells treated with Compound 4b and positive control CPT.
3.2.5 Assessment of mitochondrial membrane potential (ΔΨ m)
Mitochondrial membrane integrity is one of the indicators of apoptosis initiation and progression. Disruption of MMP lead to release of proapoptotic factors, such as cytochrome c and other molecular factors which trigger the apoptosis in cells. When apoptosis is triggered, the failure in the MMP corresponds with the release of cytochrome c into the cytosol through the permeability transition pores of the mitochondria, which in turn activated the other downstream molecular factors of the apoptotic cascade. Upon treatment with compound 4b and CPT, increase in mitochondrial membrane potential was observed Figs. 8 and 9.
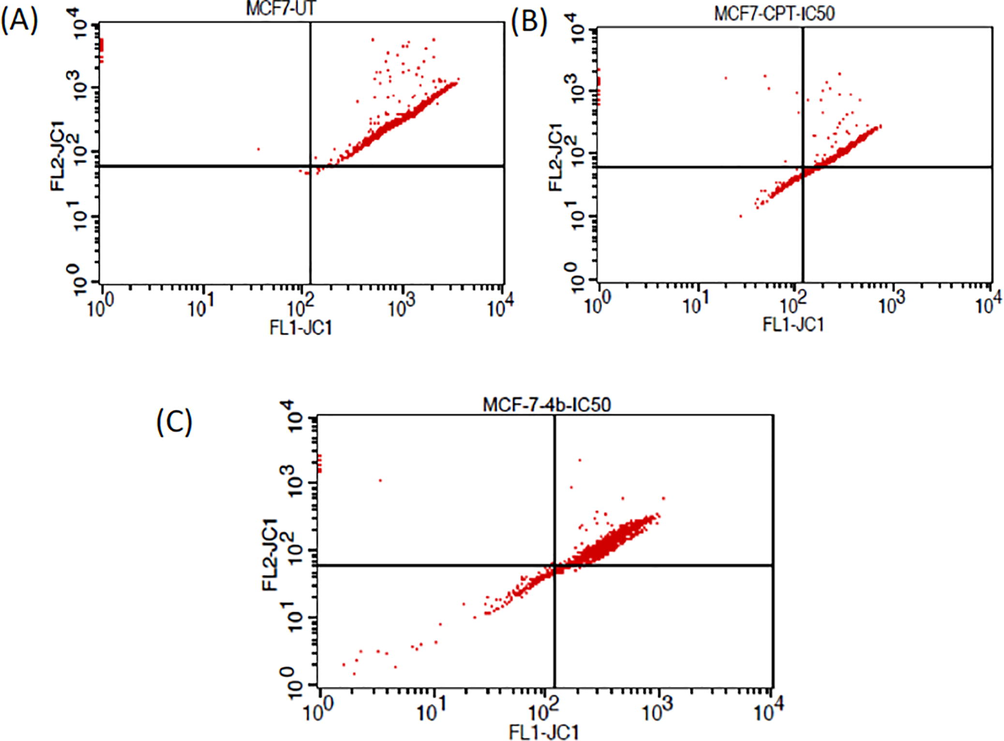
- Graph of FLT-JC1 Vs FLT-JC2 against MCF-7 cell lines upon treatment with compound 4b and CPT at IC50 value; (A) Control untreated MCF-7 cells; (B) MCF-7 Cells treated with standard drug CPT at IC50 value; (C) MCF-7 Cells treated with 4b at IC50 value.
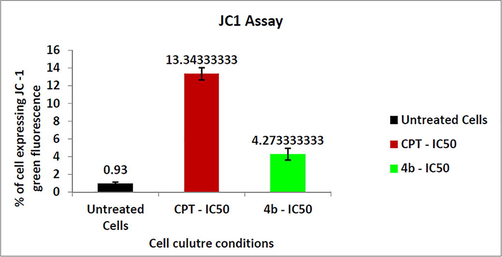
- Bar graph representing the % of cells expressing JC1 green fluorescence.
3.2.6 TUNEL assay
Fragmentation of the DNA is a pathological hallmark of apoptosis. In dead cells, DNA is cleaved by an endonuclease (CAD) that fragments the double-stranded DNA into nucleosomal units, which are multiples of about 200 bp oligomers. There was a significant increase in the population of DNA-fragmented MCF-7 cells by Compound 4b at IC50 concentration for 48 hrs. of treatment. The detection of excessive DNA damage in individual cells was widely determined by the TUNEL assay method. For this purpose, the APO-Direct TUNEL assay kit was utilized. Percentage of cells expressing FITC-dUTP for untreated cells, test sample, and CPT treated cells depicted in Figs. 10 and 11. Treatment of compound 4b and CPT (at IC50) for 48 hrs revealed that compound 4binduced DNA fragmentation in 24.61%cells, CPT in 82.94% cells as compared to untreated control cells (0.155%). This result suggests that in MCF-7 cells, DNA fragmentation was initiated by endonucleases to enable cells to undergo apoptosis.
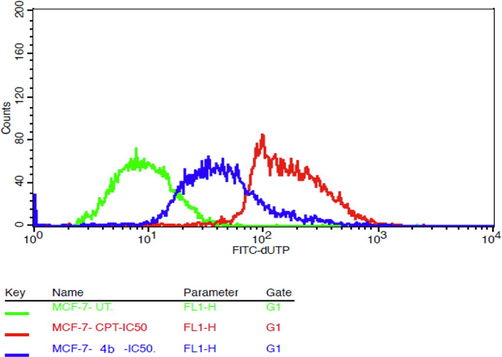
- Overlay of fluorescence intensities of FITC-dUTP.
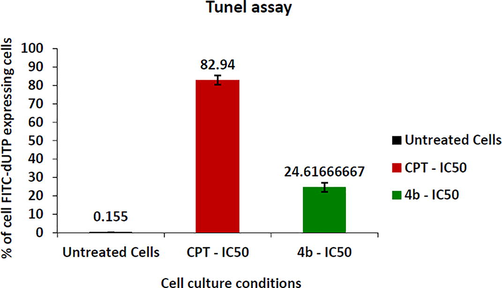
- Graph depicting the FITC-dUTP quantification in MCF-7 cells treated with Compound 4b and positive control CPT.
4 Conclusions
A highly efficient synthesis of a series of polycyclic cage-like heterocycles have been accomplished in good yields by 1,3-dipolar cycloaddition of N-unsubstituted 3,5-bis(E)-arylmethylidene]tetrahydro-4(1H)-pyridinones, acenaphthenequinone and 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid followed by annulation reaction strategy. The compounds induced concentration dependent reduction of growth of MCF-7 and NCI-H460 cell lines indicating the significant cytotoxic activity against breast and lung cancer lines. The results revealed that compound 4b able to inhibit MCF-7 and NCI-H460 cell line with IC50 values 10.86 and 9.17 µM respectively. Compound 4b was further screened for apoptosis and cell cycle analysis. The results reveal that compound 4b was able to induce apoptosis at early stage in MCF-7 cell line and also arrest cell line which is equivalent to standard drug camptothecin. Further, compound 4b also increase threshold of MMP and % of cells expressing FITC-dUTP which are important indicators of apoptosis in cancer cells upon drug treatment. These in vitro anticancer activity results revealed that compound 4b may be a potential molecule to develop as a drug candidate. The presence of multiple biologically active structural units in these cage-like hybrid heterocycles, enhances their potential for further exploration.
Acknowledgment
The project was supported by Researchers Supporting Project number (RSP-2020/231), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Almansour, A.I., Kumar, R.S., Arumugam, N., Kotresha, D., Menendez, J.C., Anti-Cancer compound, US Patent US 10,357,485 B1 Jul. 23, 2019.
- The Isoquinoline Alkaloids. Amsterdam: Harwood Academic; 1998. p. :255-361.
- Role of cellular glutathione and glutathione S-transferase in the expression of alkylating agent cytotoxicity in human breast cancer cells. Biochem. Pharmacol.. 1994;47:1079-1087.
- [Google Scholar]
- Principles of Medical Pharmacology (7th ed.;). Toronto: Saunders Elsevier:; 2007. p. :778.
- The Story of MCF-7 Breast Cancer Cell Line: 40 years of Experience in Research. Anticancer Res.. 2015;35:3147-3154.
- [Google Scholar]
- Design, synthesis and cytotoxic properties of novel 1-[4-(2-alkylaminoethoxy)phenylcarbonyl]-3,5-bis(arylidene)-4-piperidones and related compounds. Eur. J. Med. Chem.. 2007;42:71-80.
- [Google Scholar]
- 1-[4-(2-Aminoethoxy)phenylcarbonyl]-3,5-bis-(benzylidene)-4-oxopiperidines: A novel series of highly potent revertants of P-glycoprotein associated multidrug resistance. Bioorg. Med. Chem. Lett.. 2008;18:3484-3487.
- [Google Scholar]
- 1,5-diaryl-3-oxo-1,4-pentadienes: a case for antineoplastics with multiple targets. Curr. Med. Chem.. 2009;16:2001-2020.
- [Google Scholar]
- Antileukemic Evaluation of Some Mannich Bases Derived from 2-Arylidene-1,3-diketones. Eur. J. Med. Chem.. 1983;18:248-254.
- [Google Scholar]
- 3-Arylidene-1-(4-nitrophenylmethylene)-3,4-dihydro-1H-naphthalen-2-ones and related compounds displaying selective toxicity and reversal of multidrug resistance in neoplastic cells. Bioorg. Med. Chem. Lett.. 2005;15:1633-1636.
- [Google Scholar]
- Dyke, S.F., Quessy, S.N. In The Alkaloids; Rodrigo, R. G. A., ed.; Academic: New York, 1981, vol. 18, pp. 1.
- Hoshino, O., In The Alkaloids; Cordell, G.A., Ed.; Academic: San Diego; 1998; Vol. 51, pp 324−424.
- Design, synthesis and in vitro mechanistic investigation of novel hexacyclic cage-like hybrid heterocycles. Molecules. 2019;24:3820.
- [Google Scholar]
- In vitro mechanistic investigation of polycyclic cage-like heterocyclic hybrid possessing diverse pharmacophoric units. J. King Saud Univ. Sci.. 2020;32:2406-2413.
- [Google Scholar]
- Isolation, total synthesis and biological activity of phenanthroindolizidine and phenanthroquinolizidine alkaloids. Synthesis 2001:2365-2378.
- [Google Scholar]
- Love, S.M., Dr. Susan Love's breast book. Da Capo Lifelong Books; 2015 Sep 8.
- Lyon, International Agency for Research on Cancer. Latest global cancer data: Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in International Agency for Research on Cancer 2018 France 2018 Sep
- Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods.. 1983;65:55-63.
- [Google Scholar]
- 1-p-chlorophenyl-4,4-dimethyl-5-diethylamino-1-penten-3-one hydrobromide, a sulfhydryl-specific compound which reacts irreversibly with protein thiols but reversibly with small molecular weight thiols. Anal. Biochem.. 1989;177:237-243.
- [Google Scholar]
- Supramolecular exo-functionalized palladium cages: Fluorescent properties and biological activity. Dalton Trans.. 2016;45:8556-8565.
- [Google Scholar]
- Evaluation of new palladium cages as potential delivery systems for the anticancer drug cisplatin. Chemistry (Weinheim an der Bergstrasse, Germany). 2016;22:2253-2256.
- [Google Scholar]
- The biology of breast cancer at the cellular level. Biochimica et biophysica acta. 1984;738(3):103-123.
- [Google Scholar]
- Risk prediction models for selection of lung cancer screening candidates: A retrospective validation study. PLoS Med.. 2017;14:e1002277
- [Google Scholar]
- Chemosensitization by buthionine sulfoximine in vivo. Int. J. Radiat. Oncol. Biol. Phys.. 1986;1986(12):1183-1186.
- [Google Scholar]
- Waste PET plastic derived ZnO@NMC nanocomposite via MOF-5 construction for hydrogen and oxygen evolution reactions. J. King Saud Univ. Sci.. 2020;32:2397-2405.
- [Google Scholar]
- Cell Proliferation Assays. In: eLS. Chichester: John Wiley & Sons Ltd:; August 2014.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.101238.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







