Translate this page into:
Development of saline loaded mask materials, evaluation of the antimicrobial efficacy and survivability of selected bacteria on these mask materials
⁎Corresponding author. afraa@hct.edu.om (Afraa Said Al Adawi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Surgical face masks have been recommended by World Health Organization (WHO) during the COVID-19 pandemic. Nowadays wearing masks have become a norm and lifestyle around the globe. The present investigation was carried out to evaluate the feasibility of developing masks loaded with analytical grade sodium chloride (NaCl), Iodized salts (IS) and Omani sea salt (OSS) with or without sodium bicarbonate (NaHCO3).
Methods
The saline loaded masks were prepared by soaking the middle layer of the mask in 30% (w/v) saline solutions (NaCl, IS, OSS) with or without 10% NaHCO3 for 24 h followed by drying at room temperature. The prepared saline solutions and its combinations were evaluated for antimicrobial efficacy against the bacteria like Escherichia coli, Pseudomonas aeruginosa, Proteus vulgaris, Salmonella typhi, and Staphylococcus aureus, and antifungal activity against the Penicillium spp. and Rhizopus spp. by agar diffusion. Optical microscopy was employed to observe the formation of salt crystal in the mask material. Survivability of S. aureus and P. aeruginosa was tested on the mask material loaded with 30% OSS + 10% NaHCO3 at particular time intervals.
Results
The results showed that a combination of 30% OSS + 10% NaHCO3 exhibited promising antimicrobial activity against all the bacteria as well as Rhizopus spp. compared to the 30% IS + 10% NaHCO3. Moreover, the middle layer of the mask loaded with saline solutions of 30% OSS + 10% NaHCO3 or 30% IS + 10% NaHCO3 have antibacterial activity, particularly for oral microbiome. On dehydration, the masks materials showed the presence of a significant amount of salt crystals. Survivability tests showed that both S. aureus and P. aeruginosa were killed within 3 h of contact with the salt crystals on the mask materials.
Conclusions
A combination of 30% OSS + 10% NaHCO3 possessed significant antimicrobial activities on the tested microorganisms. Presence of a significant amount of salt crystals on dehydration of the saline loaded masks can be used as an effective protective barrier to infectious respiratory agents.
Keywords
Surgical mask
Omani sea salt
Sodium bicarbonate
Antimicrobial activity
Salt crystals
1 Introduction
The wearing of face mask has been recommended by the World Health Organization (WHO, 2020) as protection strategy against respiratory pathogens particularly during the pandemic. Various reports suggested the use of face masks as an effective barrier to curb the aerosol spread of respiratory infections including COVID-19, influenza and other viruses (Occupational Safety and Health Administration (OSHA), 2009; Long et al., 2020; Chermey and Potter, 2020). However, these days the general public is following the usage of disposable surgical face masks as directed by the authorities (Lipp and Edwards, 2005; Canadian Agency for Drugs and Technologies in Health (CADTH), 2013; Vincent and Edwards, 2016). Also, surgical face masks were originally developed to contain and filter droplets containing microorganisms expelled from the mouth and nasopharynx during surgery, therefore the primary purpose of a surgical mask is to provide protection for the patient from the surgical team. At the same time masks have been advocated as a barrier to protect the surgical team from the patient (Vincent et al., 2016).
Previous studies highlighted the efficacy of saline solutions in direct and indirect medical application (Passanha et al., 2014). They are used as intravenous solutions (Tonog and Lakhkar, 2019), and are found to be effective in nasal irrigation, cystic fibrosis and upper respiratory tract infections (URTIs). The practice of gargling with salt water was found to either alleviate symptoms or even help to prevent URTIs (King et al., 2015, Osborn, 2018, Fletcher, 2019). In cells infected with reticuloendotheliosis virus (REV), saline solution was shown to interfere with viral protein synthesis and further release of virus from infected cells. Saline solution inhibited the protein synthesis of Influenza B virus infecting Madin-Darby Canine Kidney (MDCK) cells (Bishop et al., 1976; Tsumrumi et al., 1983). In addition to this, it has also been found to enhance viral adsorption to micro porous filters (Lukasik et al., 2000). High salt concentration was found to promote the interferon beta (IFNβ) production and its signaling in both human and mouse macrophages and consequentially increased antiviral resistance (Goralski and Donaldson, 2014; Grandjean-Lapierre et al., 2017; Zhang et al., 2017). All these facts underline the role of saline solutions in the management and control of various infections.
Sodium bicarbonate (NaHCO3) or baking soda finds applications in dentistry due to its bactericidal property (Myneni, 2017; Madeswaran and Jayachandran, 2018). Its low cost, easy availability, low abrasion property, as well as antibacterial and highly biocompatible natures (Myneni, 2017) that made it a chemical of suitable choice for the current investigation.
The current pandemic situation combined with the unavailability of sufficient units of personal protective equipment (PPE) as surgical masks, calls the attention of the scientific community to explore the development of cost effective and efficacious PPE. Currently available PPE pose a potential risk of primary or secondary infection and transmission. Herein, the development and antimicrobial efficacy of a potential reusable surgical mask loaded with saline (NaCl, IS, OSS) solutions and NaHCO3 were explored based on the work by Quan et al. (2017).
2 Materials and Methods
2.1 Materials
Commercially available (CA) surgical masks, iodized salt (IS) and Omani sea salt (OSS) were purchased from the local market. Some of the masks were also procured from the Ministry of Health (MOH), Sultanate of Oman. Sodium chloride (NaCl), NaHCO3, polysorbates (Tween 20) were of analytical grade. Nutrient agar (NA), potato dextrose agar (PDA), and nutrient broth were used as the culture media, and 0.5 McFarland was used to standardize the survivability of bacteria.
2.2 Methods
2.2.1 Loading of CA and MOH masks with different saline solutions
A 30% (w/v) NaCl stock solution containing 1% (v/v) Tween 20 was prepared using deionized water and stirred well to dissolve the contents. A 10 % (w/v) NaHCO3 solution was prepared individually and in combination with the prepared saline stock. A 30% OSS (w/v) solution and 30% IS (w/v) solution was prepared along with a combo of 10% NaHCO3 solution. The prepared solutions were stored in separate glass containers. The mask material (whole mask and the middle layer of the mask) was soaked in these solutions for 24 h. These mask materials were flipped at regular intervals using sterile forceps to ensure proper loading of the components. After 24 h, the soaked mask materials were air dried at room temperature. All the experiments were carried out under strict aseptic conditions.
2.2.2 Evaluation of the antimicrobial activity
2.2.2.1 Agar well diffusion method
Agar well diffusion method was used to evaluate the antimicrobial efficacy of the salt powders as well as the saline solutions and their combinations. Bacteria used in this study included E. coli, P. aeruginosa, P. vulgaris, S. typhi, and S. aureus and fungi included Penicillium spp. and Rhizopus spp. Wells of standard size (6 mm) were incised at specified distances in the agar plate using a sterile cork borer. Overnight broth cultures of the bacterial isolates were swabbed on separate nutrient agar plates. In addition to this, one of the plates were also swabbed with oral microbiome from a healthy individual using a sterile cotton swab. In separate wells, 100 µl of the three stock solutions, as well as their combo with 10% NaHCO3 were added. Similarly, a 10% NaHCO3 solution was also added to a separate well. To study the efficacy of the powder materials, 10 mg of each of the salts was added into a separate well in the plate. All the plates were incubated at 37 °C overnight. After incubation, the zones of inhibition (mm) were measured and recorded. Antifungal activity was carried out in PDA. The fungal plates were incubated for 72–120 h at room temperature and the zone of inhibition was measured and recorded. All the experiments were carried out in triplicates.
2.2.2.2 Evaluation of the antimicrobial activity of mask materials by agar disc diffusion
The plain three-layered mask material, middle layer (control) as well as the whole material and the middle layer was loaded individually with 30% NaCl, 30% IS, 30% OSS and 10% NaHCO3. Simultaneously, 30% NaCl + 10% NaHCO3, 30% OSS + 10% NaHCO3 and 30% IS + 10% NaHCO3 were also used for loading separate mask material and middle layer material. These materials were cut into discs of standard diameter (5 mm) using a sterile cork borer. These discs were placed on separate NA plates swabbed with the bacteria, oral microbiome and fungi in PDA plates. The discs were placed on the agar surface at a specified distance using sterile forceps. The conditions of incubation were maintained as in the previous experiment for bacteria and fungi. After incubation, the zone of inhibition around the wells were measured and recorded. All the experiments were carried out in triplicates.
2.3 Microscopic observation
The plain and saline (30% OSS + 10% NaHCO3) loaded surgical masks materials that have been soaked for 24 h were observed under an optical microscope (40X, 100X) to study the presence/absence of loaded salt crystals as well the general structure of the mask material.
2.4 Survivability of bacteria on saline loaded mask material
Tests for the bacterial survivability on the MOH and CA mask material of both middle layer and whole mask loaded with 30% OSS + 10% NaHCO3 and controls were carried out using 2 cm2 cut pieces of the mask material. These pieces were placed in glass bottles and sterilized by autoclaving and later these sterile pieces were soaked in sterile 30% OSS + 10% NaHCO3 solution and kept at room temperature for 24 h. After 24 h, the pieces were carefully transferred to a sterile container and dried overnight in a sterile cabinet. To standardize the number of bacteria in the culture, 0.5 McFarland, spectrophotometrically equated S. aureus (SA) and P. aeruginosa (PA) nutrient broth tubes were used. From the 0.5 McFarland equated culture broth, 50 µl was placed on each of the four sets (1 control + 4 saline loaded pieces in each set) of sterile mask pieces (labelled as 0 h, 0.5 h, 3 h and 24 h) of saline loaded middle layer, whole mask material (both MOH and CA) as well as the control placed in separate sterile petri plates. Simultaneously, the same amount of broth culture of both the bacteria were loaded on separate mask materials (4 sets viz., 1 control + 4 saline loaded pieces in each set). Immediately after loading the mask materials with bacteria, the loaded area of the first set of mask materials was gently touched using sterile cotton swab moistened with sterile water and this was inoculated on to sterile nutrient agar in a quadrant swabbing pattern. These plates were labeled as 0 h and kept for incubation at 37 °C overnight. Simultaneously another set of sterile mask materials (saline loaded and control) were loaded with bacteria as mentioned above and these mask pieces were placed on nutrient agar using a sterile forceps by keeping the bacteria loaded side in contact with the surface of the agar. This was considered as 0 h sample and these plates were incubated at 37 °C overnight. The mask pieces loaded with bacteria were kept in room temperature (37 °C) for 0.5 h (2nd set) for 3 h (3rd set) and 24 h (4th set) and they were yielded to swabbing technique as well as direct placement on nutrient agar as mentioned earlier. On incubation particular intervals, presence of bacterial colonies in the plates yielded to swabbing techniques were observed. After incubation, in case of the plates yielded to direct placement of mask pieces, presence of bacterial growth was confirmed by gently removing the mask materials from the agar surface using a sterile forceps and observing bacterial growth both on the surface of the mask pieces those had contact with the agar surface as well as on the agar surface itself. To confirm the absence of any growth (bacteria/fungi) in the powdery material from the agar surface was sub-cultured on to nutrient agar and potato dextrose agar incubated at 37 °C overnight and 4 days at 28 °C respectively. To make the study tangible 50 µl of 0.5 McFarland equated culture broth of each bacteria was separately swabbed on the nutrient agar in a quadrant pattern. In the swabbing technique as well as direct placement technique of the mask material, growth was considered in a scale of no growth = 0, slight = +, moderate = ++, abundant = +++ (Mapetla, 2019) both cases.
2.5 Statistical analysis
The data from the study were analyzed using IBM SPSS Statistical Package for Social Science for Windows Version 20.0. Armonk, NY: IBM Corp. The analysis of variance (ANOVA) and the general linear model were used to determine the significance among the parameters. Any means with significant differences were subjected to Tukey’s (HSD) at 5% level. However, t-test was used to test further with the parameter of two treatments means.
3 Results
The present study was carried out to enhance the efficiency of the mask materials with different saline solutions (30% NaCl, 30% IS, 30% OSS) as well as their combo with 10% NaHCO3 and evaluate the antimicrobial efficacy of the saline loaded mask materials.
3.1 Antimicrobial activity of the powdered salts and saline solutions
The antimicrobial activity extended by (a) powdered salts (NaCl, IS and OSS) and (b) saline solutions (30% NaCl, 30% IS and 30% OSS) is shown in Fig. 1. The zone of inhibition highlighted the following trend in the sensitivity of bacteria to all the salt powders viz., oral microbiome > E. coli > P. aeruginosa > S. typhi > P. vulgaris. Iodized salt showed comparatively better zone of inhibition activity against P. vulgaris. In case of all the bacteria the OSS showed comparable efficacy. There existed a significant (P < 0.05) difference between antimicrobial efficacy extended by all the salt powders to the oral microbiome compared to the rest of the bacteria except P. vulgaris. However, both the fungi showed resistance to all the salt powders. In addition to this, none of the saline solutions (30% NaCl, 30% IS and 30% OSS) studied showed any inhibitory effect on bacteria, oral microbiome and fungi.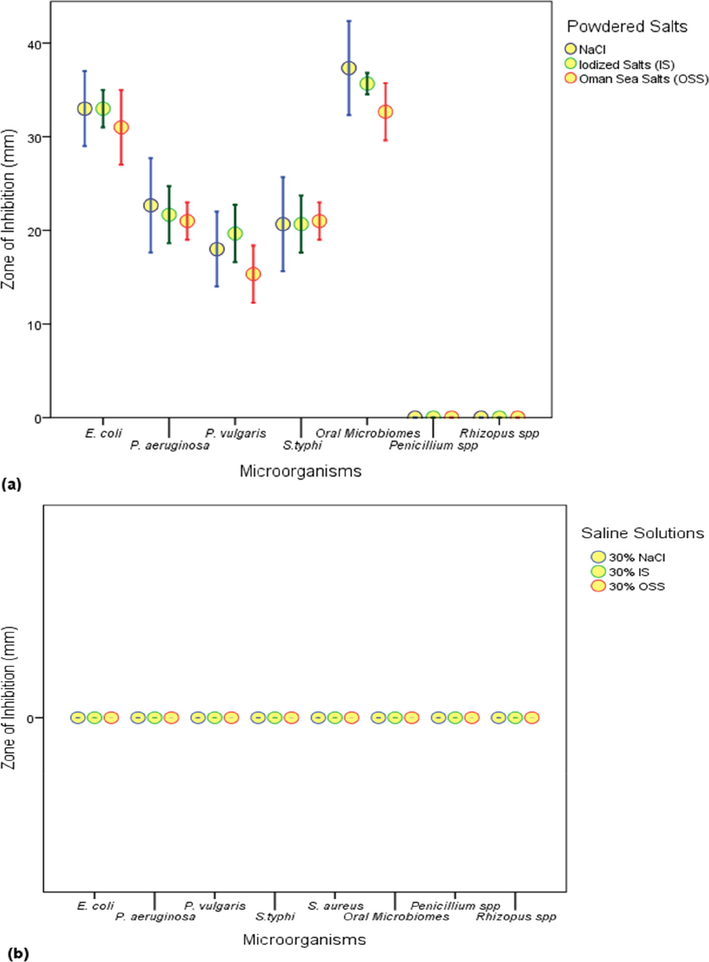
Antimicrobial activity extended by (a) salt powders (NaCl, IS and OSS) and (b) saline solutions (30%) NaCl or IS or OSS.
3.2 Antimicrobial activity of NaHCO3 powder and its solutions
Sodium bicarbonate in powder form extended better antibacterial efficacy than its solutions (Fig. 2). The zone of inhibition highlighted the following trend in the sensitivity of bacteria to NaHCO3 powder as S. typhi > E. coli > oral microbiome > S. aureus > P. aeruginosa > P. vulgaris. It was noted that 5% NaHCO3 solution did not have any inhibitory effect on any bacteria other than the oral microbiome. In case of 10% NaHCO3 solution the zone of inhibition exhibited a trend in the following manner, oral microbiome > E. coli > S. typhi > P. aeruginosa. Neither S. aureus nor P. vulgaris were sensitive to 10% NaHCO3 solution. However, the NaHCO3 powder, 5% and 10% NaHCO3 solutions had almost similar inhibitory effects on the oral microbiome. Both 5% and 10% NaHCO3 solutions showed almost similar activity against Penicillium spp. Rhizopus spp was more sensitive (40 mm) to the NaHCO3 powder compared to the Penicillium spp (24.7 mm). There was a significant difference (P < 0.05) between the efficacy of the NaHCO3 powder and both the NaHCO3 (5% and 10%) solutions in case of Rhizopus spp.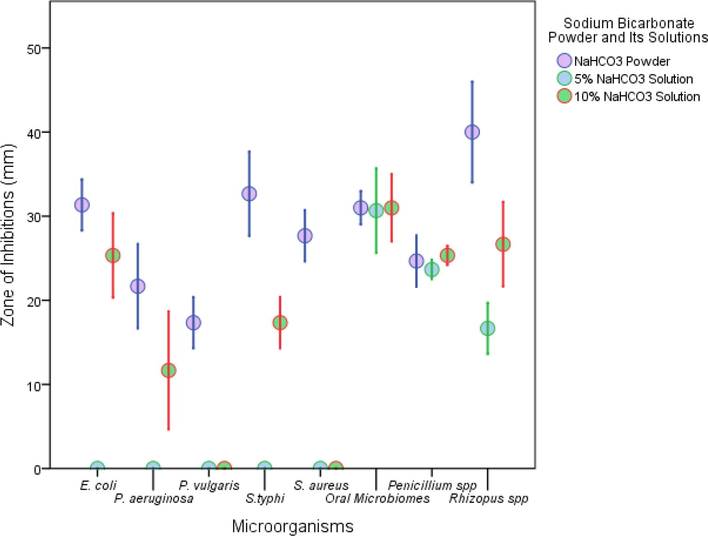
Antimicrobial activities using NaHCO3 powder and its solutions.
3.3 Antimicrobial activity of saline solutions with 10% NaHCO3
Fig. 1(b) showed that none of the saline solutions (30%) NaCl or IS or OSS had any inhibitory effect by itself on all the microbes under this study. However, when 10% NaHCO3 was added in 30% saline solutions (OSS and IS), remarkable antimicrobial activities were observed, except in the case of Penicillium spp. It is evident from Fig. 3, that 30% OSS + 10% NaHCO3 saline solution exhibited better antibacterial activity than the 30% IS + 10% NaHCO3 saline solution.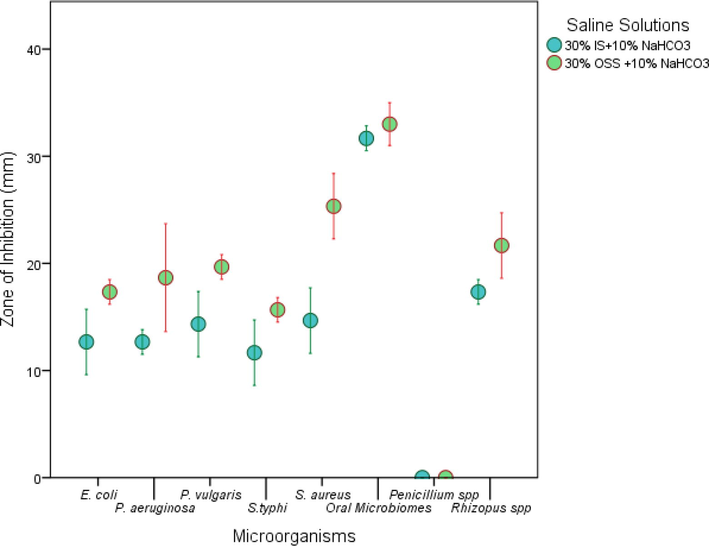
Antimicrobial activities of saline solutions (30% IS + 10% NaHCO3 and 30% OSS + 10% NaHCO3).
3.4 Antimicrobial efficacy of CA surgical mask material (wet and dried) loaded with different salts at varying soaking time intervals
Both the whole and the middle layer of the CA surgical mask in wet and dried form soaked with saline solutions (30%) NaCl or IS or OSS at varying time intervals did not exhibit any antimicrobial activity against bacteria and fungi (Fig. 4).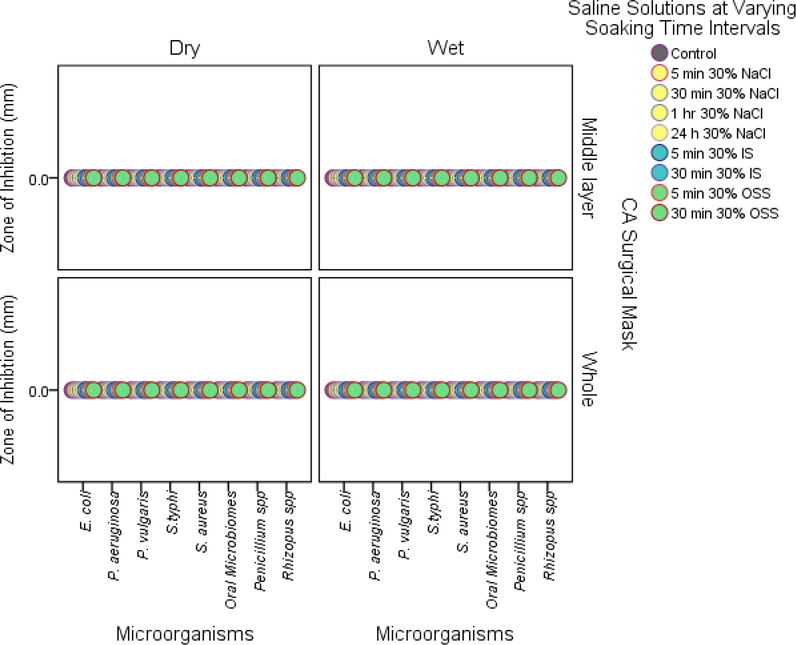
Antimicrobial efficacy of CA surgical mask material (wet and dried) loaded with saline solutions (30% NaCl, 30% IS, 30% OSS) at varying soaking time intervals.
3.5 Antimicrobial efficacy of the dried CA surgical mask material (whole and middle layer) loaded with various saline solutions with 10% NaHCO3 for a soaking time of 24 h
The whole and the middle layer of the CA surgical mask materials loaded with 30% NaCl or 30% IS or 30% OSS in combination with 10% NaHCO3 saline solution soaked for 24 h showed a zone of inhibition in oral microbiome only but did not show any zone of inhibition in either bacteria or fungi (Fig. 5). However, the middle layer of the CA surgical mask loaded with the 30% OSS + 10% NaHCO3 saline solution showed a significant difference (P < 0.05) in the zone of inhibition in oral microbiome compared to the CA surgical whole mask.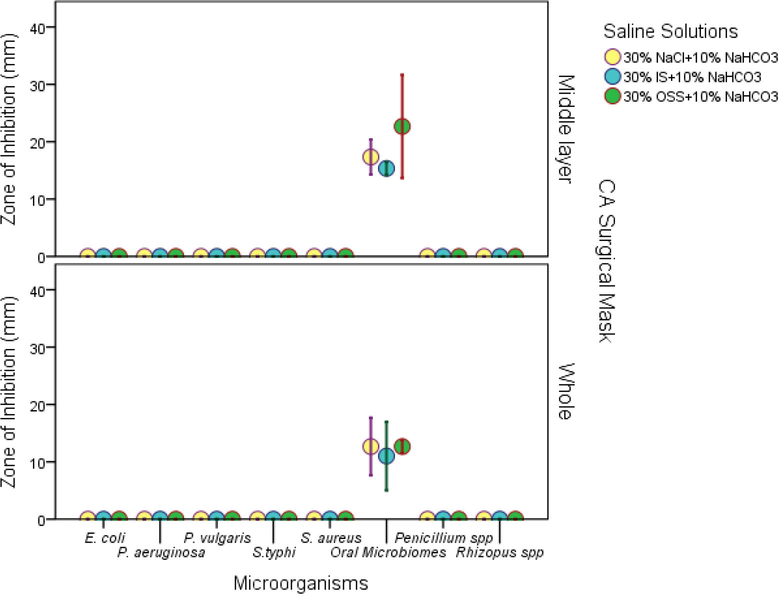
Antimicrobial efficacy of the dried whole and middle layer of the CA surgical mask materials with various saline solutions (30%) NaCl or IS or OSS + 10% NaHCO3) at 24 h soaking time.
3.6 Antimicrobial efficacy of the dried MOH surgical mask material (whole and middle layer) loaded with the OSS solution itself and in combination with 10% NaHCO3
As mentioned in Fig. 1(b), none of the 30% saline solutions had any antimicrobial activity against microorganisms under this study. However, 30% OSS + 10% NaHCO3 saline solution showed antimicrobial activity as reflected in Fig. 3. In addition to this, Fig. 6 revealed the antimicrobial effect of dried MOH surgical masks soaked for 24 h with 30% OSS with and without 10% NaHCO3. It was noted that, the whole and middle layer of the dried MOH surgical mask loaded with OSS solution alone, did not extend any antimicrobial activity except to the oral microbiome. This underlined the combinatorial effect of 30% OSS + 10% NaHCO3 saline solution on the oral microbiome.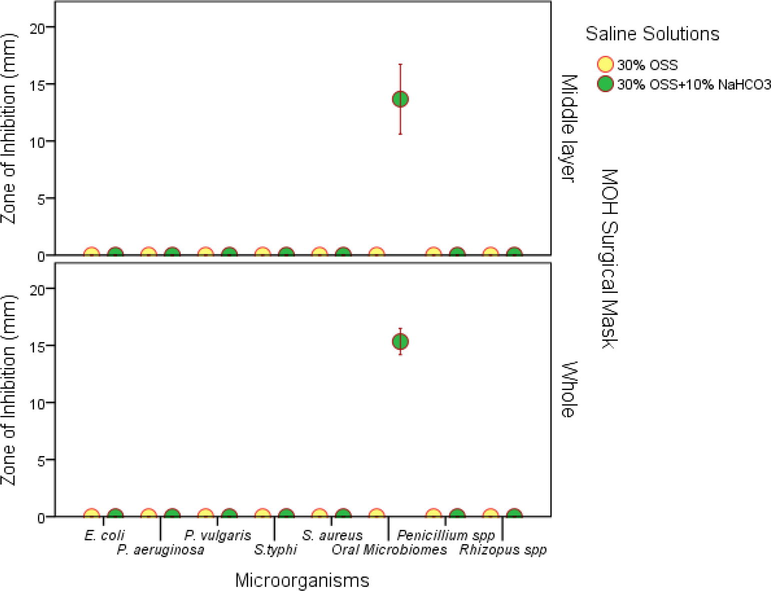
Antimicrobial efficacy of dried MOH surgical mask material (whole and middle layer) loaded with the saline solutions (30% OSS or 30% OSS + 10% NaHCO3) at 24 h soaking time.
3.7 Microscopic observation
Results of the optical microscopy (40X, 100X) of the plain surgical mask materials and the saline solutions (30% OSS + 10% NaHCO3) loaded surgical mask materials (dried middle layer) after 24 h is shown in the Fig. 7. The presence of salts crystals was found in the saline loaded middle layer surgical masks, but not in the plain layer surgical masks materials.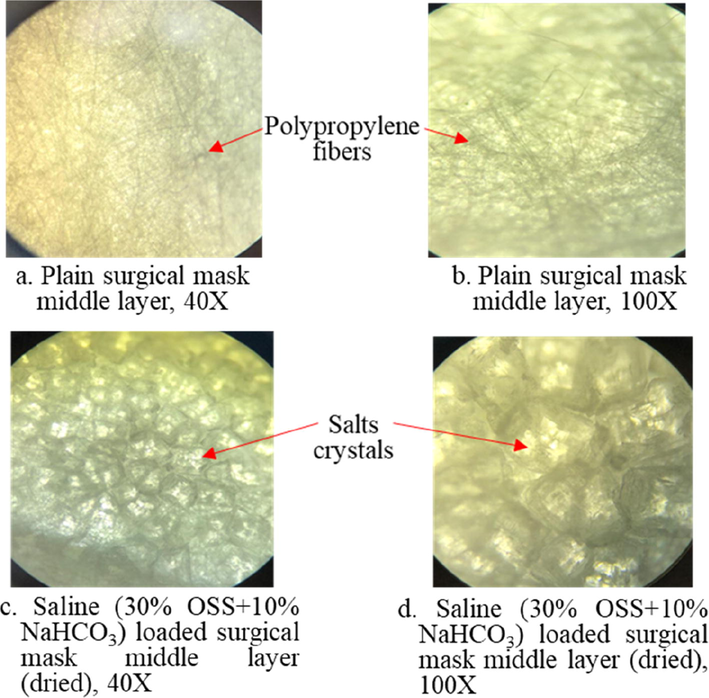
Optical microscopy (40X, 100X) of the plain middle layer surgical mask materials and the saline (30% OSS + 10% NaHCO3) loaded surgical mask materials (dried middle layer) after 24 h.
3.8 Survivability of bacteria on saline loaded mask material
The results obtained for the bacterial survivability on the saline solution (30% OSS + 10% NaHCO3) loaded MOH and commercially available mask materials (middle layer and whole) either by swabbing or direct placement of mask material on nutrient agar is arranged in Table 1 and Fig. 8. A = Swabbing, B = direct placement of mask material on nutrient agar. No growth = 0, slight = +, moderate = ++, abundant/luxuriant = +++.
Samples
Contact Time (h)
0
0.5
3
24
A
B
A
B
A
B
A
B
MOH Middle Control - PA
+++
+++
++
++
+
++
+
+
MOH Middle Saline loaded - PA
+
++
+
0
0
0
0
0
MOH Whole Control - PA
+++
+++
++
++
+
++
+
+
MOH Whole Saline loaded - PA
++
++
+
0
0
0
0
0
MOH Middle Control -SA
++
++
++
+
+
+
+
+
MOH Middle Saline loaded - SA
+
+
+
0
0
0
0
0
MOH Whole Control -SA
++
++
++
+
+
+
+
+
MOH Whole Saline loaded -SA
+
+
+
0
0
0
0
0
CA Middle Control -PA
+++
+++
++
++
+
++
+
+
CA Middle Saline loaded - PA
++
++
+
0
0
0
0
0
CA Whole Control -PA
+++
+++
++
++
+
++
+
+
CA Whole Saline loaded -PA
++
++
+
0
0
0
0
0
CA Middle Control -SA
++
++
+
+
+
+
+
+
CA Middle Saline loaded - SA
+
+
+
0
0
0
0
0
CA Whole Control - SA
++
++
+
+
+
+
+
+
CA Whole Saline loaded - SA
++
+
+
0
0
0
0
0
0.5McFarland PA
+++
+++
+++
+++
+++
+++
+++
+++
0.5McFarland SA
++
++
++
++
++
++
+++
++
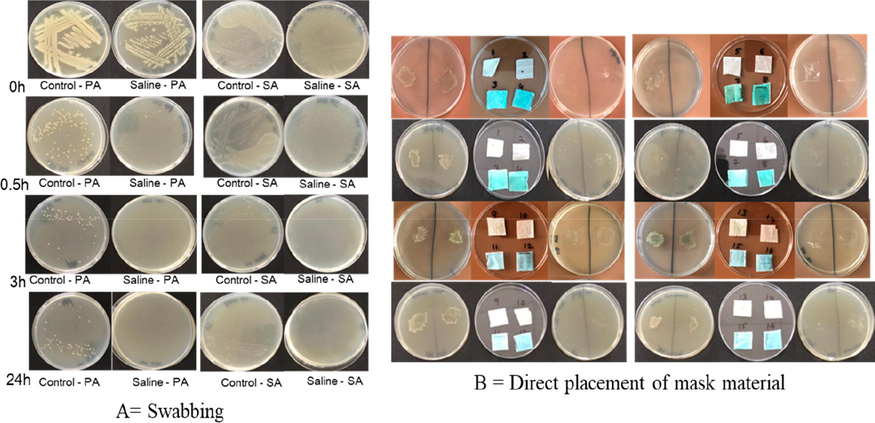
Survivability of bacteria on saline loaded mask materials either by swabbing or direct placement of mask material on nutrient agar.
The Table 1 pertaining to the survivability test based on the swabbing inoculation on nutrient agar shows that all the control mask pieces (without saline solutions) loaded with S. aureus and P. aeruginosa resulted in growth of bacteria irrespective of the time gap from 0 h to 24 h, whereas the 30% OSS + 10% NaHCO3 loaded samples labelled 0 h and 0.5 h resulted in the presence of bacterial colonies and comparatively lesser in numbers than the controls, but no presence of colonies were observed at 3 h or 24 h. In case of all the control mask pieces (0 h; 0.5 h, 3 h and 24 h) loaded with bacteria placed on the nutrient agar showed mat like growth of bacteria (Fig. 8) once it was removed using forceps. But in case of all the 30% OSS + 10% NaHCO3 treated mask pieces loaded with both PA and SA showed only bacterial growth in the case of the 0 h sample whereas 0.5 h, 3 h and 24 h did not show any presence of colonies on the agar surface. A white deposit was noted on the surface of the agar once the mask piece was removed and that could be the powdery material associated with the mask. Absence of bacterial and fungal growth was confirmed in the powdery material from the agar surface as there was not any growth either on the nutrient agar or the potato dextrose agar in which the powdery residue was sub-cultured.
4 Discussion
The present study was conducted to investigate the possibility of developing reusable masks loaded with analytical grade NaCl, IS and OSS with or without NaHCO3. In this in vitro study, it was noted that salt powders (NaCl, IS and OSS) showed significant inhibitory effect on bacteria as compared to 30% saline solutions (NaCl, IS and OSS) which did not show any antibacterial and antifungal activities. Absence of activity with 30% solution (w/v) may be due to the decreased diffusion of salt particles through the agar. However, Parfentjev and Catelli (1964) reported that 0.85% NaCl containing broth culture could significantly inhibit S. aureus. Hajmeer et al., (2006) mentioned that S. aureus maintained its cellular structure and no severe cell wall or plasma membrane damage and/or shrinkage was observed with 10% NaCl, whereas 10% NaCl could cause extensive cellular damage to E. coli O157:H7. The lowest sensitivity exhibited by P. vulgaris to all the salts (NaCl, IS and OSS) under study could be suggestive of a comparatively more salt tolerance in this bacterial species (Mansouri and Pahlavanzadeh, 2009). The antibacterial effect showed by OSS powder can be considered for future applications as it is cheaper and is naturally available resource considering that Oman has a vast coastline stretch of 3,165 km (MOECA, 2014). The significant efficacy extended by the OSS may be contributed to the presence of other minerals in the sea salt as it may not be stringently processed as in the case of the iodized salt (Zeratsky, 2019). Sea salt can be help tackle problems related to diseases such as cardiovascular disease, diabetes, cancer, and skin problems (Nani et al., 2016).
In the current study, we have also investigated NaHCO3 alone and in combination with other salts. The NaHCO3 alone showed comparable results in powder form except for S. aureus. Inclusion of only 10% NaHCO3 solutions in this study is justified by its solubility property (7.8 to 10%). Even though other bacteria and fungi under study responded differently to NaHCO3 powder and its solutions, nevertheless Penicillium spp and the oral microbiome individually responded to these treatments in a similar manner. Previous studies have suggested the broad-spectrum antimicrobial effect of NaHCO3 on bacteria including the potent respiratory pathogen, Mycobacterium tuberculosis (El Badrawy et al., 2018). The bicarbonate ion was identified as the probable cause of NaHCO3 mediated inhibition Dobay, et al., (2018). Gutiérrez-Huante et al., (2019) revealed that iron deficiency may also be involved in the inhibition of bacterial growth by NaHCO3 (Gutiérrez-Huante et al., 2019). However, in some cases, pH elevation also played a significant role (Corral et al., 1988). Scientists have found that the virucidal efficacy of NaHCO3 was also enhanced when it was used in combination with aldehydes or hydrogen peroxide (Malik and Goyal, 2005). In addition, oral administration and wash with NaHCO3, significantly decreased the morbidity and mortality of the cattle inflicted with foot and mouth disease (FMD) virus underlining its role as prophylactic and therapeutic agents (Hossain, et al., 2015).
Dobay et al., (2018) reported that NaHCO3 (100 mmol/l) significantly inhibited the growth of planktonic bacteria whereas NaCl (100 mmol/l) did not have any influence on the bacterial growth. Furthermore, the HCO3 − not only impeded the planktonic growth of different bacteria but also impeded biofilm formation by P. aeruginosa. All these make NaHCO3 an interesting component for its application on masks.
The inclusion of 10% NaHCO3 with the saline solution was to investigate the synergistic effect of NaHCO3 with other salt solutions. This study highlighted 30% OSS + 10% NaHCO3 saline solution is an effective combination from the antibacterial perspective as well as its effect on Rhizopus spp, compared to the 30% IS + 10% NaHCO3 saline solution. However, both these combinations did not affect Penicillium spp.
When the presence of bacteria in droplets from nose and mouth was discovered along with their role in disease transmission, masks have been used as a protective barrier to respiratory diseases (Borkow et al., 2010). The middle layer of CA surgical mask material soaked for 24 h with individual saline solutions (30%) NaCl or IS or OSS could not inhibit microbial growth, whereas, the middle layer CA surgical mask material soaked with individual saline solution (30%) NaCl or IS or OSS with 10% NaHCO3 could inhibit the oral microbiome. Since the results did not show any significant difference with the antimicrobial efficacy of (30%) NaCl or IS or OSS with 10% NaHCO3, further investigations were concentrated only on the 30% OSS with 10% NaHCO3, combo loaded whole and middle layer of the mask in both CA and MOH surgical masks which antimicrobial activity against oral microbiome only. The significant activity extended by the functionalized middle layer material to the oral microbiome could be related to the nature of this material, as reflected in terms of the abundance of impregnated salt crystals in the middle layer material seen under the optical microscope. This could be due to the absorption of salt crystals occurs in materials soaked after dehydration (Xie et al., 2007). Consequently, the microbes could be killed when they encounter the dry salt crystals on polypropylene that derived from saline combo (30% OSS + 10% NaHCO3). The report by Quan et al. (2017) confirmed that salt treated masks showed the presence of crystals on the mask surface that can be served as a blocker or trap for whatever aerosol or moisture from the environment, thereby preventing the entry of pathogens to mouth and nose. Polypropylene possesses bacterial resistance and is highly impermeable to water. Hence, Tween 20 was used to enhance the adsorption of salt solution on the hydrophobic surface of the face mask as the mask surface is hydrophobic (Xie et al., 2007).
This study included the evaluation of survivability on two important bacteria viz., S. aureus and P. aeruginosa on the saline loaded as well as control mask materials by swabbing inoculation as well as by direct placement of the bacterial loaded mask materials with and without saline on nutrient agar. It was understood that there existed a significant difference in the survivability of both the bacteria at 0 h and 0.5 h with respect to 3 h and 24 h while in contact with 30% OSS + 10% NaHCO3 as evident from the Table 1 and Fig. 8. These results are significant from the view point that S. aureus is a ubiquitous pathogen associated with a wide range of infections affecting the respiratory tract that range from asymptomatic colonization to fulminant necrotizing pneumonia (Parker and Prince, 2012) whereas P aeruginosa is a formidable opportunistic pathogen that can cause invasive and fulminant infections, such as acute pneumonia (Faure et al., 2018). Even though surgical masks are used to reduce bacterial shedding from the mouth, nose and face these masks could be the source of bacterial shedding with extended wearing time. Therefore, controlling airborne contamination and reducing microbial shed from personnel may help to decrease the incidence of surgical site infections (SSIs) (Zhiqing et al., 2018) transmitted especially via medical personnel in hospital settings. In this juncture the killing effect of 30% OSS + 10% NaHCO3 crystals present on the mask material is noteworthy and gives scope for detailed studies in this direction. The differences in the presence or absence of bacterial colonies in the nutrient agar inoculation following 0.5 h by swabbing or direct placement of the mask material treated with 30% OSS + 10% NaHCO3 and bacteria could be due to the inhibition of bacterial growth by the combined effect of direct contact and slow diffusion of salt from the mask material while in contact with the agar. A microbial growth qualitative test was followed instead of counting the colonies, as the usage of sterile water containing swab in the transference of bacteria to the agar would have contributed to the reduction in the number of bacterial cells in every subsequent steps viz., at 0 h, 0.5 h, 3 h and 24 h).
Drying is the major stress for airborne bacteria and may strongly diminish their survival probability during aerosolization (transport) (Potts, 1994). Microbial cells encountering the salt crystals may be killed or damaged due to the high salt concentration present on the surface. Water diffuses out of the cell leading to dehydration or loss of the structural integrity or enzyme and protein malfunction, resulting in bacterial cell death (Walsh, 2019). The beneficial properties of NaCl and NaHCO3 (Myneni, 2017; Fletcher, 2019) very well justify the incorporation of these chemicals for developing effective reusable antimicrobial face masks. In this context, the loaded salt particles may be considered as a biocidal agent and the use of biocidal masks may significantly reduce the risk due to hand or environmental contamination and the subsequent infection (Borkow et al., 2010).
The killing of the influenza virus in droplets on evaporation on contact with salt crystals in the mask material was established by Quan et al. (2017). To underline the findings of this study from the perspective of the efficacy of saline (30% OSS + 10% NaHCO3) loaded mask, the salt recrystallization virus negating system results of Quan et al. (2017) would be apt to emphasize in this juncture.
The present study combines the idea of the virucidal properties of NaCl solution as per Quan et al. (2017) and the bactericidal properties of NaHCO3, Dobay et al. (2018). Thus, making the saline coated surgical mask as a reusable, efficient antimicrobial, and cost-effective protective barrier against infectious agents. This study emphasized the value addition of masks by functionalizing its materials with 30% OSS + 10% NaHCO3 combination. Further studies may be carried out by modifying the composition of the saline solution to be loaded in the mask material.
The varying nature of microbes can influence their potential ability to penetrate the face mask. Material properties including shape, size, and their surface characteristics should be considered while designing chemical compound loaded mask materials.
5 Conclusion
Wearing masks is a proven effective barrier for protection from airborne illnesses. The recent Covid-19 pandemic showed an increased demand for PPE. Therefore, the present work was carried out to develop safe, economically feasible and reusable masks. This investigation confirmed that salt powders (NaCl, IS and OSS) extended significant antibacterial activity but not any antifungal effect. A combo of saline solutions (30% OSS + 10% NaHCO3) impregnated in the middle layer surgical masks materials after 24 h soaking possessed significant antimicrobial activities particularly against the oral microbiome as exhibited by the presence of salt crystals that would lead to the practical application as a reusable PPE. Moreover, killing of the pathogens like S. aureus and P. aeruginosa within 3 h of contact with the saline loaded mask materials underscored bactericidal effect of the salt functionalized mask.
Funding
This work was supported by Higher College of Technology, Ministry of Manpower Muscat, Sultanate of Oman. This study was also financially supported by the Researchers Supporting Project number (RSP-2021/371), King Saud University, Riyadh, Saudi Arabia.
Acknowledgements
We are highly grateful to the Vice Chancellor of UTAS Muscat Dr. Said Bin Hamad Al-Rubaiee for his motivation and support during the study. We would also like to express our sincere thanks to the Former Dean Dr. Ali Saif Salim Al Harthy (Deputy Vice Chancellor) for his motivation, initiation of this research and continuous encouragement throughout the study. We are also very thankful to Dr. Nasser Salim Al Bimani (Assistant Dean for Academic Affairs, HCT) for his ideas and discussion on the research. We would like to express our sincere gratitude to Dr. Abdul Hakim Al-Ismaili, former Director General, Technical Education, Ministry of Manpower for his motivation to conduct this research work. In addition to this we express our gratefulness and acknowledgements to our colleague Dr. Syed Najmul Hejaz Azmi for his constructive comments and advice on the manuscript. Sincere thanks and appreciation are also due to all the staff members of the Applied Sciences Department who were involved in the brainstorming sessions during the initiation of this work. The authors would also like to acknowledge the funding support by the Researchers Supporting Project number (RSP-2021/371), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effect of medium of lowered NaCl concentration on virus release and protein synthesis in cells infected with reticuloendotheliosis virus. J. Virol.. 1976;17(2):446-452.
- [Google Scholar]
- A novel anti-influenza copper oxide containing respiratory face mask. PloS One. 2010;5(6):e11295.
- [CrossRef] [Google Scholar]
- Canadian Agency for Drugs and Technologies in Health (CADTH). (2013). Use of Surgical Masks in the Operating Room: A Review of the Clinical Effectiveness and Guidelines [Internet]. https://www.ncbi.nlm.nih.gov/pubmed/24741720.
- Chermey, K and D. Potter. (2020). Does wearing a mask protect you from the flu and other viruses? https://www.healthline.com/health/cold-flu/mask.
- Corral, L. G., L. S. Post and T. J. Montville. (1988). Antimicrobial Activity of Sodium Bicarbonate. https://doi.org/10.1111/j.1365-2621.1988.tb09005.x
- Bicarbonate Inhibits Bacterial Growth and Biofilm Formation of Prevalent Cystic Fibrosis Pathogens. Front. Microbiol.. 2018;9:2245.
- [CrossRef] [Google Scholar]
- El Badrawy MK, M.A. Elela, A.M. Yousef, N.T. Abou El-Khier, T.T. Abdelgawad, D.A. Abdalla, and A. Moawad. (2018). Effect of sodium bicarbonate 8.4% on respiratory tract pathogens. Chest Lung Res. 2018: 1(1):3-7. https://www.pulsus.com/scholarly-articles/effect-of-sodium-bicarbonate-84-on-respiratory-tract-pathogens.pdf.
- Faure E., Kwong K., Nguyen D. (2018). Pseudomonas aeruginosa in chronic lung infections: how to adapt within the host?. Front. Immunol. 2018;9:2416. Published 2018 Oct 22. doi:10.3389/fimmu.2018.02416.
- Fletcher, J. (2019). What to know about gargling with salt water. https://www.medicalnewstoday.com/articles/325238.
- Hypertonic saline for cystic fibrosis: worth its salt? Expert Rev. Respir. Med.. 2014;8(3):267-269.
- [Google Scholar]
- Cystic fibrosis respiratory tract salt concentration: An exploratory cohort study. Medicine. 2017;96(47)
- [Google Scholar]
- The antibiotics potentiator bicarbonate causes upregulation of tryptophanase and iron acquisition proteins in Escherichia coli. Lett. Appl. Microbiol.. 2019;68(1):87-95.
- [CrossRef] [Google Scholar]
- Hajmeer M., Marsden J. L., Fung D.Y.C., Enis C. (2006). Impact of sodium chloride on Escherichia coli O157: H7 and Staphylococcus aureus analyzed using transmission electron microscopy. Food Microbiol. 23(5):446-52. DOI: 10.1016/j.fm.2005.06.005.
- Efficacy of sodium bicarbonate and determination of haematological parameters against foot-and-mouth disease virus in cattle. Suranaree J. Sci. Technol.. 2015;22(1):43-50.
- [Google Scholar]
- King D., Mitchell B., Williams C.P., Spurling G.K. (2015). Saline nasal irrigation for acute upper respiratory tract infections. https://www.ncbi.nlm.nih.gov/pubmed/25892369.
- Disposable surgical face mask: a systematic reviewed. Can. Oper. Room Nurs. J.. 2005;23(3):33-38.
- [Google Scholar]
- Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med.. 2020;13(2):93-101.
- [CrossRef] [Google Scholar]
- Influence of salts on virus adsorption to microporous filters. Appl. Environ. Microbiol.. 2000;66(7):2914-2920.
- [Google Scholar]
- Sodium bicarbonate: A review and its uses in dentistry. Indian J Dent Res.. 2018;29(5):672-677.
- [Google Scholar]
- Virucidal efficacy of sodium bicarbonate on a food contact surface against feline calicivirus, a norovirus surrogate. Int. J. Food Microbiol.. 2005;109(1–2):160-163.
- [CrossRef] [Google Scholar]
- Hemolysin production, salt tolerance, antibacterial resistance, and prevalence of extended spectrum β-lactamases in Proteus bacilli isolated from clinical and environmental sources. Jundishapur J. Microbiol.. 2009;2(3):97-104.
- [Google Scholar]
- Mapetla, K. (2019). Cultural Characteristics of Bacteria. https://www.studocu.com/en-za/document/sefako-makgatho-health-sciences-university/public-health/lecture-notes/cultural-characteristics-of-bacteria/7931803/view.
- MOECA [Ministry of Environment and Climate Affairs]. (2014). 5th National Report to the Convention of Biological Diversity (CBD) 2014. P20. https://www.cbd.int/doc/world/om/om-nr-05-en.pdf.
- Effect of baking soda in dentifrices on plaque removal. J. Am. Dental Assoc.. 2017;148(11):S4-S9.
- [Google Scholar]
- Nani, S. Z. M., Majid F. A. A., Jaafar A. B., Mahdzir A., Musa M. N. (2016). Potential health benefits of deep sea water: A review. Evid. Based Complement. Altern. Med. 2016, Article ID 6520475, 18 pages. http://downloads.hindawi.com/journals/ecam/2016/6520475.pdf.
- Occupational Safety and Health Administration (OSHA). (2009). OSHA Fact Sheet: Respiratory Infection Control: Respirators versus Surgical Masks. https://www.osha.gov/Publications/OSHA3219.pdf.
- Osborn, C. O. (2018). Everything You Need to Know About Making and Using Homemade Saline Solution. https://www.healthline.com/health/make-your-own-saline-solution.
- Tolerance of Staphylococcus aureus to sodium chloride. J. Bacteriol.. 1964;88(1):1-3.
- [Google Scholar]
- Immunopathogenesis of Staphylococcus aureus pulmonary infection. Semin. Immunopathol.. 2012;34(2):281-297.
- [CrossRef] [Google Scholar]
- The use of NaCl addition for the improvement of polyhydroxyalkanoate production by Cupriavidus necator. Bioresour. Technol.. 2014;163:287-294.
- [Google Scholar]
- Potts, M. (1994). Desiccation tolerance of prokaryotes. Microbiol. Rev., 58 (4): 755–805. https://pubmed.ncbi.nlm.nih.gov/7854254/.
- Quan, F.S., Rubino I., Lee S.H., Koch B., Choi H.J. (2017). Universal and reusable virus deactivation system for respiratory protection. https://www.nature.com/articles/srep39956.
- Tonog P., Lakhkar A.D. (2019), Normal saline. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2020 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545210/.
- Effect of high salt treatment on influenza B viral protein synthesis in MDCK cells. Microbiol. Immunol.. 1983;27(6):519-529.
- [Google Scholar]
- Vincent, M and P. Edwards. (2016). Disposable surgical face masks for preventing surgical wound infection in clean surgery. Cochrane Database Syst. Rev. 2016(4). Art. No.: CD002929. DOI: 10.1002/14651858.CD002929.pub3. https://www.cochrane.org/CD002929/WOUNDS_disposable-surgical-face-masks-preventing-surgical-wound-infection-clean-surgery.
- Walsh, E. (2019). How to kill bacteria with salt. https://sciencing.com/kill-bacteria-salt-12029250.html.
- World Health Organization (WHO). (2020). Advice on the use of masks in the community, during home care, and in health care settings in the context of COVID-19. https://apps.who.int/iris/bitstream/handle/10665/331493/WHO-2019-nCoV-IPC_Masks-2020.2-eng.pdf.
- Improvement of antifouling characteristics in a bioreactor of polypropylene microporous membrane by the adsorption of Tween 20. J. Environ. Sci.. 2007;19(12):1461-1465.
- [Google Scholar]
- Zeratsky, K. (2019). What's the difference between sea salt and table salt? https://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/expert-answers/sea-salt/faq-20058512.
- Zhang, W.C. L.J. Du, X.J. Zheng, X.Q. Chen, C. Shi C, B.Y. Chen, X.N. Sun, C. Li, Y.Y. Zhang, Y, Liu, H. Xiao, Q. Leng, X. Jiang X, Z. Zhang, S. Sun, S.Z. Duan. (2017). Elevated sodium chloride drives type I interferon signaling in macrophages and increases antiviral resistance. J. Biol. Chem. 293(3):1030-1039. https://www.ncbi.nlm.nih.gov/pubmed/29203528.
- Surgical masks as source of bacterial contamination during operative procedures. J. Orthop. Transl.. 2018;14:57-62.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102125.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







