Translate this page into:
Design, stereoselective synthesis and anticancer efficacy of a new class of functionalized pyrrolizidine heterocyclic hybrids
⁎Corresponding author. sraju@ksu.edu.sa (Raju Suresh Kumar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Novel spirooxindole-pyrrolizidines has been obtained by a cycloaddition reaction and are tested for their anticancer potentials in vitro using HepG2 cells (cancer cells) and the most active heterocyclic hybrids are verified for their toxicity against L929 cells (non-cancer cells).
Abstract
Eight new functionalized spirooxindole-pyrrolizidine heterocyclic hybrids has been obtained from N-pyridinylmethyl-bisarylmethylidene-pyridinones, Isatin and L-Proline by a multi-component cycloaddition reaction. Such synthesized derivatives are being characterized systematically with the aid of instrumental facilities like FT-IR, NMR spectroscopy, and Mass spectrometry. Following this, all the compounds are tested for the anticancer potentials in vitro using HepG2 cells (cancer cells). The compound with no substitution on the aryl rings (phenyl rings), displayed the highest activity among the spirooxindole-pyrrolizidines. Further the most active heterocyclic hybrids are verified for toxicity effects against L929 cells (non-cancer cells; at 200 µg/mL for 24 h). Finally, the cell viability losses for these most active compounds (at its IC50 value) are addressed by assaying the activity of free radicals, apoptosis, and caspases.
Keywords
Cycloaddition reaction
Spirooxindole-pyrrolizidines
Anticancer activity
ROS generation
Caspase-3 activity
Apoptosis
1 Introduction
Cancer is a widespread group of diseases with abnormal growth of unwanted cells and the second most deadly disease worldwide (https://www.who.int/health-topics/cancer#tab=tab_1). Of many different kinds of cancers, hepatocellular carcinoma (HCC) is a serious form of cancer disease that can feast from the liver to other organs and is the foremost liver cancer accounting for >90 % of all cases (Sia et al., 2017; Villanueva, 2019; Llovet et al., 2016; Global Burden of Disease Liver Cancer Collaboration, 2017). Due to its very high impedance, this cancer type has become the 6th most common malignant illness worldwide and the 2nd main source of cancer-related death (Waller et al., 2015; World Health Statistics, 2021). Therefore, these statistics are demanding for the researching of novel therapeutic pathways for the medical practitioners and the development of more effective and targeted anticancer drugs to the medicinal chemists (Tang et al., 2004; Blume, 2003).
In view of that, there have been a number of pharmacologically active moieties which are under investigation as potential anticancer agents and one among them is the spiro heterocycles (Al-thamili et al., 2020; Kumar et al., 2023; Sachdeva et al., 2022; Hassan and Abou-Amra, 2022; Bora et al., 2021; Bin et al., 2015). Spiro heterocycles have engrossed noteworthy attention in recent years owing to their profuse biological profiles attributed mainly to their adaptability and structural resemblance to vital pharmacophores. Since many of the classified compounds available in most of the bioactive molecules are belonging to the spiro heterocycles family and so are considered to have privileged structural frameworks in the drug discovery research. One of the spiro derivatives, spirooxindole illustrates a class of compounds resulting from isatin owning vibrant biological properties. The significant feature of spirooxindoles with pyrrolizidine structural units linked at the C-3 position of oxindole ring renders them as optimistic entrants in drug discovery (Panda et al., 2023; Zhou et al., 2020). These spirooxindole significantly inhibits the tumor growth by acting against the phosphodiesterases (PDEs), an ubiquitous enzyme family that control the stages of intracellular second messengers like cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP) (Stratakis, 2012). The PDEs are being classified into 11 different isoenzyme types (PDE1–PDE11) where they regulate the signal transduction pathways that have a control over various intracellular pathways such as the cell proliferation, differentiation, apoptosis, inflammation, vasoconstriction etc. Also, increased levels of cAMP in some of the cancer cells like breast and colon may lead to the inhibition of cell growth, restrict cell migration, and apoptosis (Azevedo et al., 2014; Ahmed et al., 2022). Although the increased cAMP levels in the cancer cells may lead to the inhibition of diseased cell proliferation, the drugs associated with such intracellular protein increase are not commonly recommended because of the high toxicity issues (Barakat et al., 2018). Hence there has been a demand for the development of novel PDE regulators with minimal toxicity effects in cancer therapy.
Therefore, by considering the promising anticancer potentials of spiro compounds in view (Panda et al., 2023; Zhou et al., 2020; Stratakis, 2012), we have recently reported the anticancer potentials of spirooxindole derivatives engrafted with diverse sub-units (Al-thamili et al., 2020; Kumar et al., 2020; Kumar et al., 2021; Kumar et al., 2020; Kumar et al., 2020). The present work is aimed to investigate the efficacy of spirooxindole-pyrrolizidine heterocyclic hybrids in vitro. For that, we designed, synthesized, and explored the cancer cell proliferation behaviors of the new class of spirooxindole-pyrrolizidine heterocyclic hybrids. From the results analysis, the heterocyclic hybrids engrafted N-pyridylpiperidine and with α,β-unsaturated ketone unit are investigated to have significantly improved the anticancer potentials because of the enhanced pharmacodynamic and pharmacokinetic profiles.
2 Results and discussion
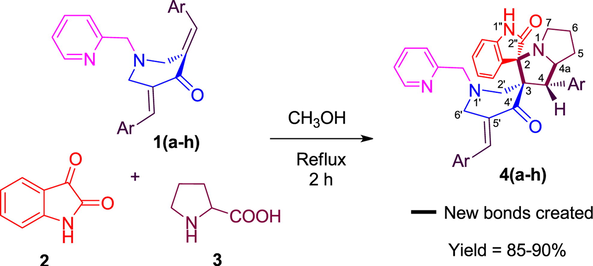
As presented in the Scheme 1, we synthesized spirooxindole-pyrrolizidine heterocyclic hybrids by performing the one-pot cycloaddition reaction of N-pyridinylmethyl substituted pyridinones 1(a–h), isatin 2 and (L)-proline 3 in methanol in good yields. The reaction condition for the efficient construction of 4a was optimized primarily by carrying out the reaction of 1a, 2 and 3 in various solvents as briefed in Table 1. Development in the reaction was observed time to time and after completion, the obtained precipitate was filtered and dried. Among the solvents employed, the optimal result was observed in methanol under reflux temperature for 2 h, all other solvents are not fruitful as methanol in terms of reaction time and yield.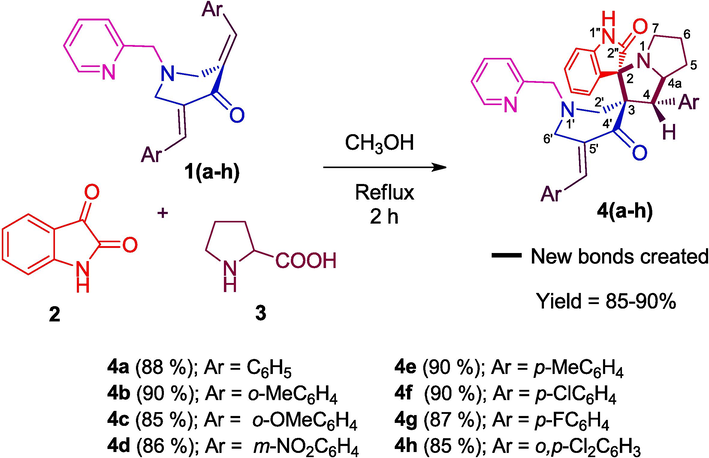
Schematic depiction of the formation of spirooxindole-pyrrolizidines 4(a–h).
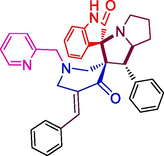
Structural elucidation of 4a was done with the support of FT-IR and 1D & 2D NMR spectroscopic data (Figs. 2a & 2b and supporting Information). In the IR spectrum, the primary functional groups viz. N—H, C⚌O and C⚌C was identified by the presence of absorption peaks at υmax 2359, 2341, 1712, and 1590 cm−1. H-4 and H-4a protons resonated as a doublet at 4.42 ppm (J = 11.5 Hz) and a multiplet at 4.76–4.80 ppm. The signals at 53.22 ppm and 65.94 ppm were allocated to C-4 and C-4a respectively. The 5-CH2 protons appeared as multiplets at 1.66–1.73 ppm and 2.08–2.14 ppm. Similarly, the multiplet at 1.76–1.94 ppm was due to 6-CH2 whereas the 7-CH2 protons appeared as multiplets at 2.61–2.64 ppm and 3.24–3.29 ppm. The carbon signals at 28.13 ppm, 25.18 ppm and 47.96 ppm were allotted respectively to C-5, C-6 and C-7. The spiro carbons C-3 and C-2 resonated at 70.56 ppm and 74.55 ppm. 2′-CH2 protons resonated as a doublet and a multiplet respectively at 2.02 ppm 3.80–3.82 ppm. The 6′-CH2 protons appears as doublet of doublets and a doublet at 2.90 ppm and 3.40 ppm respectively. The pyridinylmethyl protons, 7′-CH2 appear as two doublets at 3.35 ppm and 3.85 ppm. From HMQC spectra, the carbon signals at 56.56 ppm and 53.80 ppm were assigned to C-2′ and C-6′ while the C-7′ showed a peak at 63.43 ppm. The peaks at 179.37 ppm and 199.44 ppm were assigned to oxindole and piperidone carbonyl carbons. The peaks around 6.65–8.45 ppm were attributed to the aromatic protons while the aromatic carbons appear around 109–157 ppm. Fig. 1 depicted the important 1H & 13C chemical shifts of 4a and Figs. 2a & 2b represent respectively the 1H and 13C NMR spectrum of 4a.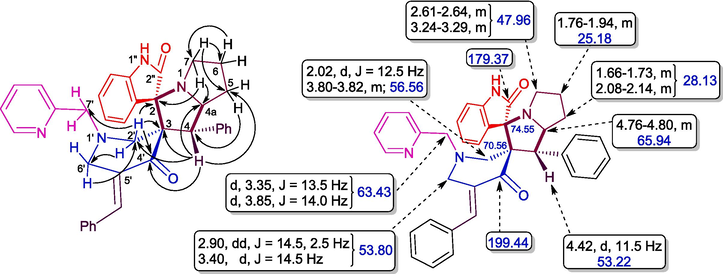
Selective HMBCs and 1H & 13C chemical shifts of 4a.
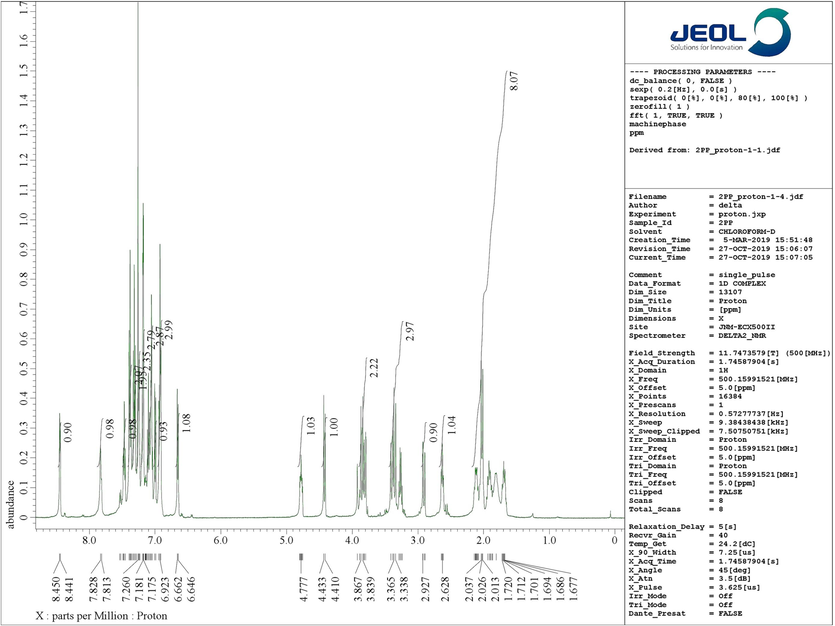
1H NMR spectrum of 4a.
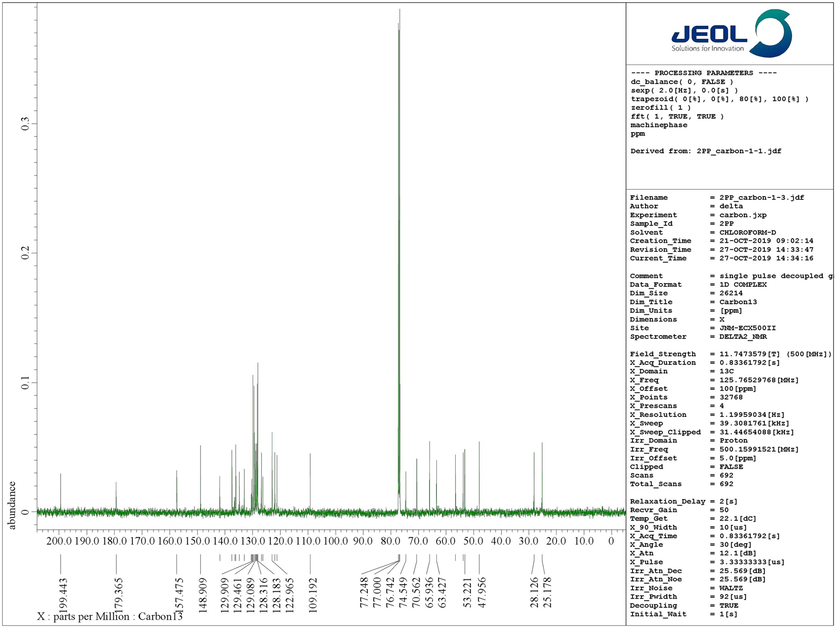
13C NMR spectrum of 4a.
Later, the remaining dipolarophiles 1(b–h) were also used to obtain spirooxindole-pyrrolizidine heterocyclic hybrids substituted with different groups in the aryl ring in good yields by adopting the same reaction methodology.
Scheme 2 depicts the reasonable mechanism for the efficient construction of 4(a–h). Firstly, the resonating forms of azomethine ylide, A and B (Kumar et al., 2020) was generated through the decarboxylative condensation of 2 and 3. Then, addition of ylide A to the C⚌C bond of 1(a–h) as shown in scheme 2 afford the cycloadducts 4(a–h). Addition of ylide B to the C⚌C bond was not observed due to the possible steric hindrance exerted by the oxindole and aryl rings.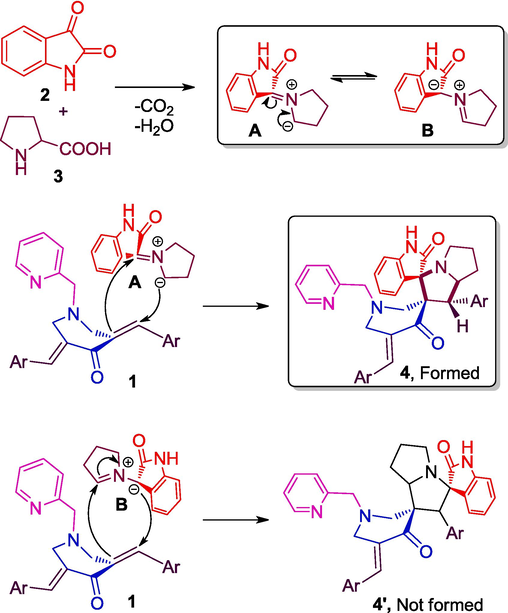
Illustration of feasible mechanism towards the formation of 4(a–h).
The efficacy of synthesized compounds 4(a–h) was tested against the in vitro cell culture systems and by taking two different cell cultures (cancerous and non-cancerous) over a 24 h period (Table 2). The reason for selecting one cancer and other non-cancer cell for our study is that the most anticancer agents (chemotherapeutic) act against the highly aggressive cancer cells and at the same time they have potential toxicological effects on the healthy normal cells too. Therefore, to understand the influence of synthesized compounds on both type of cells and associated cell physiology onto the cells, we selected the HepG2 human hepatic cells under the cancer cell category while the L929 mouse subcutaneous connective tissue from the non-cancer cells category. Further, the detailed cell culturing and the assay protocols of cell viability and their proliferation (MTT assay), flow cytometry involved apoptosis, cell-involved oxidative stress status, and the activity of caspases are presented as supplementary data in the Supporting Information.
Entry
Compound
IC50 values (µM) HepG2 cells; 24 h
IC50 values (µM) L929 cells; 24 h
1
14.09 ± 2.8
124.72 ± 4.3
2
41.66 ± 3.4
3
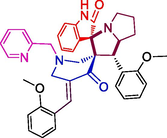
112.66 ± 5.2
4
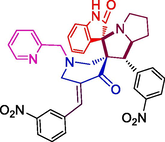
27.59 ± 2.3
139.51 ± 5.7
5
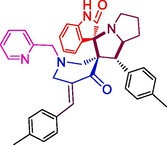
106.9 ± 4.8
6
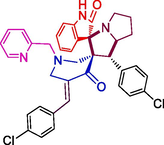
134.22 ± 5.3
7
36.12 ± 3.1
8
33.52 ± 3.8
Fig. 3 shows the assessment of percentage cell viability of HepG2 cells after their treatment with 4(a–h) in the concentration range of 12.5–200 µg/mL where 15 µg/mL of Melphalan (Mel) was employed as the positive control and the cells without any treatment as negative control. As provided in the graph, it is noticed that the testing compounds 4(a–h) are causing a significant reduction of cells to lose their viability. We observed almost all the compounds are reducing the cells viability with an increase of concentration and with the highest activity being observed for 4a (IC50 of 14.0 µg/mL) followed by 4d (IC50 of 27.5 µg/mL) (the IC50 values were calculated from the log concentration vs cell viability plots). Further, the tested compounds 4(a–h) are following the order of IC50 values as: 4a > 4d > 4 h > 4 g > 4b > 4e > 4c > 4f. Such an observation of order can be explained by taking the structures of spirooxindole-pyrrolizidine heterocyclic hybrids, i.e., the increase in the substitution of groups onto the basic structure is causing a reduction in the activity due to the decreased solubility in aqueous cell culture media and associated decrease in the testing compound’s interaction with that of cellular proteins. Based on this phenomenon, we observed the reactivity order as follows: no substituent > meta nitro > ortho para di chloro > para fluoro > ortho methyl > para methyl > ortho methoxy > para chloro substituent.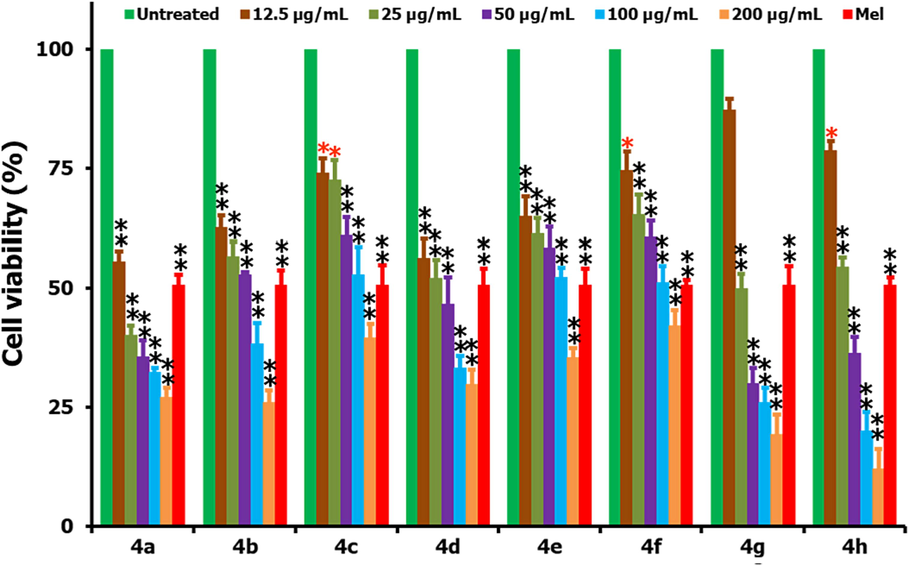
Cell viability (%) studies of the testing compounds 4(a–h) by comparing with the positive and negative controls for a 24 h time frame. From the figure, * and ** stands for the significance (p < 0.05) and highly significance (p < 0.01) values (respectively) when compared to the untreated controls.
In a similar way, the cellular effects of two highly active compounds 4a and 4d are being studied by taking the non-cancer L929 cells where we compared the results by taking the untreated negative and positive control (Mel of 15 µg/mL) over the same 24 h period (Fig. 4). This can be informed from the figure that the tested compounds are causing a reduction of non-cancer cell’s viability too, but not in the same way like HepG2 cancer cells, i.e., significant losses to the cell’s viability was noted at the high concentrations only. The IC50 values calculated for the two tested compounds 4a and 4d towards the non-cancer L929 cells are 124.7 µg/mL and 139.5 µg/mL, respectively. As compared to the IC50 values of 4a and 4d against the non-cancer H929 cells, the obtained values are quite high, which in other words providing an information that the growth of cancer cells can be controlled without much damage to the non-cancer cells by engaging the current heterocyclic hybrids (4a and 4d) as the potential therapeutic agents.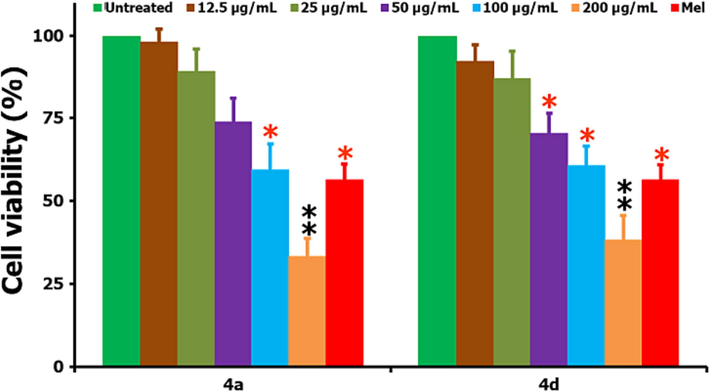
Cell viability (%) studies of non-cancer L929 cells following the compounds 4a and 4d treatment (at IC50 concentration) and when compared to the positive and negative controls. From the figure, * stands for the significance (p < 0.05) and ** for the highly significance (p < 0.01) values (respectively) when compared to the untreated controls.
In continuation to the cell viability studies, the compound 4a which is highly active (at its IC50 value of 14 µg/mL) is being explored for the cell death mechanism by performing the apoptosis, ROS, and caspase assays. The studies of apoptosis assay results as observed from compound 4a compared to that of positive and negative controls over a 24 h period are provided in Fig. 5. From the figure, ‘A’ series corresponds to the the cells of no sample treament, ‘B’ for the compound 4a treated cells, and ‘C’ series for the Mel treatment (respectively) to HepG2 cancer cells. Likewise, the series 1, 2, and 3 in the Fig. 5 (as A-1, A-2, A-3 etc) corresponds to forward scattering (FSC) parameters, combined fluoresence activities of propidium iodide (PI) and Annexin-V-FITC, and Annexin-V-FITC activity of HepG2 cells respectively. Therefore, from the the data provided in the figure, we mearued the compound 4a treated cancer cells are having 40.8 % of live, 20.6 % of early apoptotic, 33.8 % of late apoptotic, and 4.6 % of necrotic cells (Fig. 5(B-2)). However, for the Mel compound treated cells are having 41.8 % live, 44 % early apoptotic, 10.3 % late apoptotic, and 3.7 % necrotic cells (Fig. 5(C-2)). Further, the compound 4a treated cells are having 48 % non-apoptotic and 52 % apoptotic cells (Fig. 5(B-3)) as against the Mel treated cells with 46 % non-apoptotic and 54 % apoptotic cells (Fig. 5(C-3)). This observation indicates that the compound 4a is behaving almost similar to the positive compound Mel in terms of its activity of generating equal percentage of apoptotic cells. From the analysis therefore, it can be inferred that apoptosis is the major pathway of mechanism to cause the HepG2 cancer cells to lose their viability after compound 4a treatment.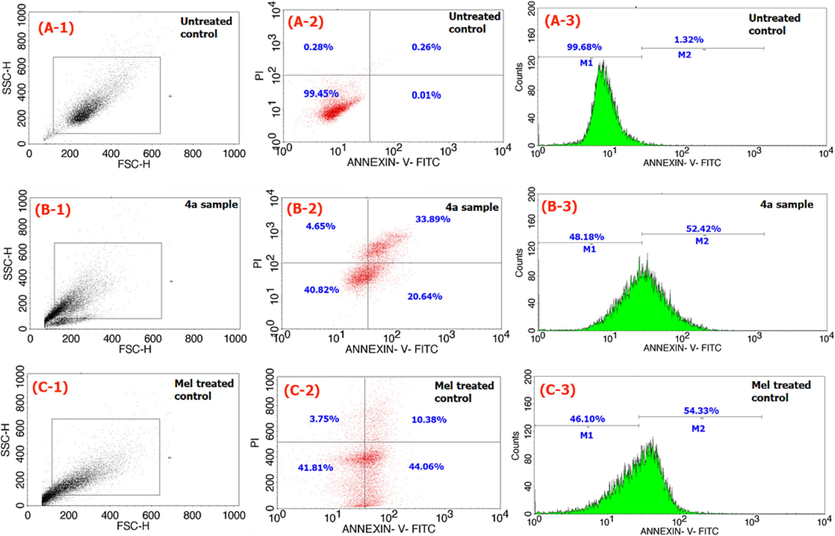
Flow cytometric analysis of apoptosis assay results comparison of compound 4a (B series) with that of no cell treatment (A series) and positive control of Mel (C series) for 24 h time frame.
Fig. 6 demonstrates the flow cytometry studies of ROS assay results for 4a (at its IC50 value) exposed cells when compared to the positive and negative controls. From the results, about 31.1 % (M2) of compound 4a exposed cancer cells are providing significant levels of ROS production as against the corresponding 69.8 % (M1) of cells with no significant ROS. These values are rather high as compared to the Mel treated cells with 10.6 % and only 0.06 % of cells with significant ROS production, and thereby providing the information that the compound 4a is inducing oxidative response to the cancer cells.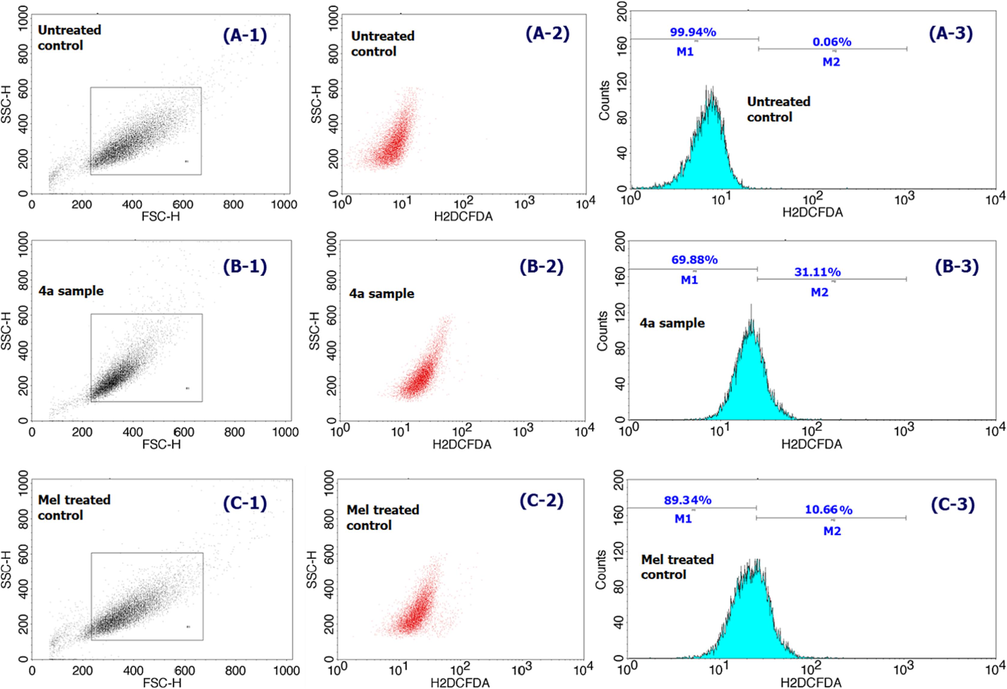
Flow cytometric analysis of ROS test results comparison of compound 4a (B series) treated HepG2 cancer cells with that of positive control Mel (15 µg/mL; C series) and non-treated cells (A series) over a 24 h period.
To investigate the association of caspases in 4a treated cells (at its IC50 value), the caspase-8 activity assay was performed and compared the results with that of positive and negative controls as shown in Fig. 7. It can be observed from the analysis that the compound 4a (Fig. 7c) treated cells are having significant amount of caspases and this is very high as compared with the cells of no treatment (Fig. 7a) and a little high against Mel treated sample (Fig. 7b). From the analysis therefore, it can be mentioned that the HepG2 cancer cells are expressing the caspases due to the toxic action of compound 4a which further can be scrutinized for the generation of complete cell death particularly in the cancer cells with slight loss to the normal cells.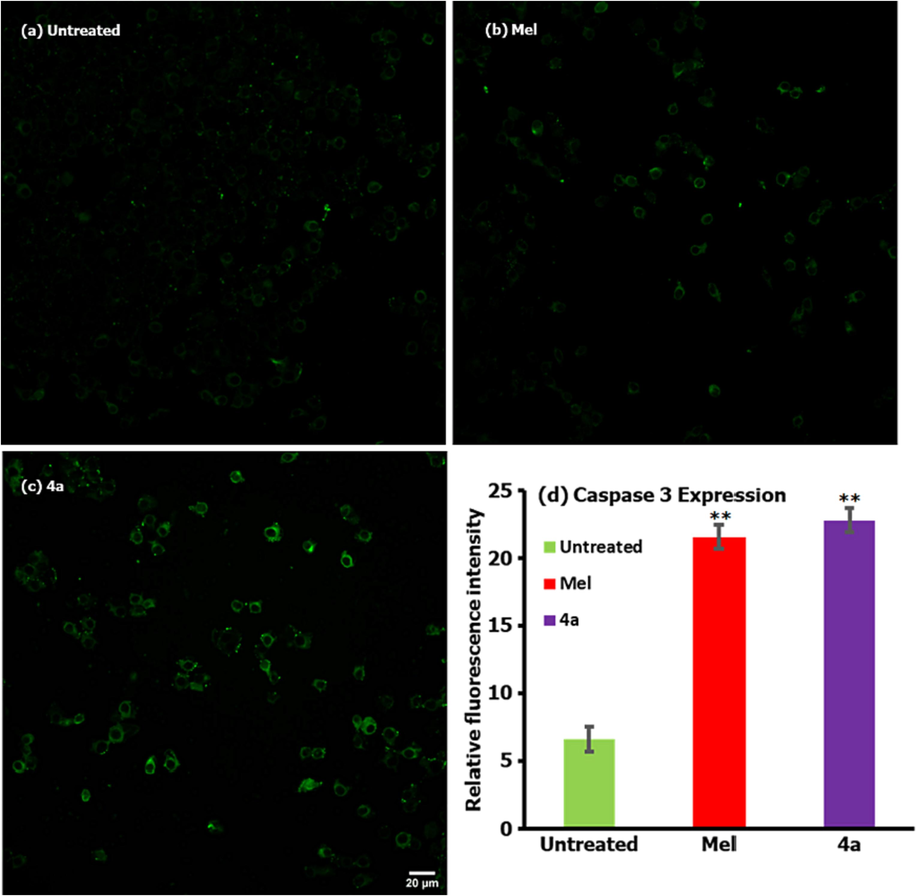
Caspase-3 activity assay followed by treatment of 4a when compared with positive and negative controls.
In the present study, we have synthesized and characterized various spirooxindole-pyrrolizidine heterocyclic hybrids with change of substituents and evaluated their in vitro biological effects following the incubation with a cancer and non-cancer cell line at a change of concentration (12.5–200 µg/mL) over a 24 h period. We observed from the incubation studies on cancer cells that all the compounds have anticancer effects, i.e., a reduction of viability for all the treatments was noticed and with the highest activity being observed for compound 4a. For the compound 4a, the least IC50 value (from the log concentration vs cell viability plot) of 14 µg/mL was calculated and such an observation of least value for this compound 4a is attributed to its aqueous solubility as against all other derivatives. In a similar way, the cell viability effects of this compound against the non-cancer cells were investigated to have very high levels of IC50 values and thereby providing a room for the safety use of this compound towards therapeutic applications. Further tests of flow cytometry provided the evidence that compound 4a is prompting the apoptotic pathway with the release of ROS and caspase-8, and all these assays confirm the potential anticancer activity of the synthesized spirooxindole-pyrrolizidine heterocyclic hybrids.
3 Experimental
The detailed experimental procedure and data has been provided in the Supporting Information as supplementary data.
4 Preparation of spirooxindole-pyrrolizidines 4(a–h)
The dipolarophiles 1(a–h), the diketone Isatin 2 and the amino acid (L)-Proline 3 in an equimolar ratio in 5 mL of methanol was stirred under reflux temperature for 2 h. Subsequently, after the reaction was complete, the mixture was discharged into water (50 mL), the obtained product was purified by crystallization using methanol, to afford compounds 4(a–h) in good yields.
5 Conclusion
In conclusion, an effective synthesis of functionalized spirooxindole-pyrrolizidine heterocyclic hybrids have been produced employing cycloaddition reaction of a new class of substituted dipolarophiles with azomethine ylide produced in situ from the diketone, Isatin and the amino acid, (L)-Proline. The in vitro biological assays for the synthesized spirooxindole-pyrrolizidine heterocyclic hybrids of all the derivatives have confirmed for the anticancer activity against the HepG2 cells. The compound with no substitution on the aryl rings (phenyl rings), displayed the highest activity among the spirooxindole-pyrrolizidines. The change of efficiency with respect to the substitution (highest for the basic structure without any substituent) can be linked to the sample soluble capacity in the growth media and is associated intracellular protein interaction. Further assays studied with the highly potent spirooxinddole-pyrrolizidine heterocyclic compound like the apoptosis, ROS generation, and the influence of caspases are supporting the occurrence of programmed cell death in the cancer HepG2 cells. Finally, the study provides an experimental proof for the incorporation of spirooxindole-pyrrolizidine heterocycles as potential anticancer agents with controlled toxicity effects towards the cancer and non-cancer cells, where the pharmacokinetic parameters are chosen based on the kind of derivative which in turn a dependent of the substituent.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, “Ministry of Education” in Saudi Arabia for funding this research (IFKSUOR3-488-1).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- cAMP signaling in cancer: a PKA-CREB and EPAC-centric approach. Cells.. 2022;11(13):2020.
- [Google Scholar]
- In vitro molecular biology studies of spirooxindole heterocyclic hybrids. Processes. 2020;8:1473.
- [Google Scholar]
- Functionalized N-pyridinylmethyl engrafted bisarylmethylidenepyridinones as anticancer agents. Processes. 2020;8:1154.
- [Google Scholar]
- Clinical and molecular genetics of the phosphodiesterases (PDEs) Endocr. Rev.. 2014;35:195-233.
- [Google Scholar]
- Substituted spirooxindole derivatives as potent anticancer agents through inhibition of phosphodiesterase 1. RSC Adv.. 2018;8(26):14335-14346.
- [Google Scholar]
- Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem.. 2015;97:673-698.
- [Google Scholar]
- Molecular therapy and prevention of hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int.. 2003;2:11-22.
- [Google Scholar]
- Anticancer potential of spirocompounds in medicinal chemistry: a pentennial expedition. Eur. J. Med. Chem.. 2021;215:113263.
- [Google Scholar]
- Global Burden of Disease Liver Cancer Collaboration, 2017. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level. JAMA Oncol. 3, 1683–1691.
- Novel indoline spiro derivatives ss anticancer agents; design, synthesis, molecular docking studies, and ADME evaluation. Polycycl. Aromat. Compd. 2022:1-21.
- [Google Scholar]
- [Bmim]Br accelerated one-pot three-component cascade protocol for the construction of spirooxindole-pyrrolidine heterocyclic hybrids. Molecules. 2020;25:4779.
- [Google Scholar]
- In vitro mechanistic exploration of novel spiropyrrolidine heterocyclic hybrids as anticancer agents. Front. Chem.. 2020;8:465.
- [Google Scholar]
- A one-pot three-component synthesis and investigation of the in vitro mechanistic anticancer activity of highly functionalized spirooxindole-pyrrolidine heterocyclic hybrids. Molecules. 2020;25:5581.
- [Google Scholar]
- Caspase dependent apoptotic activity of polycyclic cage-like heterocyclic hybrids. Saudi J. Biol. Sci.. 2020;27(12):3290-3300.
- [Google Scholar]
- Ionic liquid mediated synthesis and in vitro mechanistic exploration of polycyclic cage-like heterocyclic hybrid. J. Heterocycl. Chem.. 2021;58(2):580-588.
- [Google Scholar]
- Nitrogen containing heterocycles as anticancer agents: a medicinal chemistry perspective. Pharmaceuticals. 2023;16:299.
- [Google Scholar]
- Spirooxindole: a versatile biologically active heterocyclic scaffold. Molecules. 2023;28:618.
- [Google Scholar]
- Oxygen- and sulphur-containing heterocyclic compounds as potential anticancer agents. Appl. Biochem. Biotechnol.. 2022;194:6438-6467.
- [Google Scholar]
- Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152:745-761.
- [Google Scholar]
- Cyclic AMP, protein kinase A, and phosphodiesterases: proceedings of an international workshop. Horm. Metab. Res.. 2012;44:713-715.
- [Google Scholar]
- A decade’s studies on metastasis of hepatocellular carcinoma. J. Cancer Res. Clin. Oncol.. 2004;130(4):187-196.
- [Google Scholar]
- Hepatocellular carcinoma: a comprehensive review. World J. Hepatol.. 2015;7:2648-2663.
- [Google Scholar]
- World Health Statistics, 2021. Monitoring Health for the SDGs – World, ReliefWeb, n.d. Retrieved December 7, 2021, from https://reliefweb.int/report/world/world-health-statistics-2021-monitoring-health-sdgs.
- An overview of spirooxindole as a promising scaffold for novel drug discovery. Expert Opin. Drug Discov.. 2020;15:603-625.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.103009.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
Supporting Information







