Translate this page into:
Cytotoxic effect of polystyrene nanoplastics in human umbilical vein endothelial cells (HUVECs) and normal rat kidney cells (NRK52E)
⁎Corresponding author. mahamed@ksu.edu.sa (Maqusood Ahamed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Plastics play a crucial role in nearly each aspect of societal production and everyday life, therefore making them one of the most widespread pollutants on a global scale. Because synthetic plastics are not entirely biodegradable, they often remain in the environment and fragment into micro- and nanoplastic particles. The detrimental impacts of nanoplastics on the environment and human health have received substantial worldwide interest in recent years. However, the effects of nanoplastics on human health have not yet been fully explored. Our study aimed to investigate the cytotoxic effects of polystyrene nanoplastics on two distinct mammalian cell lines: normal rat kidney cells (NRK52E) and human umbilical vein endothelial cells (HUVECs). Results showed that polystyrene nanoplastics generate cytotoxicity in both NRK52E and HUVECs in a concentration- and time-dependent manner. Additionally, polystyrene nanoplastics induced pro-oxidant levels (e.g., reactive oxygen species and hydrogen peroxide) and reduced antioxidants (glutathione content and glutathione peroxidase enzyme activity) in both types of cells. We also found that polystyrene nanoplastics cause apoptosis in both NRK52E and HUVECs, as shown by the activation of the caspase-3 enzyme and the loss of mitochondrial membrane potential. Interestingly, it was noticed that the vulnerability of HUVECs cells against polystyrene nanoplastics was a little higher than that of NRK52E cells. Also, the cell toxicity caused by polystyrene nanoplastics in NRK52E and HUVECs was effectively alleviated by co-exposure to a reactive oxygen species inhibitor, N-acetyl-cysteine. This suggests that oxidative stress could be one of the possible pathways by which polystyrene nanoplastics cause cell toxicity. The present work warrants future study to explore the toxicity mechanisms of polystyrene nanoplastics in appropriate in vivo models.
Keywords
Plastic waste
Human health
Kidney toxicity
Blood vessels
ROS
Apoptosis
Caspase-3
1 Introduction
Plastics have become extensively utilized in recent times, covering diverse parts of our everyday life and industrial production, therefore making them one of the most widespread pollutants on an international scale (Banerjee et al., 2022). Currently, the global use of plastics has surged to more than 400 million tons per year (Wang et al., 2023). By 2050, it is projected that there will be almost 12,000 million tons of plastic trash (Wen et al., 2022). Efforts are underway to tackle this problem by implementing international agreements on plastic waste management and suggesting the adoption of a circular bio-economy for plastics (Alimi et al., 2022). However, the persistent nature of plastics poses challenges to completely eliminating them (Li et al., 2022). These plastic products that are made by humans are inevitably thrown away into the nearby environment, particularly those that are used only once, and then they are broken down into very small pieces called microplastics (<5 mm) or even smaller pieces called nanoplastics (<100 nm) through processes such as mechanical wear and tear, degradation caused by exposure to ultraviolet radiation, and degradation caused by biological factors (Singh et al., 2022). Nanoplastics pose a greater risk to organisms compared to microplastics because of their smaller particle size, which enables them to pass through cell membranes in a random manner (Oliveri Conti et al., 2020). According to reports, micro- and nanoplastics have been found in various environments worldwide, such as land, air, oceans, sediments, and freshwater. This presence of micro- and nanoplastics poses a danger to both the environment and humans (Li et al., 2022). Upon ingestion, micro- and nanoplastics infiltrate and amass in different tissues and organs, such as the stomach, gut, liver, and kidneys (Jeyavani et al., 2022; Rist et al., 2017). This can lead to physical damage, such as blockages or perforation, as well as trigger oxidative stress, inflammatory reactions, and metabolic dysfunctions (Fournier et al., 2020; Ragusa et al., 2021) Micro- and nanoplastics have been identified in various human organs and bodily fluids, including the lung, kidney, placenta, urine, intestine, and blood (Fournier et al., 2020; Li et al., 2023). However, the impact of these micro- and nanoplastics on human health remains uncertain.
Polystyrene is one of the most commonly used plastics that is produced by the polymerization of styrene monomers. Main sources of polystyrene nanoplastics include discharge from various products like cosmetics, paints, biomedical items, electronics, and pharmaceutical products (Kik et al., 2020). Polystyrene nanoplastics are non-degradable and persist in the environment for several years, leading to significant environmental pollution (Yan et al., 2023). Polystyrene nanoplastics can be exposed to organisms through direct consumption of water and food, accidental ingestion while feeding or drinking, or transmission along the food chain (Gupta et al., 2022). Research on the toxicity of polystyrene nanoplastics in various biological systems is limited.
Polystyrene nanoplastics can enter the gastrointestinal tract through the consumption of contaminated food and/or water (Xu et al., 2023). After absorption, nanoplastics can enter the bloodstream and target important vital organs like the liver and kidneys, disrupting their activities and leading to toxicity (Banerjee et al., 2022; Li et al., 2023). Nanoplastics entering the cardiovascular system may come into contact with human blood vessels (Cepulis et al., 2023). Therefore, the impact of nanoplastics on the blood vessels has received significant attention. Endothelial cells form a thin layer covering the interior of human blood vessels. Endothelial cells are a focus of nanotoxicity/nanomedicine research due to two specific aspects. First, edothelial cells are the initial point of interaction with nanoplastics upon entering the bloodstream before they are transported to specific organs (Setyawati et al., 2015). Secondly, endothelial cells are crucial in controlling blood vessel constriction, blood clot formation, internal balance, immune cell attraction, and hormone movement (Tomaszewski et al., 2015). Endothelial cell dysfunction is linked to a variety of cardiovascular illnesses. Evaluating the toxicity of nanoplastics on endothelial cells involved in the development of cardiovascular illnesses can offer valuable insights into the harmful effects of nanoplastics on cardiovascular systems. Human umbilical vein endothelial cells (HUVECs) are a commonly utilized in vitro model for studying endothelial cells (Cao et al., 2017).
On the other hand, the kidney is often another target organ for nanoscale materials including nanoplastics due to its toxin-excreting capabilities (Li et al., 2023). The NRK52E is a cell line that has been stably immortalized and originates from rat kidney tubules. The NRK52E cell line is considered a suitable model for studying the effects of different nanoscale materials or xenobiotics on kidney damage and toxicity mechanisms (Dev et al., 2022; Lateef et al., 2023).
Here, two toxicologically relevant discrete cell lines (HUVECs and NRK52E) were chosen to explore the cytotoxic potential of polystyrene nanoplastics. N-acetyl-cysteine (NAC) helps replenish the intracellular GSH pool to enhance antioxidant functions. It has been established that NAC is effective in scavenging ROS and is commonly used as a pharmaceutical drug or nutritional supplement for a variety of health conditions. In this study, during some experiments, cells were exposed to both NAC and nanoplastics to investigate the potential mechanisms of nanoplastic toxicity via the oxidative stress pathway.
2 Materials and methods
2.1 Polystyrene nanoplastics
Polystyrene nanoplastics of 20 nm diameter was acquired from Huge-Biotechnology Company (Shanghai, China). Polystyrene nanoplastics were diluted in serum-free cell culture media to achieve required concentrations (1–200 µg/ml) for characterization of aqueous behavior and cytotoxicity experiments. Aqueous behavior of polystyrene nanoplastics in culture medium was characterized by dynamic light scattering (DLS) (Zeta-Sizer HT, Malvern Instruments).
2.2 Cell culture and exposure of polystyrene nanoplastics
HUVECs and NRK52E cells were acquired from ATCC (USA). Both kinds of cells were cultured in DMEM supplied with 10 % FBS, 100 µg/ml streptomycin, and 100 U/ml penicillin. Cells were cultured in a humidified incubator at 37 ˚C with a continuous supply of 5 % CO2. In order to carry out toxicity experiments, cells were exposed to polystyrene nanoplastics for 24, 48, and 72 h. Nanoplastics dilutions were sonicated for 15 min at 40 W before being exposed to cells.
2.3 Measurement of toxicity parameters
The MTT assay was performed in accordance with the procedure that Mosmann had previously documented (Mosmann, 1983), with slight modifications (Ahamed et al., 2021). The quantification of ROS within cells was achieved by employing 2‘-7‘-dichlorodihydrofluorescein (H2DCFDA) in conjunction with a microplate reader (Synergy-HT Biotek) (Ahamed et al., 2021). The intracellular concentration of GSH was determined through the protocol provided by Ellman (Ellman, 1959). To evaluate the activity of the GPx enzyme, the experimental procedures outlined by Rotruck and co-workers were implemented (Rotruck et al., 1973). The quantitative analysis of MMP was performed following the earlier described protocol (Creed and McKenzie, 2019) with minor adjustments as outlined before (Ahamed et al., 2021). The caspase-3 enzyme activity was assayed employing a BioVision colorimetric kit. The estimation of protein content was performed utilizing the protocol described by Bradford (Bradford, 1976).
2.4 Statistical assessment
Results were analyzed by one-way analysis of variance (ANOVA), followed by Dunnett‘s multiple comparison procedure. Data were presented as the mean ± SD of three separate tests (n = 3). The p < 0.05 was assigned as statistically significant.
3 Results and discussion
3.1 DLS characterization of polystyrene nanoplastics
The zeta potential and particle size distribution of polystyrene nanoplastics are presented in Table 1. Polystyrene nanoplastics were diluted into deionized water and culture medium from the stock suspension. A zetasizer Nano ZS was used to measure the zeta-potential (surface charge) of polystyrene nanoplastics in deionized water and complete culture medium (DMEM with FBS supplementation) at different aging periods (0, 4, 8, and 24 h). Results showed that zeta-potential values were in the range of −27 mV to −31 mV in different aging periods. Zeta potential data indicated that polystyrene nanoplastics were fairly stable in both kinds of aqueous medium. DLS data showed that the hydrodynamic size of polystyrene nanoplastics in both kinds of aqueous media was found to be in the range of 23 nm to 31 nm at different aging periods (0, 4, 8, and 24 h). DLS data also indicated that the hydrodynamic size of polystyrene nanoplastics did not change significantly within 0–24 h aging period. This also indicates that the dispersed nanoplastic suspensions were stable over the selected period that was used in toxicity studies. Earlier studies found that the higher stability of colloidal suspensions of nanostructured materials is associated with their better interaction with biological systems (Khorrami et al., 2018; Shi et al., 2022).
Suspension preparation time
Deionized
water
Culture
medium
Zeta potential
Hydrodynamic size
Zeta potential
Hydrodynamic size
0 h
−31.2 mV
23.4 nm
−29.3 mV
27.4 nm
4 h
−30.1 mV
24.8 nm
−28.8 mV
29.3 nm
8 h
−28.9 mV
26.4 nm
−27.5 mV
30.1 nm
24 h
−29.8 mV
29.3 nm
−26.8 mV
31.4 nm
3.2 Cytotoxic response of polystyrene nanoplastics
The potential toxicity of polystyrene nanoplastics could be a significant hurdle in diverse applications; hence, it is necessary to study the toxicological profile of theses nanomaterials. Here, we investigated the toxicity of polystyrene nanoplastics in two distinct cell lines. NRK52E and HUVECs were treated with different concentrations of nanoplastics (1–200 µg/ml) for various exposure times (24–72 h), and cytotoxicity was assessed through a MTT assay. Results showed that polystyrene nanoplastics induced dose- and time-dependent cytotoxicity in both NRK52E (Fig. 1A) and HUVECs (Fig. 1B). The IC50 values of polystyrene nanoplastics in NRK52E cells were 55, 49, and 27 µg/ml for 24, 48, and 72 h, respectively. Moreover, the IC50 values of the same nanoplastics for HUVECs were 52, 41, and 23 µg/ml for 24, 48, and 72 h, respectively (Table 2). These results further indicated that HUVECs were marginally more susceptible to polystyrene nanoplastics exposure in comparison to NRK52E cells. Recently, Shi and co-workers showed that 80 nm polystyrene nanoplastics induced a dose-dependent cell viability reduction of human lung cells (A549) in the concentration range of (2.5–400 µg/ml) (Shi et al., 2022). The cytotoxic effects of polystyrene nanoplastics in different cells have also been reported in other studies. Another study found that 50 nm polystyrene nanoplastics induce dose-dependent cytotoxicity in human liver cells (HepG2) in the concentration range of 10–100 (He et al., 2020).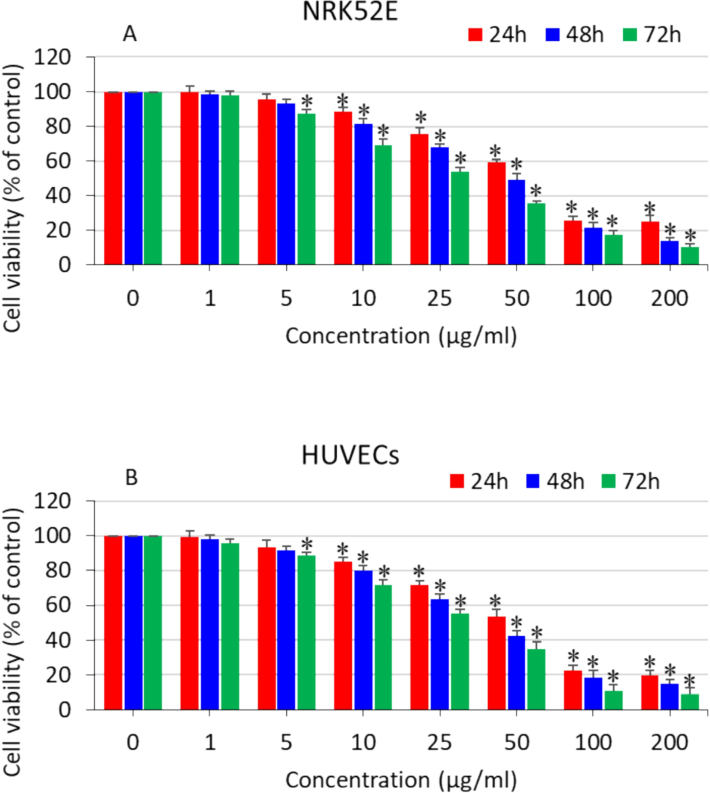
Dose- and time-dependent cytotoxicity of polystyrene nanoplastics in two distinct cell lines. (A) NRK52E cells and (B) HUVECs. *significantly different from the controls (p < 0.05).
Exposure time
NRK52E
HUVECs
24 h
55 µg/ml
52 µg/ml
48 h
49 µg/ml
41 µg/ml
72 h
27 µg/ml
23 µg/ml
3.3 Oxidative stress response of polystyrene nanoplastics
The ROS are byproducts of cellular metabolism and play crucial roles in cell signaling and maintaining internal stability. Nevertheless, an organism experiences an overabundance of ROS during periods of stress, which can result in damage due to oxidative stress. As a result, the cellular oxidative damage leads to the breakdown of lipids, proteins, and DNA through oxidation. The superoxide anion (O2•-), hydrogen peroxide (H2O2), and the hydroxyl radical (HO•) are the three main components of ROS. The precise methods by which nanoplastics generate toxicity have not yet been fully understood. The global scientific community is currently investigating the mechanism of the oxidative stress pathway caused by nanostructured materials, which leads to toxicity. Nanoplastics might cause oxidative stress through two mechanisms: by promoting the production of pro-oxidants or by impairing the cellular antioxidant defense system. In this study, explored the oxidative stress response of NRK52E and HUVECs cells after being exposed to polystyrene nanoplastics. Both types of cells were exposed 10, 25, 50, and 100 µg/ml of polystyrene nanoplastics for 24 h and determined the several parameters of pro-oxidants (ROS and H2O2) and antioxidants (GSH and GPx). Results demonstrated that nanoplastics induced ROS generation (Fig. 2A) and H2O2 level (Fig. 2B) in both NRK52E and HUVECs in a dose-dependent manner.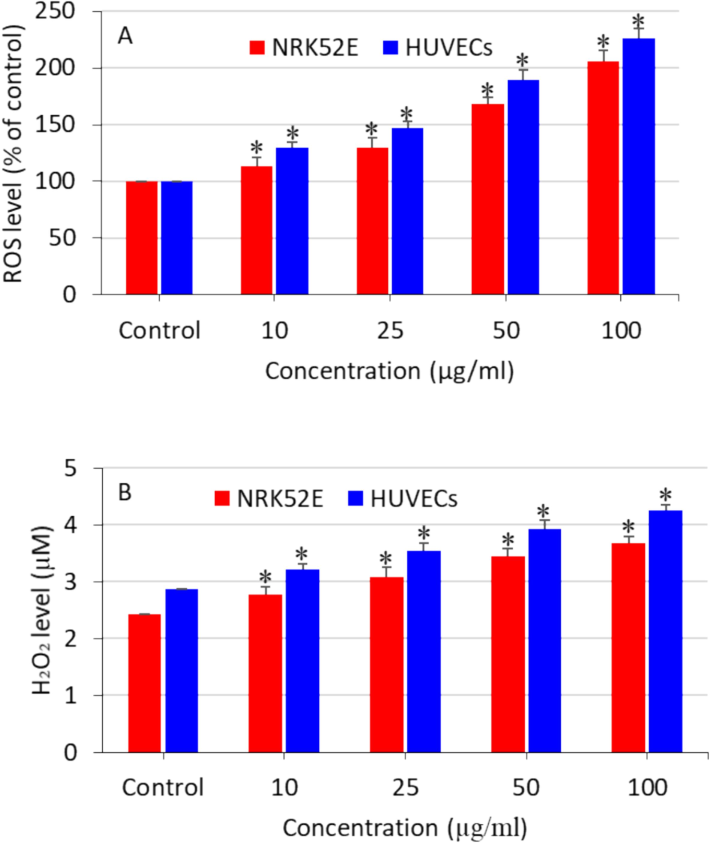
Polystyrene nanoplastics-induced pro-oxidants generation in NRK52E cells and HUVECs. (A) Intracellular ROS generation and (B) H2O2 production. *significantly different from the controls (p < 0.05).
The balance between the production of oxidants and their removal by antioxidant defense mechanisms is highly fragile. Hence, we further conducted a more in-depth analysis on the impact of polystyrene nanoplastics on the antioxidant molecule GSH and the enzyme GPx in NRK52E and HUVECs. The antioxidant biomarkers have a crucial function in eliminating the extremely reactive free oxygen radicals (Chen et al., 2020). The antioxidant molecule GSH catalyzes the reduction of H2O2 into singlet oxygen and water with the catalysis of GPx enzyme. The results showed that the levels of the antioxidant GSH and the activity of the GPx enzyme were depleted with increasing doses of nanoplastics in both NRK52E and HUVECs (Fig. 3A and B). Moreover, oxidative stress generated by polystyrene nanoplastics in HUVECs was slightly higher than NRK52E cells. Present results are supported by other investigators where they observed that toxicity of polystyrene nanoplstics was mediated via oxidative stress pathway (He et al., 2020; Liu et al., 2020).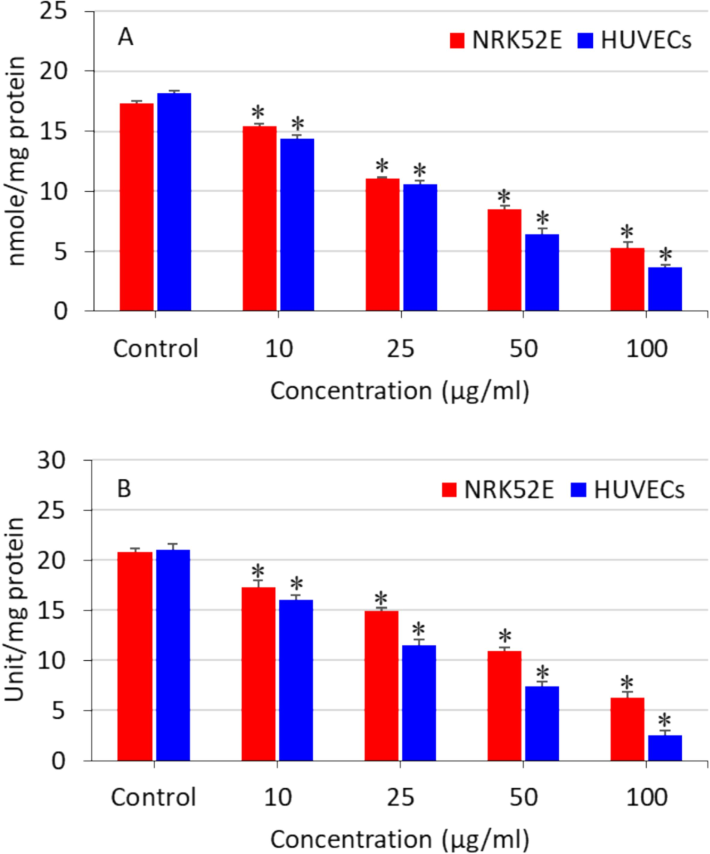
Polystyrene nanoplastics-induced antioxidants depletion in NRK52E cells and HUVECs. (A) GSH level and (B) GPx enzyme activity. *significantly different from the controls (p < 0.05).
3.4 Apoptosis response of polystyrene nanoplastics
Apoptosis is a kind of programmed cell death that sustains the homeostasis of tissues (Al Olayan et al., 2020). Programmed cell death might be triggered by several factors, including nutrient deficiency, growth factors, and external stimuli (Li et al., 2021). Excessive ROS generation in the cells can also act as a signalling molecule for the initiation of apoptosis (Yao et al., 2019). The antioxidant molecule GSH has also been associated with apoptosis stimulation (Malla et al., 2020). Hence, we further explored the effects of polystyrene nanoplastics on the apoptosis biomarkers of NRK52E and HUVECs. Cells were treated for 24 h with 10, 25, 50, and 100 µg/ml of nanoplastics, and caspase-3 enzyme activity and MMP level were determined in order to explore the apoptosis effect of polystyrene nanoplastics. As we can see in Fig. 4, the activity of the apoptotic enzyme caspase-3 was dose-dependently higher in both types of cells as compared to the controls. Moreover, polystyrene nanoplastics were also found to induce dose-dependent MMP loss in both NRK52E and HUVECs (Fig. 5). Interestingly, the apoptosis response of polystyrene nanoplastics against HUVECs was slightly higher than that of NRK52E cells. Previous studies also observed that polystyrene nanoplastics exposure to different cultured cells cause caspase-3 gene activation and MMP loss (Banerjee et al., 2022; Liu et al., 2022). A recent study also reported that polystyrene nanoplastics induced lung damage through apoptosis pathway (Wu et al., 2024).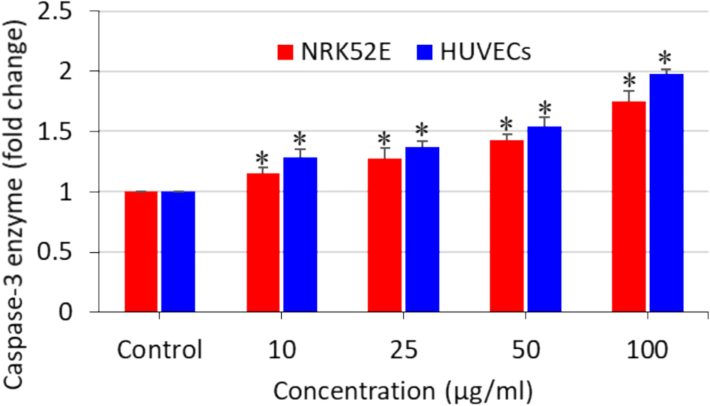
Polystyrene nanoplastics-induced caspase-3 enzyme activity in NRK52E cells and HUVECs. *significantly different from the controls (p < 0.05).
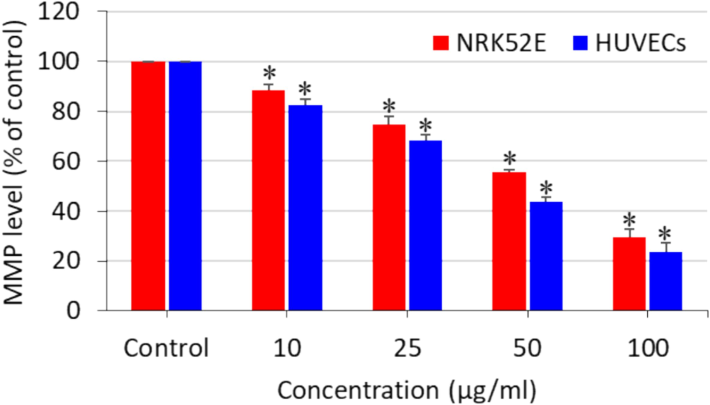
Polystyrene nanoplastics-induced MMP loss in NRK52E cells and HUVECs. *significantly different from the controls (p < 0.05).
3.5 Possible mechanisms of cytotoxicity induced by polystyrene nanoplastics
There is a scarcity of research that explores the potential mechanisms of toxicity induced by polystyrene nanoplastics. Some investigators observed that ROS generation and oxidative stress could be possible pathways for toxicity generated by polystyrene nanoplastics in biological systems (Liu et al., 2020; Yan et al., 2023). In this work, we further explored the role of ROS in polystyrene nanoplastics-induced toxicity in NRK52E and HUVECs. Both the cells were treated for 24 h with 50 µg/ml of polystyrene nanoplastics with or without co-exposure to the ROS inhibitor N-acetylcysteine (NAC). Results demonstrated that polystyrene nanoplastics-induced ROS in both NRK52E and HUVECs was significantly alleviated by NAC (Fig. 6A). Moreover, polystyrene nanoplastics-induced cytotoxicity in both cell types was effectively reverted by NAC co-exposure (Fig. 6B). These results indicate that polystyrene nanoplastics-induced cytotoxicity in both NRK52E and HUVECs was mediated via the oxidative stress pathway. A recent research has also observed that NAC effectively reduce the polystyrene nanoplastic-induced apoptosis in vitro and in vivo (Wu et al., 2024).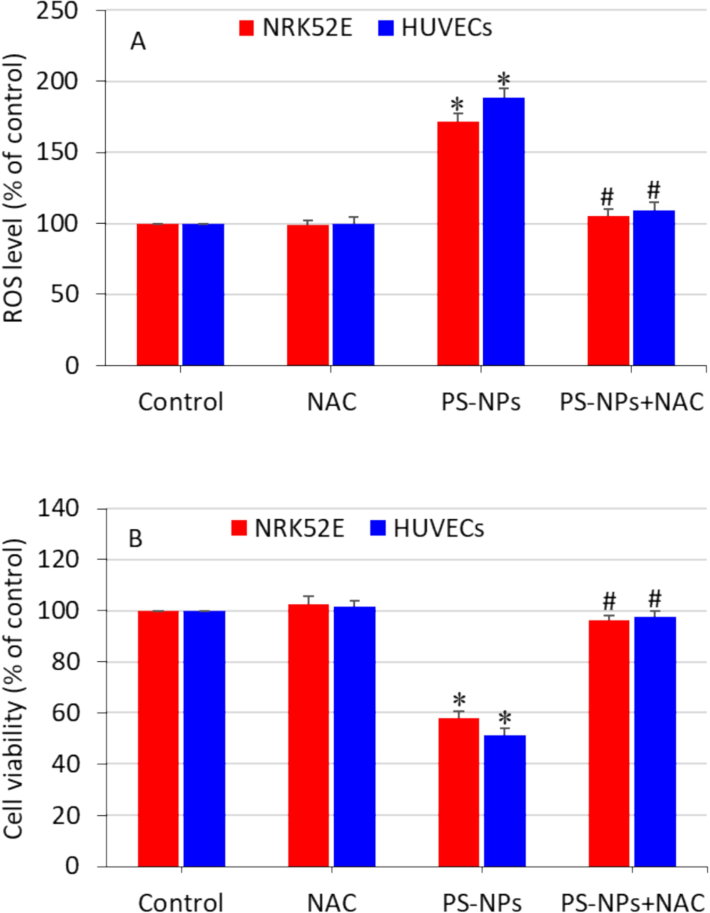
Role of ROS in polystyrene nanoplastics-induced toxicity in NRK52E cells and HUVECs. Cells were treated with a moderate concentration of nanoplastics (50 µg/ml) with or without antioxidant NAC (2 mM) for 24 h. (A) MTT cell viability and (B) intracellular ROS level. *significantly different from the controls (p < 0.05). # protective effect of NAC against nanoplastics-induced cytotoxicity and ROS generation.
4 Conclusion
We observed that polystyrene nanoplastics generate cytotoxicity in normal rat kidney cells (NRK52E) and human umbilical vein endothelial cells (HUVECs) in a concentration- and time-dependent manner. Interestingly, it was found that HUVECs were slightly more susceptible to polystyrene nanoplastics exposure as compared to NRK52E cells. Polystyrene nanoplastics were further observed to induce pro-oxidants (ROS and GSH) generation and antioxidants (GSH and GPx) depletion in both NRK52E and HUVECs. Caspase-3 enzyme activation and MMP loss in both cell types suggest the apoptosis response of polystyrene nanoplastics. Besides, cytotoxicity generated by polystyrene nanoplastics was efficiently alleviated by NAC co-exposure, which indicates that oxidative stress might be one of the possible mechanisms of nanoplastics-induced cell toxicity. This study necessities further investigation into the toxicity mechanisms of polystyrene nanoplastics in appropriate animal models.
CRediT authorship contribution statement
Maqusood Ahamed: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Mohd Javed Akhtar: Writing – review & editing, Software, Resources, Methodology, Investigation, Formal analysis.
Acknowledgment
The authors acknowledge the Research Institute/Centre supporting program (RICSP-24-1), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Co-exposure of Bi2O3 nanoparticles and bezo[a]pyrene-enhanced in vitro cytotoxicity of mouse spermatogonia cells. Environ. Sci. Pollut. Res. 2021
- [CrossRef] [Google Scholar]
- Protocatechuic acid mitigates cadmium-induced neurotoxicity in rats: Role of oxidative stress, inflammation and apoptosis. Sci. Total Environ.. 2020;723:137969
- [CrossRef] [Google Scholar]
- Weathering pathways and protocols for environmentally relevant microplastics and nanoplastics: What are we missing? J. Hazard. Mater.. 2022;423:126955
- [CrossRef] [Google Scholar]
- Effects of polystyrene micro/nanoplastics on liver cells based on particle size, surface functionalization, concentration and exposure period. Sci. Total Environ.. 2022;836:155621
- [CrossRef] [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [CrossRef] [Google Scholar]
- The use of human umbilical vein endothelial cells (HUVECs) as an in vitro model to assess the toxicity of nanoparticles to endothelium: a review. J. Appl. Toxicol.. 2017;37:1359-1369.
- [CrossRef] [Google Scholar]
- Cepulis, ˇ, Kazlauskait, M.;, Lazutka, J.;, Bernabò, I., Javed, M.B., Sharifan, H., Bacci, G., Babonaiṫ, M., Cepulis, M., Uraṫ E Kazlauskaiṫ, J.¯, Rimantas Lazutka, J., 2023. Evaluation of In Vitro Genotoxicity of Polystyrene Nanoparticles in Human Peripheral Blood Mononuclear Cells. Toxics 2023, Vol. 11, Page 627 11, 627. https://doi.org/10.3390/TOXICS11070627.
- Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020
- [CrossRef] [Google Scholar]
- Measurement of Mitochondrial Membrane Potential with the Fluorescent Dye Tetramethylrhodamine Methyl Ester (TMRM) Methods Mol. Biol.. 2019;1928:69-76.
- [CrossRef] [Google Scholar]
- Ochratoxin A treated rat derived urinary exosomes enhanced cell growth and extracellular matrix production in normal kidney cells through modulation of TGF-β1/smad2/3 signaling pathway. Life Sci.. 2022;298:120506
- [CrossRef] [Google Scholar]
- Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part. Fibre Toxicol.. 2020;17:1-11.
- [CrossRef] [Google Scholar]
- Gupta, C., Kaushik, S., Himanshu, Jain, S., Dhanwani, I., Mansi, Garg, S., Paul, A., Pant, P., Gupta, N., 2022. Bioaccumulation and toxicity of polystyrene nanoplastics on marine and terrestrial organisms with possible remediation strategies: A review. Environmental Advances 8, 100227. https://doi.org/10.1016/J.ENVADV.2022.100227.
- Cytotoxic effects of polystyrene nanoplastics with different surface functionalization on human HepG2 cells. Sci. Total Environ.. 2020;723:138180
- [CrossRef] [Google Scholar]
- Jeyavani, J., Sibiya, A., Bhavaniramya, S., Mahboob, S., Al-Ghanim, K.A., Nisa, Z. un, Riaz, M.N., Nicoletti, M., Govindarajan, M., Vaseeharan, B., 2022. Toxicity evaluation of polypropylene microplastic on marine microcrustacean Artemia salina: An analysis of implications and vulnerability. Chemosphere 296, 133990. https://doi.org/10.1016/J.CHEMOSPHERE.2022.133990.
- Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed.. 2018;13:8013-8024.
- [CrossRef] [Google Scholar]
- Polystyrene nanoparticles: Sources, occurrence in the environment, distribution in tissues, accumulation and toxicity to various organisms. Environ. Pollut.. 2020;262:114297
- [CrossRef] [Google Scholar]
- Lateef, R., Marhaba, Mandal, P., Ansari, K.M., Javed Akhtar, M., Ahamed, M., 2023. Cytotoxicity and apoptosis induction of copper oxide-reduced graphene oxide nanocomposites in normal rat kidney cells. Journal of King Saud University - Science 35, 102513. https://doi.org/10.1016/J.JKSUS.2022.102513.
- Toxicity of polystyrene nanoplastics to human embryonic kidney cells and human normal liver cells: Effect of particle size and Pb2+ enrichment. Chemosphere. 2023;328:138545
- [CrossRef] [Google Scholar]
- Neuroprotective effects of protocatechuic acid on sodium arsenate induced toxicity in mice: Role of oxidative stress, inflammation, and apoptosis. Chem. Biol. Interact.. 2021;337:109392
- [CrossRef] [Google Scholar]
- Separation and identification of nanoplastics in tap water. Environ. Res.. 2022;204:112134
- [CrossRef] [Google Scholar]
- Polystyrene nanoplastic induces ROS production and affects the MAPK-HIF-1/NFkB-mediated antioxidant system in Daphnia pulex. Aquat. Toxicol.. 2020;220:105420
- [CrossRef] [Google Scholar]
- Polystyrene micro(nano)plastics damage the organelles of RBL-2H3 cells and promote MOAP-1 to induce apoptosis. J. Hazard. Mater.. 2022;438:129550
- [CrossRef] [Google Scholar]
- A Glutathione Activatable Ion Channel Induces Apoptosis in Cancer Cells by Depleting Intracellular Glutathione Levels. Angew. Chem. Int. Ed.. 2020;59:7944-7952.
- [CrossRef] [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55-63.
- [CrossRef] [Google Scholar]
- Oliveri Conti, G., Ferrante, M., Banni, M., Favara, C., Nicolosi, I., Cristaldi, A., Fiore, M., Zuccarello, P., 2020. Micro- and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environmental Research 187, 109677. https://doi.org/10.1016/J.ENVRES.2020.109677.
- Plasticenta: First evidence of microplastics in human placenta. Environ. Int.. 2021;146:106274
- [CrossRef] [Google Scholar]
- Ingestion of micro- and nanoplastics in Daphnia magna – Quantification of body burdens and assessment of feeding rates and reproduction. Environ. Pollut.. 2017;228:398-407.
- [CrossRef] [Google Scholar]
- Selenium: Biochemical role as a component of glatathione peroxidase. Science. 1973;179:588-590.
- [CrossRef] [Google Scholar]
- Understanding and exploiting nanoparticles’ intimacy with the blood vessel and blood. Chem. Soc. Rev.. 2015;44:8174-8199.
- [CrossRef] [Google Scholar]
- Cytotoxicity and Genotoxicity of Polystyrene Micro- and Nanoplastics with Different Size and Surface Modification in A549 Cells. Int. J. Nanomed.. 2022;17:4509-4523.
- [CrossRef] [Google Scholar]
- Micro (nano) plastics in wastewater: A critical review on toxicity risk assessment, behaviour, environmental impact and challenges. Chemosphere. 2022;290:133169
- [CrossRef] [Google Scholar]
- Tomaszewski, K.A., Radomski, M.W., Santos-Martinez, M.J., 2015. Nanodiagnostics, nanopharmacology and nanotoxicology of platelet–vessel wall interactions. https://doi.org/10.2217/nnm.14.232 10, 1451–1475. https://doi.org/10.2217/NNM.14.232.
- In vitro wheat protoplast cytotoxicity of polystyrene nanoplastics. Sci. Total Environ.. 2023;882:163560
- [CrossRef] [Google Scholar]
- Aging processes dramatically alter the protein corona constitution, cellular internalization, and cytotoxicity of polystyrene nanoplastics. Environ. Sci. Technol. Lett.. 2022;9:962-968.
- [CrossRef] [Google Scholar]
- Polystyrene nanoplastics-induced lung apoptosis and ferroptosis via ROS-dependent endoplasmic reticulum stress. Sci. Total Environ.. 2024;912:169260
- [CrossRef] [Google Scholar]
- Autophagic response of intestinal epithelial cells exposed to polystyrene nanoplastics. Environ. Toxicol.. 2023;38:205-215.
- [CrossRef] [Google Scholar]
- Polystyrene nanoplastics promote the apoptosis in Caco-2 cells induced by okadaic acid more than microplastics. Ecotoxicol. Environ. Saf.. 2023;249:114375
- [CrossRef] [Google Scholar]
- The toxicity of metallic nanoparticles on liver: The subcellular damages, mechanisms, and outcomes. Int. J. Nanomed. 2019
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103505.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







