Translate this page into:
Cytotoxic and antiproliferative activities of aqueous extract from aerial parts of Ampelocissus latifolia (Roxb.) Planch. on Dalton’s lymphoma cells
⁎Corresponding author. sray@zoo.buruniv.ac.in (Sanjib Ray)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Ampelocissus latifolia (Roxb.) Planch. is an Indian medicinal native herb. The present study reports the cytotoxic and antiproliferative activities of the aqueous extract from aerial parts of A. latifolia (AEAAL) on Dalton’s lymphoma (DL) cells for the first time. Here, DL cells were cultured in vitro and treated with AEAAL (0.50–6 mg.mL−1) for up to 24 h. In vitro cytotoxicity and antiproliferation were measured by MTT and trypan blue assays, fluorescence microscope, agarose gel electrophoresis, and analysis of cell cycle kinetics by flow cytometer. Results indicated significant and concentration-dependent decreased survivability of AEAAL-treated DL cells, increased percentages of apoptotic cells, DNA laddering, increased sub-G1 cell frequency, and delay in cell cycle kinetics. Here, the reduced cell viability and increased sub-G1 cell populations were significantly correlated with the apoptosis rather than necrosis. Thus, the findings of our study suggested that water-soluble phytochemicals from AEAAL possess potential cytotoxic and antiproliferative activities and can hold a good future prospect as a potential drug candidate against the progression of cancer. However, advanced studies are essential for the isolation and chemical characterization of the bioactive principles and analysis of detailed molecular mechanism for the pharmacological application.
Keywords
Anticancer herbs
Phytochemicals
Flow cytometer
MTT
Trypan blue
Sub-G1
1 Introduction
Cancer holds the third rank for causing death after cardiovascular and infectious diseases (Kelloff, 2000). It is characterized by rapid and uncontrolled proliferation of cells and results due to the alteration in the genetic and epigenetic pathways induced by various factors like microbial infections, hormonal deregulation, exposure to carcinogens, mutagens etc. (Supek and Lehner, 2015). Recent advancement on the cancer treatment is craving for the herbal formulations that might target definite molecular signaling pathways in tumor cells without upsetting the activity and survivability of the normal cells (Suh et al., 2010). Since the prehistorical time, plants act as the chief source of medicines in all the cultures. Various medicinal plants possess antiproliferative, cytotoxic, immune-modulatory, and antioxidant properties leading to anticancer activity (Chaudhuri and Ray, 2014, 2015a, 2020). Amongst all the currently available methods, the in vitro cell-based assays are useful for the rapid determination of the cytotoxic activity of thousands of different compounds. Drug chemo-sensitivity assays using cell-based systems not only form the basis of the development of anti-neoplastic drugs but also are useful for the assessment of drug response in a controlled environmental condition (Nouri and Yazdanparast, 2011). Definite pharmacological properties of the medicinal plants are attributed to the different phytochemicals like steroids, terpenoids, alkaloids, flavonoids, glycosides etc. (Chen et al., 2008a; Suh et al., 2010). Many plant-derived products have also been found to exhibit significant anticancer activity against mammalian cancer cell lines (Chen et al., 2008a,b). The compounds like paclitaxel, etoposide, camptothecin, vinblastine, vincristine, uvaribonin, 22-epicalamistrin, chalcone etc. provide a clue that plant-derived natural products possess significant anticancer activity (Cragg et al., 2009; Pettit et al., 2008). Hence, the discovery and the exploration of the plant-derived natural products with better efficacy against cancer have become the area of interest. Ampelocissus latifolia (Roxb.) Planch. [Family: Vitaceae] is used in the traditional medicinal system. It is a large herbaceous climber, with hollow stem and branches, and a tuberous root. Commonly this plant is known as the wild grape plant. This plant is also known as katti-bel, pani-bel (Hindi); okela, nadena (Marathi); Gowalia-lata (Bengali); kattukodimindri (Tamil) etc. In Indian traditional medicine, the use of plant parts as an aqueous infusion or decoction is a common practice. The aqueous decoction of the tuberous root and aerial parts of this plant is widely used to treat ulcers, dental troubles, dysentery (Patil and Patil, 2012), fractured bone, gout, indigestion, dyspepsia, and tuberculosis (Prusti and Behera, 2007; Swarnkar and Katewa, 2008). It is also applied to wounds, used as an antidote for snake bite, whooping cough, abscess, and for easy labor and delivery of a baby (Patil and Patil, 2005). This plant is rich in various phytochemicals like carbohydrates, phenolics, tannins, terpenoids, saponins, anthraquinones, flavonoids etc. (Chaudhuri and Ray, 2015a). Various chemical constituents like β-sitosterol, γ-sitosterol, hexadecanoic acid, tetracosane, heneicosane, squalene, 2-ethyl-1-hexanol, di-isobutyl phthalate, bis (2-ethylhexyl) phthalate, pterin-6-carboxylic acid, dodecane, 1-iodo-2-methylnonane, nonadecane, 22-epicalmistrin, uvaribonin, chalcone etc. are reported to be present in different organic solvent extracts of tubers of this plant (Mitra et al., 2017; Pettit et al., 2008; Theng and Korpenwar, 2015). Various scientific studies have explored antibacterial (Pednekar and Raman, 2013), anti-inflammatory (Tamilarashi et al., 2000), cytogenotoxic (Chaudhuri and Ray, 2014, 2015a), and allelopathic (Chaudhuri et al., 2015; Chaudhuri and Ray, 2015b, 2016) activities of this plant. Our recent scientific study has also explored the antioxidant activity of aqueous extract of this plant in terms of total phenolics and flavonoids (Chaudhuri and Ray, 2020). However, in the present state of knowledge, the in vitro cytotoxic and antiproliferative activities of this plant has not been well studied.
Based on the ethnopharmacological values and the traditional use of hot aqueous decoction of dried or fresh plant materials of A. latifolia and also the scientific validation of the crude aqueous extract as a good antioxidant agent, the present study finds it interesting to explore the in vitro cytotoxic and antiproliferative activities of the aqueous extract from aerial parts of A. latifolia (AEAAL) on Dalton’s lymphoma cells.
2 Materials and methods
2.1 Chemicals
Roswell Park Memorial Institute-1640 (RPMI-1640) culture medium, antibiotic solution, fetal bovine serum (FBS), trypan blue, and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] reagent were obtained from Himedia, India. Agarose was obtained from Promega Corporation, Madison, USA. Ethidium bromide and quercetin were procured from Sigma, USA and acridine orange was purchased from S.D. Fine-Chem. Ltd., India.
2.2 Collection of plant products and extract preparation
Aerial parts (creeper stem and leaf) of the plant were collected (May 2011) from Golapbag campus, The University of Burdwan, and taxonomically authenticated (Voucher specimen No.BUGBAC012) by Professor Ambarish Mukherjee, Department of Botany of the same university. After washing thoroughly, the plant materials were shade-dried and pulverized. Aqueous extraction was done by keeping 100 g ground plant material in 2 L distilled water at low heat (in a water bath set at 50 °C) for 12 h, with vigorous stirring after every 1 h. Then the extract (AEAAL) was filtered, concentrated and stored at −20 °C for future use.
2.3 Maintenance and culture of Dalton’s lymphoma (DL) cells
Dalton’s lymphoma, lymphoma of T-cell, is widely used to study the antitumor activity of an agent (Chakrabarti et al., 1984). Here, DL cells were obtained from the North Eastern Hill University, Shillong, India and maintained in vivo in Swiss albino mice (aged 2–3 months and weighing 20–25 g) by sequential cell transplantation (2 × 106 cells/mouse). Ascitic fluid was collected from the DL tumor-bearing mouse after 7–8 days of cell transfer. DL cell suspension (nearly 2 × 106 cells) was cultured in RPMI-1640 medium (supplemented with 10% heat-inactivated FBS and penicillin–streptomycin antibiotic solution) and treated with AEAAL (0.50–6 mg.mL−1) for 24 h in a sterile atmosphere maintained at 37 °C. Culture with no extract exposure was used as a negative control. After 24 h, the cells were harvested for the in vitro analysis of cytotoxic and antiproliferative effects using MTT assay, trypan blue assay, fluorescence microscope, agarose gel electrophoresis, and flow cytometer. Rules of the ‘Institutional Animal Care and Use Committee’ were strictly followed during the whole experiment and the mice were used with prior approval from the Dissection Monitoring Committee (DMC) of The University of Burdwan (No: R-S/N-1/646, Dated 30-03-2016; Under Ref. No. BU-DMC/2016/01/05(a-g) Dated 13.07.2016).
2.4 MTT assay
The AEAAL-induced cytotoxicity in DL cells was measured using the MTT assay (Mosmann, 1983) and compared with the quercetin standard. This assay is used to measure the cell viability as well as mitochondrial dehydrogenase activity. MTT reagent (100 µL of 5 mg.mL−1) was added to the cell suspension and kept in an incubator maintained at 37 °C. After 4 h, the culture medium was decanted, the formazan crystals were solubilized in 2 mL DMSO, and the absorbance was recorded at 570 nm.
2.5 Trypan blue assay
Trypan blue assay is a cell viability assay, used to discriminate the live and dead cells by differences in the color pattern (Grankvist et al., 1977). The cell suspension was mixed with 0.40% trypan blue solution (1:1), incubated for 3–5 min in 37 °C incubator, and the cell numbers were counted using a hemocytometer. Cytotoxicity (%) was calculated as:
2.6 Cellular morphology analysis by fluorescence microscopy (FM)
Typical apoptotic morphologies like cell and nuclear shrinkage, apoptotic body formation, chromatin condensation, nuclear fragmentation, membrane blebbing etc. were analyzed under a FM, after staining with acridine orange (AO) and ethidium bromide (EB) (Anter et al., 2011). Acridine orange can enter both the living and dead cells, while ethidium bromide enters only the dead cells. Blue filter excitation causes viable cells to gives green fluorescence, early apoptotic cells having perinuclear chromatin condensation give green to yellow fluorescence, late apoptotic cells having condensed or fragmented chromatin give dark red fluorescence. Necrotic cells stain red and possess a large nucleus with no condensed chromatin. Here, in this study, after 24 h of culture, the treated and untreated cells were washed in PBS (1X) and stained with AO-EB (1:1, v/v). After that, the cells were again washed and re-suspended in PBS (1X) and examined under the microscope. At least 2000 cells were scored for each group.
2.7 Agarose gel electrophoresis DNA fragmentation assay
Genomic DNA was extracted from the treated and untreated DL cells (Hameed et al., 2004) and the DNA degradation pattern was analyzed by agarose gel (1.5%) electrophoresis after staining the gel with ethidium bromide (0.50 mg.mL−1).
2.8 Flow cytometric assay for cell cycle kinetics analysis using fluorescence activated cell sorter (FACS)
Cellular DNA content can be determined after staining with propidium iodide (PI) followed by analysis using flow cytometer (Kang and Alvarado, 2009). Here, the cells (1x106 cells/2 mL) were rinsed with 1 mL of PBS by centrifugation (2000 rpm) for 3 min at 4 °C. The pellet was then suspended in 1X PBS (100 μL) and fixed in 70% alcohol. After that, the cells were again suspended in PBS, reacted with Triton X-100 (1%), RNase A (1 μg.mL−1), and PI (1 mg.mL−1, 20 µL) and kept in dark for 20 min. Then the cells were examined using FACS. 10,000 events were taken into consideration for the data analysis and the results were plotted in a histogram, where the fluorescence intensity (red fluorescence; λem: 585; nm FL-2) was plotted in the X-axis and the cell counts were in the Y-axis.
2.9 Scoring and statistical analysis
Assays were done in 3 sets and the results are depicted as Mean ± SEM (Origin software 6.0). The differences between the control and the treated groups for cellular viability were analyzed using the Student’s t-test (Origin software 6.0) and 2x2 contingency χ2-test and the level of significance was considered at p < 0.05–p < 0.001. The Correlation coefficient (R) and coefficient of determination (R2) between the cytotoxicity and apoptosis as well as necrosis was carried out (Microsoft Office Excel 2007) followed by regression analysis (pα = 0.05). IC50 values were scored by probit analysis.
3 Results
3.1 MTT assay
Cytotoxic potential of AEAAL and quercetin was analyzed by MTT assay. In this study, the treatment with AEAAL (0.50–6 mg.mL−1) and quercetin significantly (p < 0.05–p < 0.001) inhibited the DL cell proliferation in a concentration-dependent way (Fig. 1).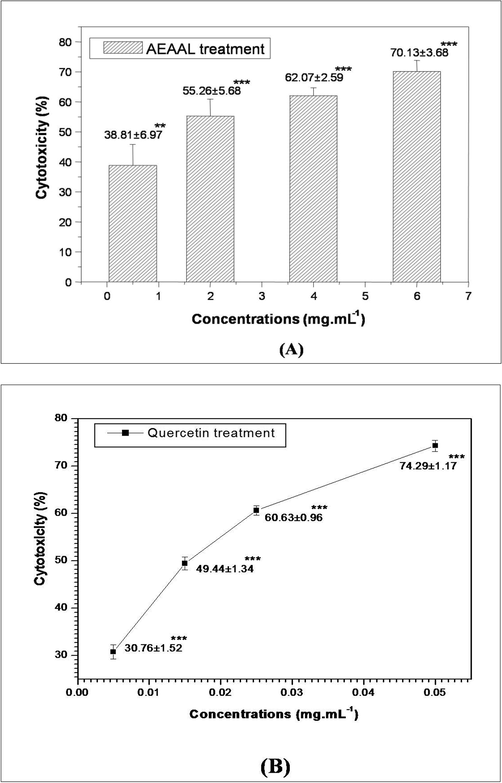
MTT assay depicting the effect of AEAAL (A) and Quercetin (B) on the viability of DL cells. ***Significant at p < 0.001 and **at p < 0.01, compared to the untreated control by Student’s t-test. Data are represented as Mean ± SEM.
IC50 values of AEAAL and quercetin were calculated as 1.63 ± 0.63 and 0.016 ± 0.001 mg.mL−1 respectively.
3.2 Trypan blue assay
Here, the data indicated concentration-dependent increased cytotoxicity towards DL cells after treatment with both AEAAL and quercetin. IC50 values were calculated as 2.32 ± 0.07 and 0.020 ± 0.002 mg.mL−1 for AEAAL and quercetin respectively (Fig. 2).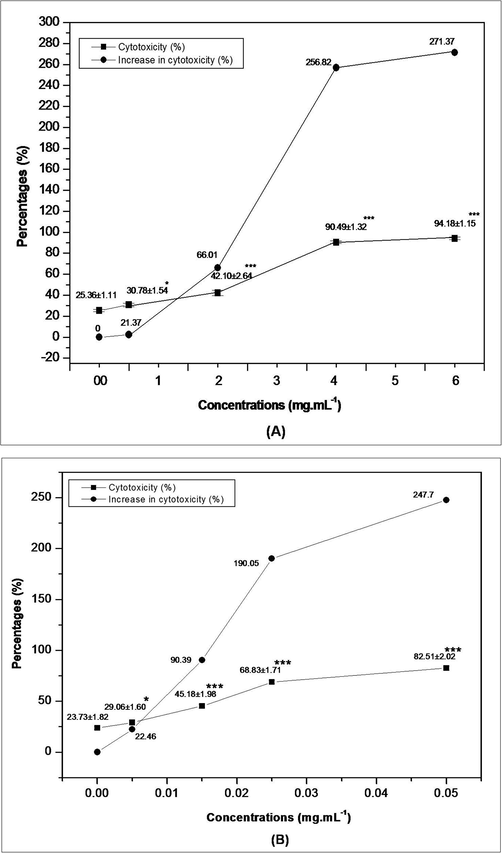
Trypan blue assay depicting the effect of AEAAL (A) and Quercetin (B) on the viability of DL cells. ***Significant at p < 0.001 and *at p < 0.05, compared to the control by 2 × 2 contingency χ2-test (d.f. = 1). Data are represented as Mean ± SEM.
3.3 Cellular morphology analysis by fluorescence microscopy (FM)
Treatment with AEAAL caused typical apoptotic morphologies in DL cells compared to the control, indicating that A. latifolia is an immense resource of natural bioactive substances with apoptotic activity on DL cells (Figs. 3 and 4).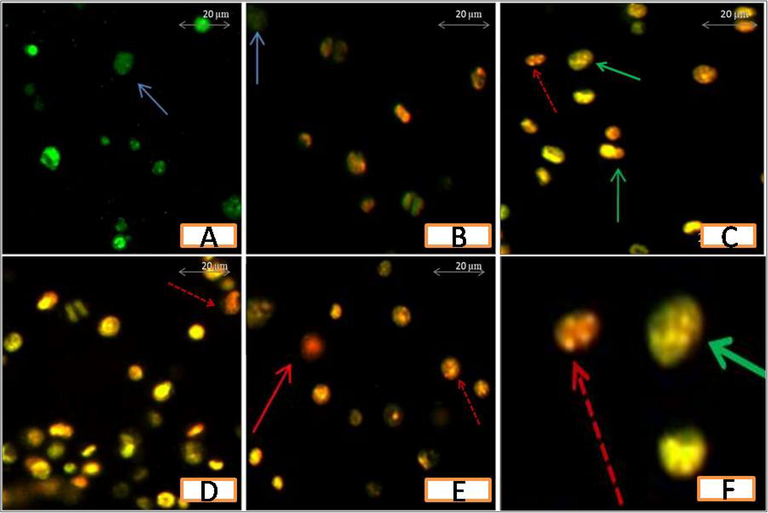
Fluorescence microscopic photomicrographs showing the effect of AEAAL on DL cells. A-E: 00, 0.50, 2, 4, and 6 mg.mL−1 of AEAAL respectively. Photomicrographs (200X) are magnified (8x) using Microsoft PowerPoint. Viable cells (blue arrow), early apoptotic cells (green arrow), late apoptotic cells (red dotted arrow), and necrotic cells (red solid arrow) are indicated. F: Enlarged view (20x) of C.
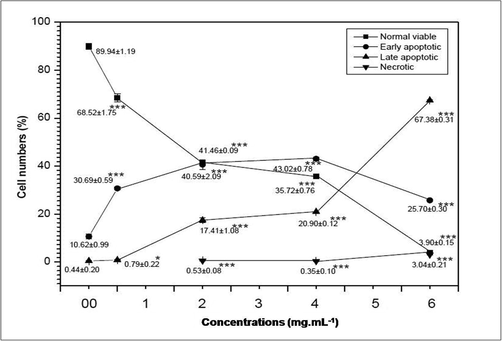
Fluorescence microscopic data showing the influence of AEAAL on the viability of DL cells. ***Significant at p < 0.001, *at p < 0.05, compared to the control by 2x2 contingency χ2-test (d.f. = 1). Data are represented as Mean ± SEM.
Results also indicated that, in comparison to the spontaneous cell death occurring in the control group, the percentages of apoptotic cells increased significantly along with a concentration-dependent gradual decrease in the percentages of the live cells in AEAAL-treated cells (Fig. 4). No necrotic cells were found after exposure to the lower concentration (0.50 mg.mL−1) of AEAAL, while treatment with higher concentrations (2–6 mg.mL−1) resulted in cellular necrosis but unlike late apoptotic cells, the percentages of necrotic cells did not follow a concentration-dependent rise.
3.4 Agarose gel electrophoresis DNA fragmentation assay
DNA degradation was analyzed after treatment with AEAAL for 24 h, where we found a ladder-like pattern with increasing concentrations of AEAAL (Fig. 5).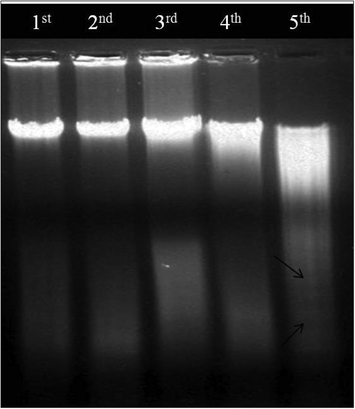
DNA fragmentation patterns of DL cells. Lane 1st–5th: 00, 0.50, 2, 4, and 6 mg.mL−1 of AEAAL respectively. Arrow point (black) indicates ladder pattern.
3.5 Flow cytometric assay for cell cycle kinetics analysis using fluorescence activated cell sorter (FACS)
Here, AEAAL-induced cell cycle alteration in DL cells was measured using flow cytometer. In the untreated DL cells, we observed 4.9, 45, 14.60, and 27.80% cells at sub-G1, G0/G1, S, and G2/M phases respectively. A concentration-dependent increased frequency of sub-G1 cells was observed after treatment with 0.50, 2, and 4 mg.mL−1 of AEAAL respectively (Fig. 6A).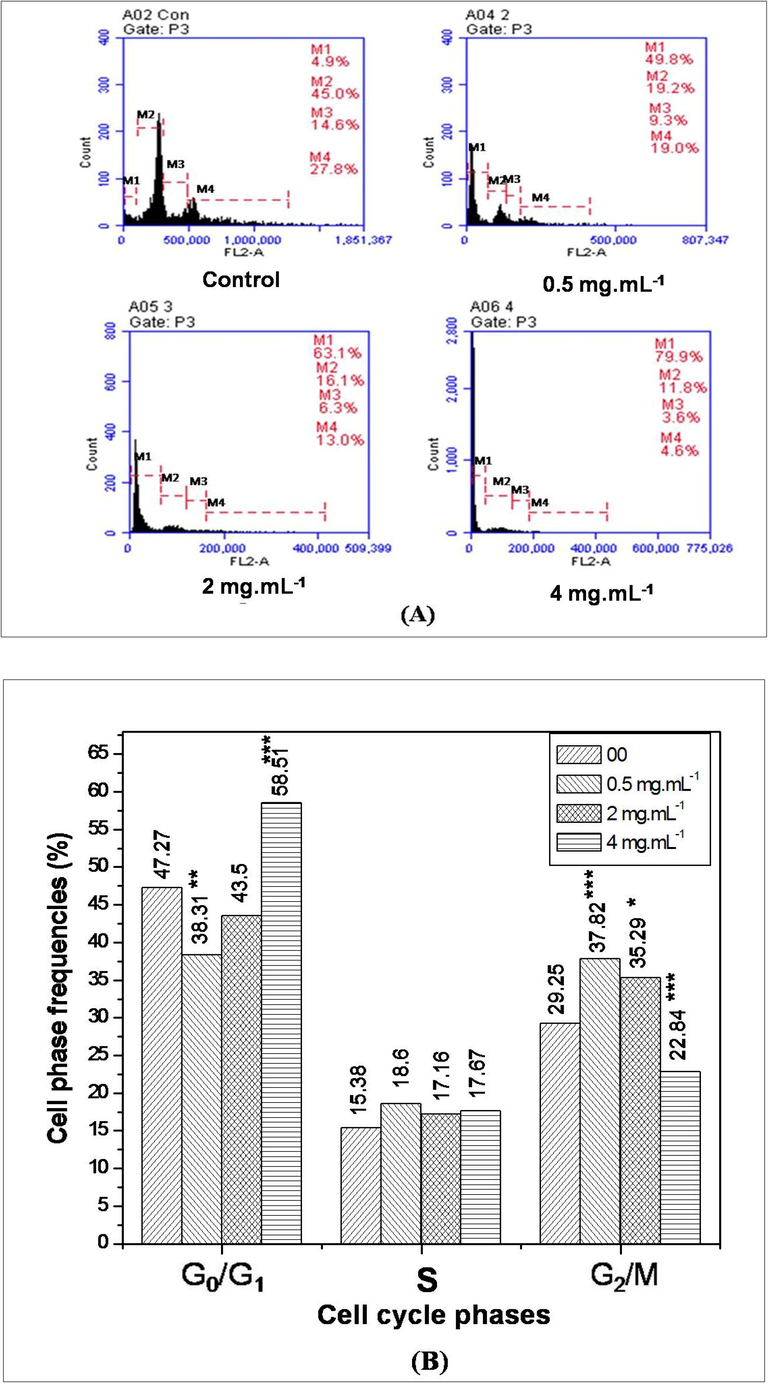
Flow cytometric study of DL cells treated with AEAAL (0–4 mg.mL−1) for 24 h. (A) Frequency distribution of total cells, where M1, M2, M3, M4 represents sub-G1, G0/G1, S, G2/M phase cell populations respectively. (B) Frequency distribution of different cells (G0/G1, S, and G2/M phase) in the cell cycle. ***Significant at p < 0.001 and *at p < 0.05, compared to the respective control by 2x2 contingency χ2-test (d.f. = 1).
The frequency distribution of cells at different phases was scored after eliminating the sub-G1 cell frequencies from the total cell count (Fig. 6B). Here, the data indicated that at 0.50 and 2 mg.mL−1, the percentages of cells in the G2/M phase were 37.82 and 35.29 respectively, significantly higher (p < 0.05–0.001) than the control (29.25%), while at 4 mg.mL−1, the cell frequency at G2/M phase were significantly (p < 0.001) decreased to 22.84%, and the frequency at G0/G1 phase was significantly (p < 0.001) increased to 58.51% in comparison to the control. Here, a concentration-wise varying response was found where, after the treatment with lower concentration (0.50 and 2 mg.mL−1), DL cells had significantly elevated number of cells at G2/M phase but the treatment with higher concentrations (4 mg.mL−1) shifted the cell population into the G0/G1 phase, with lower number of G2/M phase cells.
3.6 Correlation analysis
The results depicted that the survivability of the DL cells exhibited a significant and linear negative correlation with total apoptotic cells (R = 0.967, R2 = 0.936, p < 0.05) but the correlation with the necrotic cells was not significant (R = 0.772, R2 = 0.596, p > 0.05) (Fig. 7). Results indicated that apoptosis and necrosis contributed 93.60% and 59.60% respectively to the survivability decrease in DL cells. Moreover, the increased sub-G1 cell population with the increasing concentrations of AEAAL signifies extract-induced cytotoxicity, where the cytotoxicity was mainly caused by apoptosis rather than necrosis. Here, the data revealed that the increase in sub-G1 cell populations exhibited a significant and linear positive correlation with the apoptotic cells (R = 0.959, R2 = 0.919, p < 0.05) but the correlation with the necrotic cells was not significant (R = 0.725, R2 = 0.525, p > 0.05) (Figs. 3 and 7). Here, apoptosis and necrosis contributed 91.90% and 52.50% to the sub-G1 cell populations in DL cells. Thus the results suggested a significant and linear positive correlation between cytotoxicity and induction of apoptosis, but not to necrosis.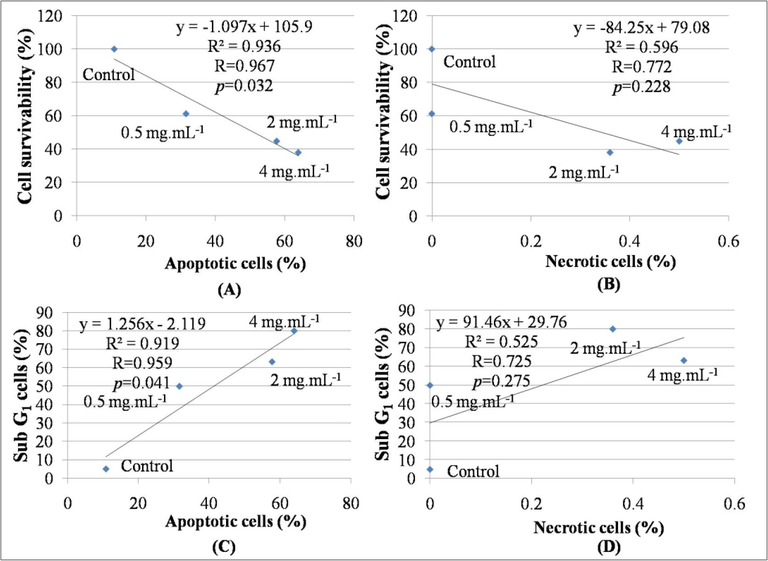
Linear negative correlation between (A): apoptotic cells (%) (X) and cell survivability (%) (Y), (B): necrotic cells (%) (X) and cell survivability (%) (Y). Linear positive correlation between (C): apoptotic cells (%) (X) and sub-G1 cells (%) (Y), (D): necrotic cells (%) (X) and sub-G1 cells (%) (Y).
4 Discussion
Chemotherapeutic drugs are considered to be the most effective modality to treat cancer. The search for plant-derived natural compounds as anticancer agents is of renewed interest (Chen et al., 2008a,b). In the present work, in vitro cytotoxic and antiproliferative activities of aerial parts’ aqueous extract of A. latifolia were determined using DL cells.
In this study, the inhibitory effect of AEAAL on DL cell survivability was detected by means of MTT assay and compared to that of the quercetin-mediated action (Fig. 1). The results indicated that both AEAAL and quercetin inhibited DL cell growth in a concentration-dependent manner, where quercetin was nearly 102 times more effective than AEAAL. There are previous study reports on the antitumor activity of the alkaloid-rich fraction of Solanum pseudocapsicum on DL cells, as shown by the decreased cell survivability in MTT assay (Vijayan et al., 2002). Previously, Chen et al. (2008b) also reported reduced viability of HT-29 and HCT-116 cells after treatment with the extract of Ephemerantha sp. as examined by MTT assay. Here, findings of the MTT assay were further substantiated with cytological validation by trypan blue test. Viable cells having intact cell membranes can exclude this dye and its uptake indicates irreversible membrane damage, leading to cell death (Grankvist et al., 1977). The results showed that both AEAAL and quercetin induced cytotoxicity towards DL cells in a concentration-dependent fashion, where quercetin showed nearly 116 times greater cytotoxicity than AEAAL (Fig. 2). Here, we have used the crude aqueous extract of A. latifolia for our analysis. A crude extract is a mixture of different phytochemicals, of which some may have cytotoxic or some have anti-cytotoxic or growth stimulatory activities. Being a mixture of different phytochemicals having different functions, the concentrations of crude extracts required to exert a specific activity is always higher than its purest form (Lin et al., 2014). Scientific exploration of the crude extract may provide a key role towards the development of therapeutically active biological agents as exemplified in the case of Camptotheca acuminate and Catharanthus roseus. The fruit aqueous extract of C. acuminate from where camptothecin, irinotecan, and topotecan have been isolated, has shown antitumor activity in human endometrial carcinoma cells (Lin et al., 2014). The efficient anticancer drugs like vinblastine and vincristine were isolated from Catharanthus roseus. The ethanolic crude extract of the underground part of this plant was reported to possess both in vivo and in vitro antitumor activities in tumor-bearing mice and Ehrlich ascites carcinoma (EAC) cells (Ruskin and Aruna, 2014). Following the staining of DL cells with AO/EB, cellular apoptosis was analyzed using FM (Anter et al., 2011). Apoptosis is an important and highly desirable aspect of anticancer drugs since by this process malignant cells are selectively removed (Sun et al., 2011). Here, AEAAL treatment caused the typical apoptotic morphologies in DL cells and the rate of apoptosis increased in a concentration-dependent manner, indicating that A. latifolia is an immense resource of natural bioactive substances with apoptotic activity on DL cells (Figs. 3 and 4). Our results are in accordance with the previous study reports showing typical apoptotic morphologies in HL-60 and Ehrlich carcinoma cells after the treatment with erianin, isolated from Dendrobium chrysotoxum (Li et al., 2001) and Eucalyptus camaldulensis (Islam et al., 2014) respectively. Thus the findings of our study suggest that AEAAL can hold significant responsibility in chemotherapeutics by causing apoptosis induction. Previously, 22-epicalamistrin, uvaribonin, and chalcone isolated from the root ethanolic extract of Phillippine Ampelocissus sp. were shown to exhibit significant growth inhibition of murine lymphocytic leukemia, breast adenocarcinoma, lung large cell, colon adenocarcinoma, and also some human cancer cell lines (Pettit et al., 2008).
The DNA degradation assay revealed a ladder-like pattern with increasing concentrations of AEAAL (Fig. 5) that further confirms the distinctive nucleosomal fragmentation, characteristics of the late apoptosis. Similar sorts of DNA laddering was found in the HL-60 cells in a concentration-reliant manner after the exposure of seed aqueous-ethanolic extract of Ziziphus auritiana (Mishra et al., 2011) and erianin (Li et al., 2001). Yang et al. (2005) also reported the occurrence of DNA ladder in COLO-205 cells at the higher concentration of denbinobin but the lower concentrations failed to induce DNA ladder formation. Our results suggest that AEAAL can not only inhibit DL cell proliferation but also induce apoptosis in DL cells.
In this study, the AEAAL-mediated inhibition of DL cell proliferation was also analyzed through flow cytometry. During the cell cycle, DNA is precisely replicated and uniformly distributed to the resulting daughter cells and it allows perfect transmission of genetic material from generation to generation (Kang and Alvarado, 2009). Arrest at the different cell cycle checkpoints and disturbance of the cell cycle events are the key mechanism of actions of the most anticancer drugs (Carnero, 2002). Cells exposed to the cytotoxic agents have perturbed cell cycle events and these alterations are measured through a flow cytometer after staining with propidium iodide (PI) and comparing with the untreated controls. Here, the results indicated that following treatment with AEAAL, the number of cells at sub G1 phase increased significantly in a concentration-dependent manner (Fig. 6). Furthermore, the number of cells at G0/G1 phase was significantly increased (p < 0.001) after treatment with AEAAL (4 mg.mL−1), while the number of cells at G2/M phase was decreased significantly (p < 0.001) compared with the control group, indicating that AEAAL can cause cell cycle arrest at the G1 phase for DL cells, prevent cell cycle progression from G1 to S phase, and induce cell apoptosis. Moreover, as compared to the control, the significantly higher G2/M frequency (p < 0.05 and 0.001) was found after treatment with the AEAAL (0.50 and 2 mg.mL−1), indicating cell cycle arrest at the G2/M phase (Ranganathan et al., 2015). Our results are in accordance with the earlier study reports by Nouri and Yazdanparast (2011), which also revealed that exposure of the drugs for a long time led to the arrest at the sub-G1 phase of the cell cycle. Quercetin was reported to possess a growth-inhibitory effect on gastric and colon carcinoma by causing G1/S arrest (Suh et al., 2010). β-sitosterol and stigmasterol were also shown to block the progression of A549 cells by G2/M arrest (Lai et al., 2010). An anticancer agent moscatilin from Dendrobium sp. was shown to arrest the HCT-116 cells at G2/M phase resulting in a reduced number of cells at G0/G1 phase, followed by an arrest at the sub-G1 phase of the cell cycle (Chen et al., 2008a). Previously, the aqueous extract of A. latifolia was shown to exert mito-depressing activity on one hand and metaphase arresting activity on the other hand in Allium cepa root apical meristem cells (Chaudhuri and Ray, 2014, 2015a). The outcomes of the flow cytometric analysis are also in agreement with the previous study reports showing AEAAL-induced antiproliferation and cytotoxicity in terms of cell cycle delay and metaphase arrest. Moreover, our data (Fig. 7) also indicate a positive linear correlation between cytotoxicity and induction of apoptosis, along with the reduced survivability and cell cycle delay. Therefore, the present study is in accordance with the previous findings of the different authors’ scientific exploration of the crude extract towards the development of newer anticancer drugs.
5 Conclusion
The valuable plant-derived drugs have been discovered as a result of scientific follow-up of the different traditionally used medicinal plants. The present study clearly demonstrated the cytotoxic and antiproliferative activities of the aqueous extract of A. latifolia and also explored the scientific basis of its ethno-pharmacological uses. Thus this study creates an avenue for further investigations on this plant for isolating the active principle(s) and to determine the pharmacological actions.
Acknowledgements
Authors acknowledge the financial support of UGC MRP [F. No. 42-563/2013 (SR) dt. 22.03.2013] and State Funded Fellowship [FC(Sc.)/RS/SF/ZOO./2011-2012/96(3) DATED 30.01.2012], infrastructural support of the Department of Zoology, The University of Burdwan, and Prof. A. Bhattacharyya of Calcutta University for extending the flow cytometric facility.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A pilot study on the DNA-protective, cytotoxic, and apoptosis-inducing properties of olive-leaf extracts. Mutat. Res.. 2011;723(2):165-170.
- [Google Scholar]
- Chromosome analysis of Dalton’s lymphoma adapted to the Swiss mouse: clonal evaluation and C-heterochromatin distribution. Cancer Genet. Cytogenet.. 1984;11(4):417-423.
- [Google Scholar]
- Allelopathic effects of aerial parts’ aqueous extract of Ampelocissus latifolia (Roxb.) Planch. in apical meristem cells. Asian J. Plant Sci. Res.. 2015;5(3):11-16.
- [Google Scholar]
- Evaluation of phytotoxic and cytogenotoxic potentials of leaf aqueous extract of Ampelocissus latifolia (Roxb.) Planch. in relation to its total polyphenol content. Int. J. Pharm. Bio. Sci.. 2014;5(4):225-235.
- [Google Scholar]
- Antiproliferative activity of phytochemicals present in aerial parts’ aqueous extract of Ampelocissus latifolia (Roxb.) Planch. on apical meristem cells. Int. J. Pharm. Bio. Sci.. 2015;6(2):99-108.
- [Google Scholar]
- Determination of effective allelopathic (inhibitory) extract fractions of Ampelocissus latifolia (Roxb.) Planch. leaf. Euro. J. Exp. Bio.. 2015;5(8):1-7.
- [Google Scholar]
- Allelopathic potential of tannic acid and its equivalent phenolics extracted from aerial parts of Ampelocissus latifolia (Roxb.) Planch. IOSR J. Agric. Vet. Sci.. 2016;9(7):90-100.
- [Google Scholar]
- In vitro free radical scavenging activities of aerial parts’ aqueous extract and extract fractions of Ampelocissus latifolia (Roxb.) Planch. in relation to total phenolics and flavonoid contents. J. King Saud Univ. Sci.. 2020;32(1):732-739.
- [Google Scholar]
- Moscatilin induces apoptosis in human colorectal cancer cells: a crucial role of c-Jun NH2-terminal protein kinase activation caused by tubulin depolymerization and DNA damage. Clin. Cancer Res.. 2008;14(13):4250-4258.
- [Google Scholar]
- Denbinobin induces apoptosis by apoptosis-inducing factor releasing and DNA damage in human colorectal cancer HCT-116 cells. Naunyn-Schmiedeberg’s Arch. Pharmacol.. 2008;378(5):447-457.
- [Google Scholar]
- Impact of natural products on developing new anticancer agents. Chem. Rev.. 2009;109(7):3012-3043.
- [Google Scholar]
- Alloxan cytotoxicity in vitro, microscope photometric analyses of trypan blue uptake by pancreatic islet cells in suspension. Biochem. J.. 1977;162(1):19-24.
- [Google Scholar]
- Rapid (100 min) method for isolating high yield and quality DNA from leaves, roots and coleoptile of wheat (Triticum aestivum L.) suitable for apoptotic and other molecular studies. Int. J. Agri. Biol.. 2004;6(2):383-387.
- [Google Scholar]
- Growth inhibition and apoptosis of Ehrlich ascites carcinoma cells by the methanol extract of Eucalyptus camaldulensis. Pharm. Biol.. 2014;52(3):281-290.
- [Google Scholar]
- Flow cytometry methods for the study of cell cycle parameters of planarian stem cells. Dev. Dyn.. 2009;238(5):1111-1117.
- [Google Scholar]
- Perspectives on cancer chemoprevention research and drug development. Adv. Cancer Res.. 2000;78:199-334.
- [Google Scholar]
- Chemical constituents and in vitro anticancer activity of Typhoniumflagelliforme (Araceae) J. Ethnopharmacol.. 2010;127(2):486-494.
- [Google Scholar]
- Erianin induces apoptosis in human leukemia HL-60 cells. Acta Pharmacol. Sin.. 2001;22(11):1018-1022.
- [Google Scholar]
- Lin, C.H., Chen, P.C., Wang, C.K., Wang, C.W., Chang, Y.J., Tai, C.J., Tai, C.J., 2014. Antitumor effects and biological mechanism of action of the aqueous extract of the Camptotheca acuminata fruit in human endometrial carcinoma cells. Evid.-Based Complement. Alternat. Med. 2014 (Article ID: 564810), 1–10.
- Mishra, T., Khullar, M., Bhatia, A., 2011. Anticancer potential of aqueous ethanol seed extract of Ziziphus mauritiana against cancer cell lines and Ehrlich ascites carcinoma. Evid.-Based Complement. Alternat. Med. 2011 (Article ID 765029), 1–11.
- Traditional uses and phytochemistry of Ampelocissus latifolia (Roxb.) Planch tuberous root through GC–MS technique. J. Economy Environ. Soc.. 2017;1(2):49-53.
- [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1–2):55-63.
- [Google Scholar]
- Proliferation inhibition, cell cycle arrest and apoptosis induced in HL-60 cells by a natural diterpene ester from Daphne mucronata. Daru J. Pharmaceut. Sci.. 2011;19(2):145-153.
- [Google Scholar]
- Ethnomedicinal practices of Nasik district, Maharashtra. Indian J. Tradit. Knowl.. 2005;4(3):287-290.
- [Google Scholar]
- Biodiversity of vulnerable and endangered plants from Jalgaon district of North Maharashtra. Asian J. Pharm. Life Sci.. 2012;2(2):144-150.
- [Google Scholar]
- Antimicrobial and antioxidant potential with FTIR analysis of Ampelocissus latifolia (Roxb.) Planch. leaves. Asian J. Pharm. Clin. Res.. 2013;6(1):157-162.
- [Google Scholar]
- Antineoplastic agents. 558. Ampelocissus sp. cancer cell growth inhibitory constituents. J. Nat. Prod.. 2008;71(1):130-133.
- [Google Scholar]
- Ethnobotanical exploration of Malkangiri district of Orissa, India. Ethnobot. Leaflets. 2007;11(1):122-140.
- [Google Scholar]
- Quercetin suppresses Twist to induce apoptosis in MCF-7 breast cancer cells. PLoS One. 2015;10(10):e0141370
- [CrossRef] [Google Scholar]
- In-vitro and in-vivo antitumor activity of Catharanthus roseus. Int. Res. J. Pharm. App. Sci.. 2014;4(6):1-4.
- [Google Scholar]
- Induction of G1/S phase arrest and apoptosis by quercetin in human osteosarcoma cells. Arch. Pharm. Res.. 2010;33(5):781-785.
- [Google Scholar]
- Coleusin factor exerts cytotoxic activity by inducing G0/G1 cell cycle arrest and apoptosis in human gastric cancer BGC-823 cells. Cancer Lett.. 2011;301(1):95-105.
- [Google Scholar]
- Differential DNA mismatch repair underlies mutation rate variation across the human genome. Nature. 2015;521(7550):81-84.
- [Google Scholar]
- Ethnobotanical observation on tuberous plants from tribal area of Rajasthan (India) Ethnobot. Leaflets. 2008;12(1):647-666.
- [Google Scholar]
- Phytochemical and pharmacological evaluation of Ampelocissus latifolia. Anc. Sci. Life. 2000;20(1–2):14-18.
- [Google Scholar]
- Phytochemical analysis of ethanol extract of Ampelocissus latifolia (Roxb.) Planch. tuberous root using UV-VIS, FTIR and GC-MS. Int. J. Pharm. Sci. Res.. 2015;6(9):3936-3942.
- [Google Scholar]
- In vitro cytotoxicity and anti-tumor properties of the total alkaloid fraction of unripe fruits of Solanum pseudocapsicum. Pharm. Biol.. 2002;40(6):456-460.
- [Google Scholar]
- Molecular mechanisms of enbinobin-induced anti-tumorigenesis effect in colon cancer cells. World J. Gastroenterol.. 2005;11(20):3040-3045.
- [Google Scholar]







