Translate this page into:
Comprehensive environmental assessment of heavy metal contamination of surface water, sediments and Nile Tilapia in Lake Nasser, Egypt
⁎Corresponding author. fehmi.boufahja@fsb.u-carthage.tn (Fehmi Boufahja)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The water of the Lake Nasser is safe for use in terms of human consumption, agricultural utilization and public sanitation. The concentration of heavy metals in fish muscles and livers meet the specifications stipulated by international limits.
Abstract
Heavy metals cause deleterious effects on human health and drastically alter the biogeochemical cycles within freshwater habitats. The main human activities leading to heavy metal contamination of various aquatic ecosystems comprise the industry, agriculture, urbanization, transport and mining. Lake Nasser is the main freshwater source in Egypt, usually polluted from upstream human activities from the hydrographic basin of the River Nile. The current study surveyed the impact of heavy metals contamination (i.e., Cd, Zn, Pb and Cu) in water, sediment and two Nile Tilapia (Oreochromis niloticus) fish organs (liver and muscles) at six sampling sites along Lake Nasser. Additionally, the effects of heavy metals bioaccumulation in the aquatic ecosystem, via water, sediments and fish organs were investigated. The conclusion is that the water of Lake Nasser is safe for use in terms of human consumption, agricultural utilization and public sanitation.
Keywords
Heavy metal contamination
Water quality
Sediment
Fish consumption
Lake Nasser
1 Introduction
Lake ecosystems comprise inland water bodies that lack any direct exchange with oceans and share many ecological and biogeochemical processes that are commonly within the classic framework definition of limnology (Bhateria and Jain, 2016).
Any alteration in lakes’ environmental status or water quality will inadvertently impact not only the water body per se, but equally the human population from adjacent areas (Hedfi et al., 2013). The human usage of lake waters, such as for drinking water supply, irrigation, fishing and clean-energy generation impact the worldwide economics, including the one of Egypt (Duan and Bastiaanssen, 2015).
Lake Nasser was formed in 1964 following the construction of the Aswan High Dam in Upper Egypt. Aswan High Dam was constructed for several purposes, such as for water storage given adequate water input along the year, to overcome floods and for clean energy (i.e. hydropower) generation. Lake Nasser is the largest source of freshwater in Egypt accounting for about 95% of the total Egyptian freshwater budget (Rizk et al., 2020). The lake is about 300 km long and represents the world's largest embankment dam (Abd Ellah, 2020). This lake is very important for the economy of Egypt, given the absence of the appreciable rainfall regime and the agriculture dependence entirely on irrigation (Rizk et al., 2020). Hence, the need for appropriate monitoring techniques to be implemented in order to check the water quality on a regular basis is of primary importance.
Among various types of pollutants, heavy metals are of major concern, given not only the wide array of compounds with multifaceted effects on lake ecosystems, but equally their various input pathways, such as the bedrock weathering, hydrodynamic processes and atmospheric deposition. The government administration of Aswan region restricted severely the use of agricultural fertilizers, discharge of household and industrial wastewater (Ideriah et al., 2012). Therefore, the main source of heavy metals in Lake Nasser comprises mainly the suspended organic matter boosted by floodwaters and those generated by improper land use along River Nile hydrographic basin, particularly in Ethiopia (Farhat and Aly, 2018). The sedimentation of suspended organic matter, to which heavy metals are intimately bound, is a complicated process, usually increasing significantly in reservoirs. The high sedimentation rates in this lake represent a very serious environmental and economic issue that must be sorted out in a sustainable manner. The main source of sediments comes from the highlands, where the erosion processes carry them downstream via the river straight into the lake. Most of the deposited sediment on the bottom of the lake comprises silt, with some traces of sands (ElKobtan et al., 2016). The heavy metals in aqueous solution do not always exhibit direct toxic effects on the environment; instead, they tend to (bio) accumulate and to persist in the environment. Moreover, these compounds are not biodegradable and cause detrimental effects on the environment and human health (Ntakirutimana et al., 2013). Several techniques were developed in time to assess the heavy metal effects bound to sediments, such as the geological accumulation index (Imam et al., 2020), sediment enrichment agent, contamination factor and degree of contamination.
Historically, the worldwide fish consumption increased directly with the interest in its nutritional value, mainly as a source of valuable proteins. Dietary guidelines in the United Kingdom recommend the consumption of fish and fish-based products at least twice a week to meet the daily requirements of polyunsaturated fatty acids (Clonan et al., 2012). Given that fish fill upper positions within aquatic food webs, the heavy metals can bioaccumulate in their biomass via food, water and sediments in significant amounts (Zhao et al., 2012), with direct toxic effects to humans (Castro-González and Méndez-Armenta, 2008). The human contamination with heavy metals via fish consumption leads sometimes to liver and kidney failures and cardiovascular diseases, to name just a few of the induced detrimental health effects.
This led to the implementation of numerous international screening approaches with the aim of estimating the quality of fish meat, as well as to survey the health of aquatic ecosystems (Meche et al., 2010). Essential metals, such as zinc and copper fulfill important physiologic roles; however, their bioaccumulation beyond certain thresholds becomes highly toxic for humans. Non-essential metals, such as lead, cadmium and mercury are nonetheless extremely dangerous for both humans and environment (Bawuro et al., 2018). These non-essential metals bioaccumulate in any given organism, leading to tissue damages, followed by a wide series of disorders and significant toxic effects along food webs. Heavy metals are non-biodegradable and once released in aquatic habitats, they are absorbed into sediment or accumulate in biota. Usually, the fish are more prone to accumulate significant amounts of heavy metals from water and food in various tissues (Weber et al., 2013). Moreover, they absorb significant concentrations of heavy metals even when their amount in aqueous form is below the detection limit of routine chemical analyses (Imam et al., 2020). Therefore, various fish organs represent important targets for properly assessing the health of the aquatic environment from this perspective. The accumulation rate of heavy metals such as lead, zinc, copper and cadmium in several tissues of the iconic fish Nile Tilapia (Oreochromis niloticus) from Lake Nasser was previously investigated (Rashed, 2001). The main findings of these studies were that these heavy metals are cycled within the lake ecosystem along with the energy and nutrients flows through food webs. However, most of these studies rather focused on one compound at a time, therefore precluding their synergic interactions within both the lake ecosystem and within fish tissues, despite a general acknowledgment of this information gap. Therefore, in the current study, we tried to fulfill this knowledge gap through a holistic investigation of the distribution of these heavy metals in water, sediment, and fish in Lake Nasser, as well as through assessing their accumulation rates in various fish organs. To understand the ecological risk related to heavy metal contamination, we calculated the metal pollution index (MPI). The MPI provides comprehensive information about the metal toxicity in a particular sample and offers an understanding of the quality of the aquatic environment. The current study comprises a continuation of previous ecotoxicological investigations carried in Lake Nasser (Rizk et al., 2020).
2 Materials and methods
2.1 Study area
Six sites were carefully selected within the main channel of Lake Nasser for water sampling, fish catching and sediment collection, starting from site Abu-Simbel (1) in the south, followed by Armina (2), Tushka (3), Korosko (4), Kalabsha (5) and High Dam site (6) situated in the northern part of the lake (Fig. 1).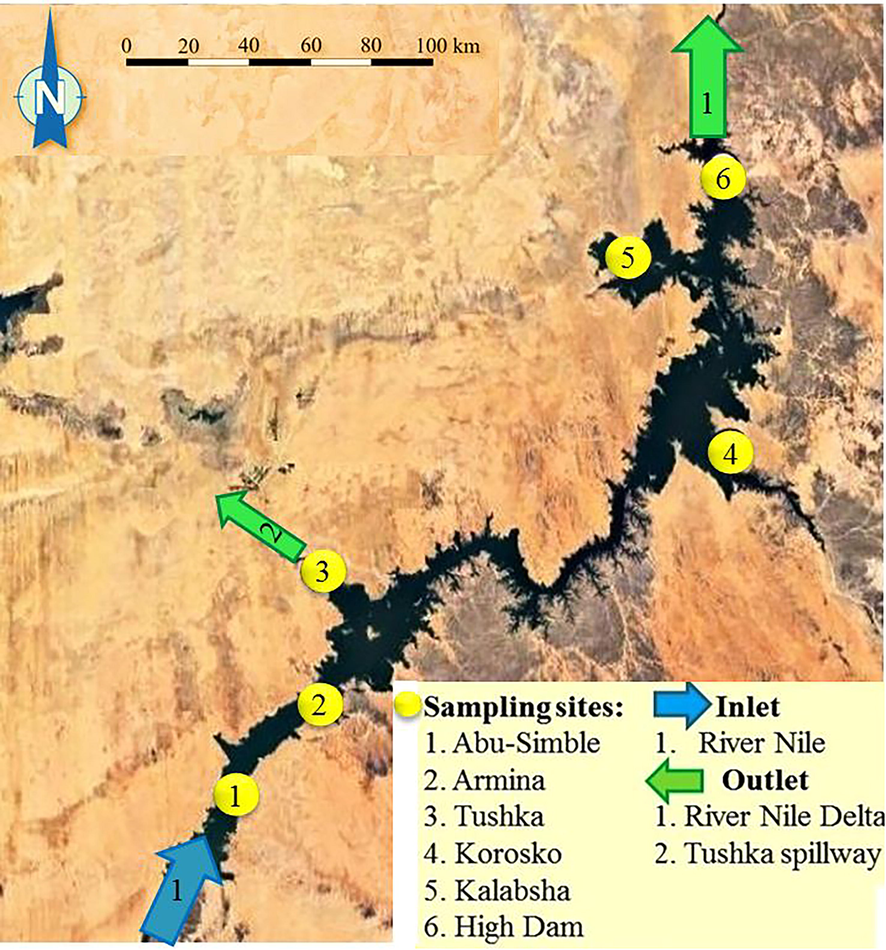
Sampling sites and main water supplies/inlets and discharge/outlet points of Nasser Lake.
2.2 Sampling procedures
2.2.1 Water samples
Water samples were collected in polyethylene bottles (2 L) according to Cornell et al. (2016). The samples were acidified with nitric acid to prevent organic matter alteration by bacterial activity and transferred in an ice-box to the laboratory of Botany Department, Faculty of Science of Port Said University.
2.2.2 Sediment samples
Three replicates of sediments were collected with the aid of an electronic vibrational core sampler tube (vibracorers), which enables sampling efficiently to a depth of eight meters (USGS, 2005). Following the packing of sediment samples in polythene bags and chilled in an ice box, they were transported to the laboratory for further analysis. The samples were dried overnight at 105 °C to constant weight for heavy metal analysis.
2.2.3 Fish samples
Nile Tilapia (Oreochromis niloticus) is a native fish species to Egypt and one of the most common fish used in the Egyptian cuisine and in fisheries in Aswan governorate. A total of 30 individuals were collected by local fisherman in March 2019.
2.3 Sample preparation
2.3.1 Water samples
The concentration of heavy metals in water samples was measured according to APHA (2012) standards, with the aid of an Atomic Absorption Spectrophotometer (AAS).
2.3.2 Sediment samples
The digestion of sediment was performed based on USEPA (2010) standards. Fifteen mL of concentrated HNO3, HF and HClO4 were mixed into a Teflon beaker with 0.5 g of sediment. The samples were sealed afterwards with a lid and kept for 3 h at room temperature, then evaporated until they turned into a liquid solution. Following that, 5 ml of HClO4 was added and vaporized to drought. Concentrated HCl (10 ml) was added to samples and placed back on a hot plate until the solution became clear. Then, it was filtered and supplemented with deionized water up to 100 ml in a volumetric flask.
2.3.3 Fish samples
Various fish organs were extracted through dissection. Muscle and liver organs were removed and their preparation for the measurement of heavy metals was carried according to Hlavay (1996). Therefore Cd, Cu, Pb and Zn contents were measured according to Krishnamurty et al. (1976). First, the samples were dried and then digested on steam bath at approximately 80 °C for 3 h, followed by the addition of 14 ml of concentrated HNO3 and 7 ml of 30 % H2O2, in this order. All samples were diluted to 50 ml with ultra-distilled water and stored in polyethylene containers at room temperature until further measurements.
2.4 Atomic Absorption spectroscopic measurement
Heavy metal concentrations were measured with the aid of an AAS (Model ICE series 3000 AAS) with a GF 5000 graphite furnace in water, sediment and fish samples. In case of fish samples, the precision of the analytical procedure was checked by using standard reference materials (dogfish muscle (DORM 4) and liver (DOLT 5) Canadian Research Council) in five replicates. The precision of analysis was calculated according to Farkas et al. (2007).
2.5 Metal pollution index (MPI)
To assess the metal pollution, the metal pollution index (MPI) was used according to (Abdel-Khalek et al., 2016) using the following equation:
1/nWhere, CM1 is the concentration value of first concerned metal, CM2 is the concentration value of second concerned metal, CM3 is the concentration of third concerned metal and CMn is the concentration of nth metal (mg/kg dry wt) in the tissue sample of a certain samples.
2.6 Model evaluation
In this study, a comprehensive evaluation method was used to evaluate the correlation between the contamination with heavy metals and sample types (i.e., water, sediment, liver and muscles) of investigated items. The main evaluation indexes included the coefficient of determination (R2), and Pearson correlation coefficient (Corr).
3 Results and discussion
Lake Nasser is the largest source of freshwater in Egypt. However, there are no point pollution sources with heavy metals in its hydrographic basin. The lake receives heavy metals via anthropogenic activities, such as fishing activities, sewage pollution, fishing boats and cruise ships, as well as others which are disposed off in wastes directly into the Sudanese main stem of River Nile (Darwish, 2013). The copper, zinc, lead and cadmium were selected in this study according to the recommendations of Egyptian Water Administration. High concentrations of copper and zinc are needed, as opposed to lead and cadmium which require low dosages, to cause severe environmental and human health issues. Humans are exposed to these heavy metals via consumption of water and fish, which bioaccumulate in various tissues, leading to both acute and chronic intoxications.
3.1 Heavy metals concentrations in water
Table 1 presents the mean concentrations of heavy metals dissolved in water in the six sampling sites of Lake Nasser. Which were below the limits imposed by the Egyptian Governmental Decree No. 92/2013 (GD, 2013) and by environmental quality standards (EQS, 2008) (Directive 2008/105/EC) of European Union, as well as by the aquatic life suitability standard of Canada (CCME, 2007).
Heavy metal concentrations (µg.L−1)
Measuring sites in Lake Nasser
limit values, µg/L
1
2
3
4
5
6
Abu-Simble
Armina
Tushka
Korosko
Kalabsha
High Dam
aEG (GD 2013)
bEQS
cCCME, 2007
Cu
0.80
2.68
6.700
2.67
1.98
4.70
10
10
4.00
Zn
7.13
7.56
11.80
7.66
9.00
9.11
10
10
50.00
Cd
0.025
0.025
0. 065
0.03
0.03
0.06
1.0
≤0.08
1.00
Pb
0.01
0.02
0.040
0.03
0.03
0.04
10
1.2
10.00
Sites 3 and 6 exhibited the highest heavy metal concentrations, whereas the site 1 showed the lowest. The measured values of heavy metals from the current study were compared to those from different countries in Table 2. In comparison with the registered heavy metal concentrations in various worldwide lakes, it can be concluded that the heavy metal concentrations in Lake Nasser are in fact significantly lower compared to Kenya (Outa et al., 2020), China (Jiang et al., 2018), USA (An and Kampbell, 2003), Lake Constance (Petri, 2006), Lake Geneva (La CIPEL, 2015) and Lake Balaton (Nguyen et al., 2005).
Lake Location /concentration (µg element.L-1)
Pb
Cd
Zn
Cu
References
Nasser Lake
0.028
0.039
8.70
3.26
current study
Constance Lake (Germany, Switzerland and Austria)
0.050
0.250
1.500
1.100
Petri (2006)
Baikal Lake (Russia)
1.5
7
1–5
1
Shiretorova et al. (2020)
Vemband Lake (India)
3.94
1.63
74.93
7.31
Shylesh Chandran et al. (2019)
Caizi Lake (China)
2.42
0.12
34.33
2.54
Jiang et al. (2018)
LosMolinos Lake (Argentina)
0.07
–
3.75
0.67
Griboff et al. (2018)
Geneva Lake (shared between Switzerland and France)
<0.050
0.005
–
0.770
La CIPEL (2015)
Lake Balaton (Hungary)
0.040
0.002
0.970
0.460
Nguyen et al. (2005)
Lake Texoma, Oklahoma–Texas (USA)
<15
20
59
24
An and Kampbell (2003)
Lake Victoria (Kenya)
1.054
0.070
144
1.568
Outa et al. (2020)
Lake Victoria (Mwanza and Winam Gulfs)
22–823
0–70
18–50
–
Ogoyi et al. (2011)
Tana Lake (Ethiopia)
3–6
30–60
110–160
110–150
Brihanu (2016)
Tanganyika Lake (Tanzania)
7–120
< 10.00
10.00
< 6.00
Chale (2002)
3.2 Assessment of environmental prominence of minerals in sediment stores in comparison to SQGs
Table 3 summarizes the measured values of heavy metals in sediment and compares them to quality guideline values, which comprise the minimum critical concentrations, which is represented by the toxic effect threshold (TET). The concentration of copper varies between 13 and 21.8 µg.g−1; high concentrations were recorded at site 3 (21.8 µg.g−1), followed by the site 6 (21 µg.g−1). However, the lowest copper concentration was recorded at site 1 (13 µg.g−1). The concentrations of zinc were high in all investigated sampling sites. To determine the state of metal pollution, the Sediment Quality Guidelines (SQGs) were applied as reference. SQGs were derived from the sediment quality pollutant concentrations using Chemical and Biological Impact Database (Jones et al., 1997; USEPA, 1999). *SQG: Sediment quality guideline (Jones et al., 1997; USEPA, 1999).
Elements concentrations (mg.kg−1)
Measuring sites/heavy metal concentrations alongside Lake Nasser
*SQG
1
2
3
4
5
6
Abu-Simble
Armina
Tushka
Korosko
Kalabsha
High Dam
aTEC
bPEC
cPEL
dSEL
eTEL
Cu
13.00
15.80
21.80
18.00
18.00
21.00
31.0
149
197
110
86
Zn
19.00
25.00
45.00
30.00
38.00
40.00
121
459
315
820
540
Cd
0.165
0.178
0.35
0.27
0.31
0.356
0.99
4.98
3.53
10.0
3.00
Pb
0.95
2.00
3.00
2.00
2.00
3.00
–
128
91
250
170
MPI
2.49
3.44
5.57
4.16
4.54
5.23
However, the usage of SQGs simultaneously with municipal data requires caution. This is important especially in the risk evaluation of heavy metal loads from sediment samples. The recorded values are well correlated with the water flow along a south to north latitudinal gradient. In conclusion, the sediment from Lake Nasser can be considered safe for agriculture activities as well as for aquatic biota (Imam et al., 2020).
3.3 Heavy metal concentrations in fish organs
Fish length and net body weight were ranged between 21.5 and 32.8 cm and 225.5–500.5 g in weight, respectively (Table S1).
Table 4 shows the concentrations of Cu, Zn, Cd and Pb in fish muscles compared to the standards of heavy metal levels of the European Community (EC, 2005), Food and Agriculture Organization (FAO, 2012), FAO/WHO limits (FAO/WHO, 2011), World Health Organization (WHO, 1989) and Ministry of Agriculture, Fisheries and Food of UK (MAFF, 2000). The copper concentrations varied between 1.2 µg.g−1 at site 1 to 9.17 µg.g−1 at site 3. The zinc concentrations exhibited the highest concentration (15.70 µg.g−1) at site 3 and the lowest (8.5 µg.g−1) at site 1.
Elements Concentration (mg.kg−1)
Measuring sites
Standard levels(mg.kg−1)
1 Abu-Simble
2 Armina
3 Tushka
4 Korosko
5 Kalabsha
6 High Dam
a EC 2005
b FAO 2012
cFAO/WHO, 2011
d WHO 1989
e MAFF 2000
Cu
1.20
2.25
9.17
2.25
5.26
6.18
10
30
30
30
20
Zn
8.50
12.78
15.70
13.20
13.65
14.9
–
30
40
100
50
Cd
0.15
0.15
0.23
0.18
0.20
0.24
0.05
0.05
0.5
1
0.2
Pb
0.10
0.20
0.50
0.20
0.20
0.50
0.20
0.5
0.5
2
2
MPI
0.63
0.96
2.02
1.02
1.30
1.82
The cadmium concentrations recorded the lowest concentrations (0.15 µg.g−1) at sites 1 and 2. In site 3 agriculture activities affect water quality, influencing directly water and sediment dwelling biota.
Table S2 shows the concentrations of cadmium, zinc and lead which are within the certified limits of standard samples. Regarding copper, both standard fish samples (DORM 4 and DOLT 5) were below certified values and within acceptable limits (96.11 % confidence level). Table S3 presents the concentrations of heavy metals in fish liver. The highest heavy metal concentrations (Cu, Zn and Cd) in fish liver were measured at site 6, in the northern part of the lake (28.90, 58.10 and 0.50 µg.g−1, respectively) whereas the lowest values were recorded at site 1, in the southern part of the lake (Cu: 13.80, Zn: 37.50 and Cd: 0.3 µg.g−1 respectively). The lowest lead concentration was recorded at site 2 (0.1 µg.g−1) and the highest at site 3 (2.1 µg.g−1).
3.4 Heavy metal distributions among Lake Nasser
Heavy metals distribution along the lake offers a better understanding of the level of lake contamination (Hasimuna et al., 2021). This way, the contamination with heavy metals and where the highest concentrations are to be expected is much better represented. An overall picture as this may help authorities to draw people's attention to the potential risk of heavy metal contamination (Turan et al., 2020). Such mapping can help with proper implementation of preventive measures. However, it is necessary to emphasize the fact that currently there are no heavy metal contamination sources in Lake Nasser hydrographic basin, providing a solid basis for the future development of agricultural activities in the region and efficient water management in Egypt.
3.4.1 Copper
Copper content in water, sediment and both fish muscle and liver has represented in Fig. 2. It highlights the lowest copper concentrations with blue, which is the best water quality in terms of contamination with this heavy metal, whereas the yellow highlights indicate high concentrations. The decreasing copper concentrations in studied samples are in liver > sediment > muscle > water. Sediment and liver samples exhibited higher concentrations in the lake (13–21.8 and 13.8–28.9 mg.kg−1 respectively), supporting the hypothesis of its bioaccumulation in fish liver and sediments. Some researchers indicated that elevated levels of heavy metals such as Zn and Cu in surface sediments come from mine effluents, vehicular emission and commercial and industrial sources (Decena et al., 2018).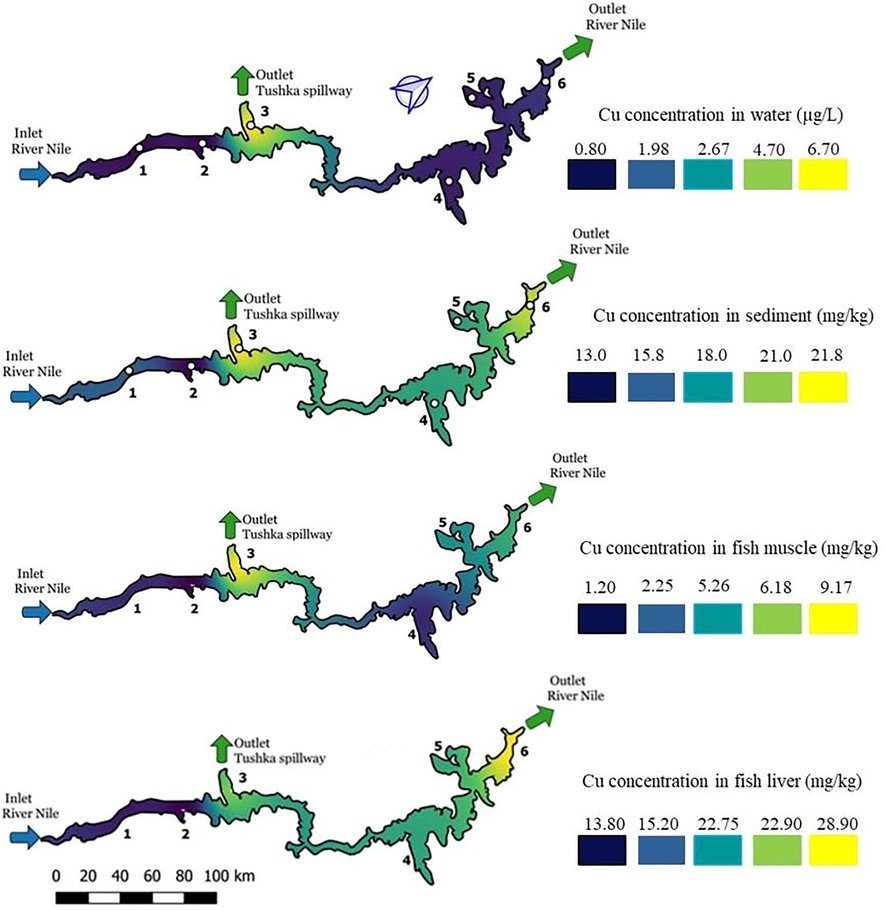
Copper distributions along the lake in water, sediment, fish muscle and fish liver.
3.4.2 Zinc
As shows in Fig. 3, zinc concentrations in water, sediment, fish muscle and fish liver has been illustrated. Blue color highlights the lowest Zn concentration, which indicates the best water quality with respect to this heavy metal, whereas the yellow indicates high concentrations. The sequence of Zn concentrations in the samples were in the order of liver > sediment > muscle > water. The results show that water exhibited the lowest concentration (Table 1), whereas sediment and liver samples exhibited the highest values (19–45 and 37.5–58.10 mg.kg−1 respectively). The results show that zinc concentrations in the edible tissues of fish were within the normal variation according to the Canadian fish standard (51.60 ± 2.80 mg.kg−1) (Table S2).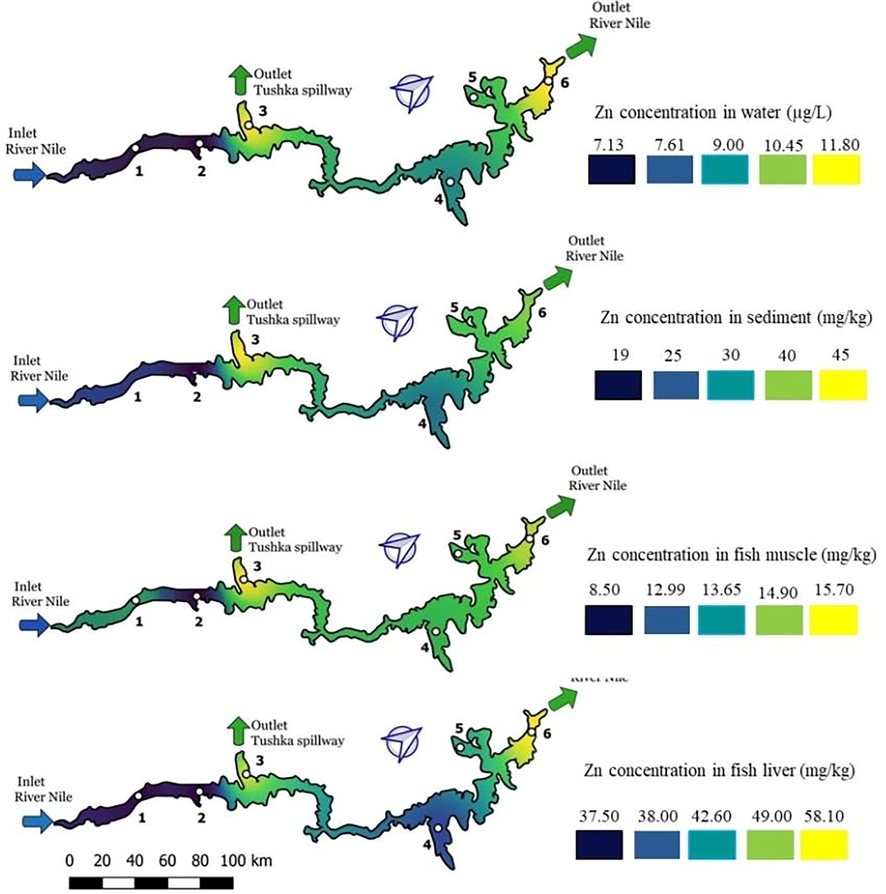
Zinc distribution along the lake in water, sediment, fish muscle and fish liver.
3.4.3 Lead
The accumulation of lead in fish tissues can induce oxidative stress due to production of Reactive Oxygen Species (ROS), which induce potential synaptic damage and several malfunction implications in fish, as neurotoxicity. Moreover, exposure to lead has a negative effect on the immune responses in fish (Lee et al., 2019). Fig. 4 highlights the lead concentrations in water, sediment, fish muscle and fish liver; the blue color refers to the lowest concentrations, indicating the best water quality with respect to this heavy metal, whereas the yellow color indicates high values. The gradient of lead concentrations in samples is in the following order sediment > liver > muscle > water. The results show that water exhibited the lowest concentrations (Table 1), whereas the sediment and liver samples exhibited the higher concentrations in the lake (0.2–3 and 0.9–2.1 mg.kg−1 respectively).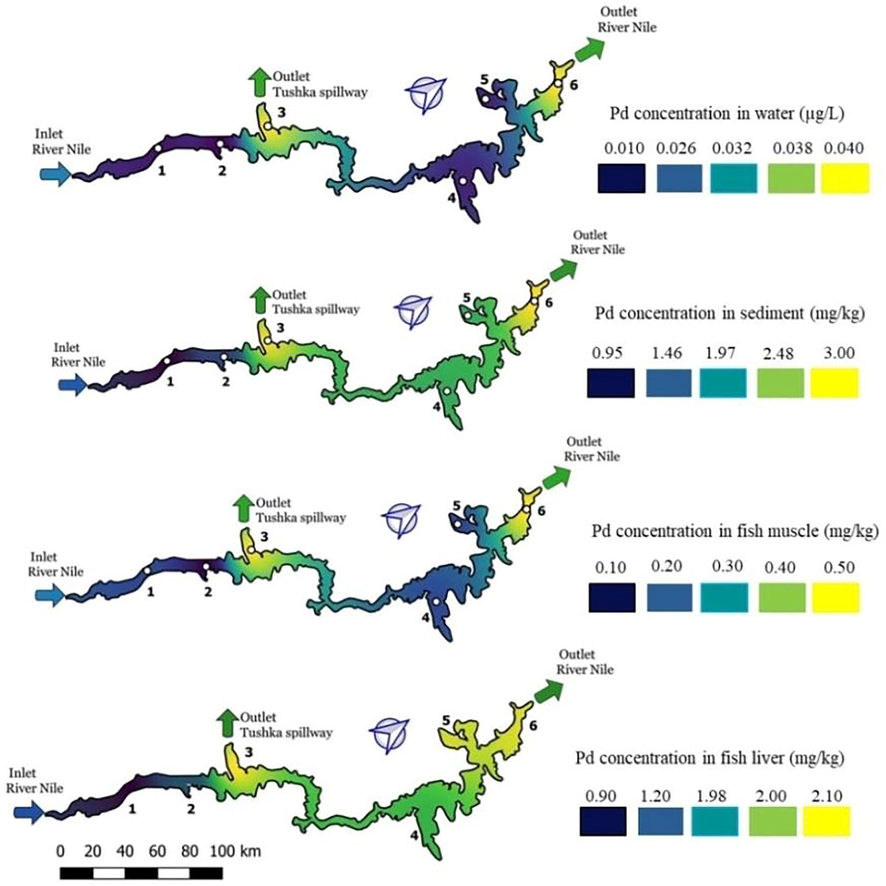
Lead distribution along the lake in water, sediment, fish muscle and fish liver.
3.4.4 Cadmium
Cadmium induces harmful effect on living cells, their membrane deterioration and destroys the DNA structure. Such severe damage result from the displacement of this heavy metals from its original binding sites (Igiri et al., 2018). Fig. 5 shows cadmium concentrations in water, sediment, fish muscle and fish liver. The blue color refers to its lowest concentrations, whereas the yellow indicates the highest values. The gradient of cadmium concentrations in samples is sediment > liver > muscle > water. The results showed that water exhibited the lowest concentrations (Table 1), whereas the sediment and liver samples exhibited the highest concentrations in the lake (0.3–0.5 and 0.18–0.356 mg.kg−1 respectively). Trace concentrations of this metal can negatively affect the metabolism and growth of fish by altering their DNA structure, causing dysfunction, disruption of cell membranes, inhibition of enzyme activities and oxidative phosphorylation. Equally, it induces direct toxic effects in humans, through food intake. Cadmium bioaccumulation was reported to induce nephrotoxicity and cause a specific renal failure as well as other associated health issues (Ismail and Rizk, 2016).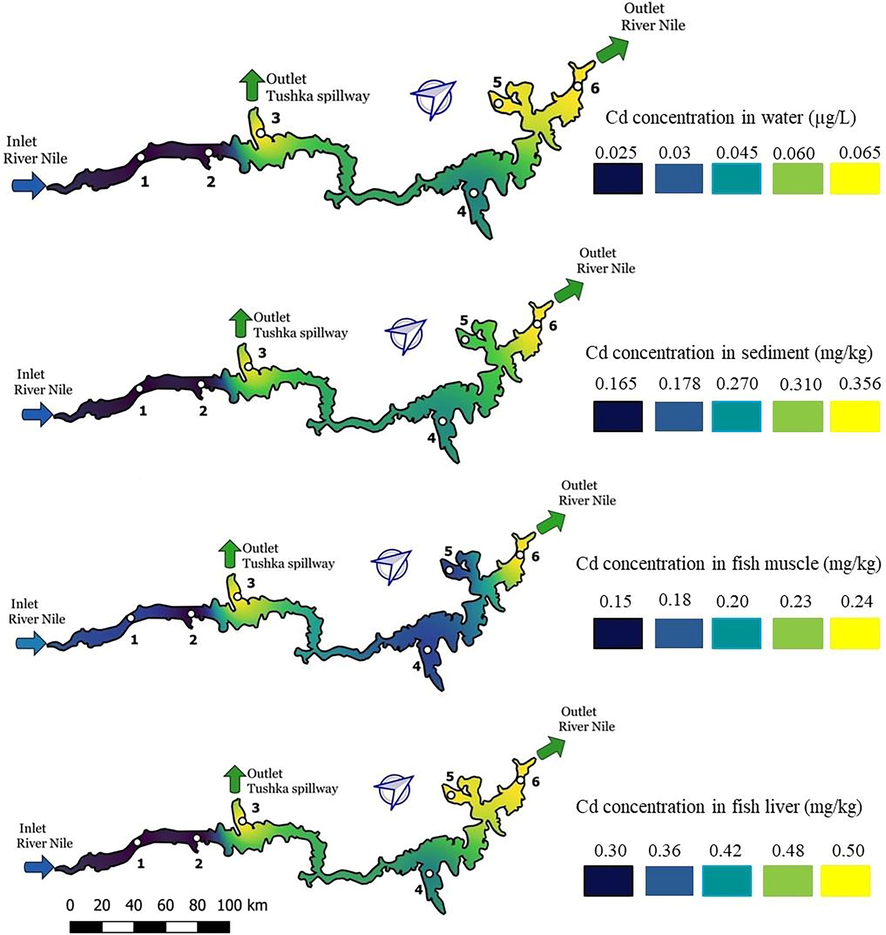
Cadmium distribution along the lake in water, sediment, fish muscle and fish liver.
The evaluated MPIs ranged from 0.63 mg/kg to 6.40 mg/kg with the mean of 3.54 mg/kg. The highest MPI value has been reported in fish liver samples at sites 6 and 3 (6.40 and 6.23 respectively) and in sediment was at sites 3 and 6 (5.57 and 5.23 respectively), while the lower MPI value were in fish Muscles at site 1 (0.63 mg/kg) (Fig. S2). The findings of MPI in the present study were much lower than that of Rutilus rutilus in Pluszne Lake (Luczynska et al., 2018).
The northern part of the lake showed the higher contamination with heavy metals compared with the southern part, except site 3 which is highlighted by yellow in Fig. 6. The high recorded values for all measured heavy metals in this site are perhaps related to the ongoing agricultural activities in Tushka region, nearby Tushka spillway outlet. The overall water quality of Lake Nasser is below maximum permissible levels of international standards and specifications. Therefore, it can be concluded that the water of Lake Nasser is safe for human consumption, agricultural activities and animal husbandry. Maintaining the water quality of the lake within these acceptable values represents an important mission for the sustainable development in Egypt, therefore all necessary actions must be implemented for the protection of Lake Nasser. Fig. S1 shows the correlation between the contamination with heavy metals and the types (i.e., water, sediment, liver and muscles) of investigated items. The cluster tree shows a high positive correlation between zinc and lead concentrations in water and its accumulation in sediment and fish liver (0.9 and 0.81; 0.94 and 0.93, respectively). The correlation between copper concentrations in water and fish muscle (0.88) was higher than that of liver samples (0.73). The copper is directed towards cell tissues in higher amounts than it does in liver, in order to participate in the protective role of the antioxidant enzymes. On the other hand, copper concentration in water exhibited a high positive correlation with sediment (0.91), given that the latter usually adsorbs the dissolved metals. In case of cadmium, the results showed a positive correlation between its concentration in water and in fish muscle, sediment and fish liver (0.91, 0.82 and 0.73 respectively). Fig. S1 confirms the increased risk for cadmium, because this element does not accumulate in the sediment in a large amount. However, it does accumulate in muscle in greater proportion. That is emphasize that Cadmium is not essential for fishes’ metabolic processes, and is potentially dangerous at lower concentrations compared to the essential metalloids (Delahaut et al., 2020). Fish organs comprise an effective target for predicting the effects of water pollutants, as a result of the positive correlation between the bioaccumulation of heavy metals in fish tissues and their concentration in water. Therefore, the current study emphasized the importance of better understanding of heavy metals bioaccumulation in fish organs. Moreover, another aim of this survey was to provide a comprehensive image of the heavy metal status in this lake. One major advantage of the current study, as opposed to other previous attempts, was the high accuracy of heavy metal measurements by using certified reference materials (CRM) within certain ranges.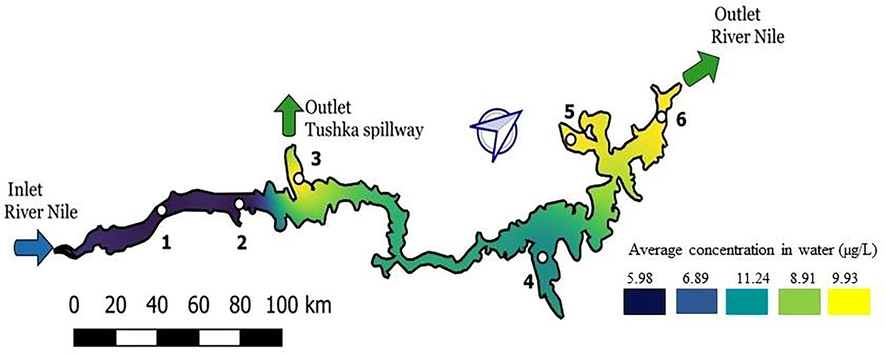
General overview on Lake Nasser of mean concentrations of heavy metal distribution in water.
4 Conclusions
Lake Nasser serves as the national water reservoir in Egypt. Heavy metal analyses in water, sediment and edible parts of fish indicated that water quality is excellent and that the fish are safe for human consumption. The sediment fulfilled a major role by absorbing dissolved metals from the aqueous form. The concentration of heavy metals in fish muscles and livers meets the specifications stipulated by international limits. Therefore, continuous monitoring and management actions must be carried to support appropriate decision-making in assessing the health of Lake Nasser. The current study also strongly suggests that legal actions are needed to be implemented by authorities to overcome pollution in the adjacent areas of Lake Nasser by reducing future pollution levels. However, given that the current survey focused solely on a single fish species, future studies should widen their scope by including other aquatic organisms, such as other common fish species and macroinvertebrates.
Funding
The authors extend their appreciation to the deanship of scientific research for funding this article by Taif University Research Supporting Project number (TURSP-2020/225), Taif University, Taif, Saudi Arabia.
Acknowledgements
This work was supported by the Tunisian Ministry of the High Education and Scientific Research. The authors extend their appreciation to the deanship of scientific research for funding this article by Taif University Research Supporting Project number (TURSP-2020/225), Taif University, Taif, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Physical properties of inland lakes and their interaction with global warming: A case study of Lake Nasser, Egypt. Egypt. J. Aquat. Res.. 2020;46(2):103-115.
- [Google Scholar]
- Assessment of metal pollution around sabal drainage in River Nile and its impacts on bioaccumulation level, metals correlation and human risk hazard using Oreochromis niloticus as a bioindicator. Turk. J. Fisheries Aquatic Sci.. 2016;2016(16):227-239.
- [Google Scholar]
- Total dissolved and bioavailable metals at Lake Texoma marinas. J. Environ. Poll.. 2003;122(2):253-259.
- [Google Scholar]
- American Public Health Association. Washington, DC, USA: Standard methods for the examination of water and wastewater; 2012.
- Bioaccumulation of Heavy Metals in Some Tissues of Fish in Lake Geriyo, Adamawa State. J. Environ. Public Health. 2018;2018:1-7.
- [Google Scholar]
- Water quality assessment of lake water: a review. Sustain. Water Resour. Manage.. 2016;2(2):161-173.
- [CrossRef] [Google Scholar]
- Macrophyte ecology, nutrient dynamics and water quality of the littoral zone, and Yitamot wetland. Ethiopia: Ababa University Addis Ababa; 2016. p. :184.
- Heavy metals: implications associated to fish consumption. J. Environ. Toxicol Pharmacol.. 2008;26(3):263-271.
- [Google Scholar]
- CCME, 2007.Canadian Council of Ministers of the Environment For the protection of aquatic life. In: Canadian Environmental Quality Guidelines, 1999, Canadian Council of Ministers of the Environment, 1999, Winnipeg.
- Trace metal concentrations in water, sediments and fish tissue from Lake Tanganyika. Sci. Total Environ.. 2002;299(1-3):115-121.
- [Google Scholar]
- The dilemma of healthy eating and environmental sustainability: The case of fish. Public Health Nutr.. 2012;15(2):277-284.
- [Google Scholar]
- Use of a UAV for water sampling to assist remote sensing of bacterial fora in freshwater environments. Undergraduate External Publications. Paper. 2016;17 Accessed 8 Mar 2020
- [Google Scholar]
- Geochemistry of the High Dam Lake sediments, South Egypt: implications for environmental significance. Int. J. Sedim. Res.. 2013;28(4):544-559.
- [Google Scholar]
- Assessing heavy metal contamination in surface sediments in an Urban River in the Philippines. Pol. J. Environ. Stud. 2018;27(5):1983-1995.
- [CrossRef] [Google Scholar]
- Toxicity and bioaccumulation of Cadmium, Copper and Zinc in a direct comparison at equitoxic concentrations in common carp (Cyprinus carpio) juveniles. PLoS ONE. 2020;15(4):e0220485.
- [CrossRef] [Google Scholar]
- A new empirical procedure for estimating intraannual heat storage changes in lakes and reservoirs: Review and analysis of 22 lakes. Remote Sens. Environ.. 2015;156:143-156.
- [Google Scholar]
- EC, 2005. European Community, Commission regulation No. 1881/2006. Setting maximum levels for certain contaminants in foodstuffs (pp. OJ, L364/5). Official J. Eur. Union.
- Sedimentological study of Lake Nasser; Egypt, using integratedimproved techniques of core sampling, X-ray diffraction and GISplatform. Cogent. Geosci.. 2016;2(1):1168069.
- [CrossRef] [Google Scholar]
- EQS, 2008. The European Parliament and the Council of the European Union. Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC of the European Parliament and of the Council, L 385; Author: Strasbourg, 2008, 84-97.
- FAO, 2012. Food and Agriculture Organization Animal Feed Resource Information System (AFRIS) The state of world fisheries and aquaculture, http://www.fao.org/ag/AGA/AGAP/FRG/afris/default.htm 28. FAO (2013) Food and Agriculture Organization Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fishery Circular No. 464. Food and Agriculture Organization 2013. pp. 5e100.
- Evaluation of certain food additives and the contaminants mercury, lead and cadmium. (JECFA). International programme on chemical safety Seventy-third report of the Joint FAO/WHO Expert Committee on Food Additives WHO technical report series. 2011;no. 960
- Effect of site on sedimentological characteristics and metal pollution in two semi-enclosed embayments of great freshwater reservoir: Lake Nasser. Egypt. J. Afr. Earth Sci.. 2018;141:194-206.
- [Google Scholar]
- Assessment of the environmental significance of heavy metal pollution in surficial sediments of the River Po.. 2007;68(4)
- GD, 2013. Governmental Decree No 92/2013. Ministry of Water Resources and irrigation amending the implementing regulations of Law 48/1982 on the Protection of the River Nile and Watercourses from Pollution and defining certain rules on the surface water monitoring and state assessment (in Arabic).
- Bioaccumulation and trophic transfer of metals, As and Se through a freshwater food web affected by antrophic pollution in Córdoba. Argentina. Ecotoxicol. Environ. Saf.. 2018;148:275-284.
- [Google Scholar]
- Variability of selected heavy metals in surface sediments and ecological risks in the Solwezi and Kifubwa Rivers, Northwestern province, Zambia. Sci. Afr. J.. 2021;12:e00822.
- [Google Scholar]
- Do trace metals (chromium, copper and nickel) influence toxicity of diesel fuel for free-living marine nematodes? Environ. Sci. Pollut. Res.. 2013;20(6):3760-3770.
- [Google Scholar]
- Identification and quantitation of inorganic and organic anthropogenic compounds in Lake Balaton and its catchment area. Report to the Prime Ministers’s Office; 1996.
- Distribution of heavy metals in water and sediment along Abonnema shoreline, Nigeria. Resource Environ.. 2012;2(1):33-40.
- [Google Scholar]
- Toxicity and Bioremediation of Heavy Metals Contaminated Ecosystem from Tannery Wastewater: A Review. J. Toxicol.. 2018;2568038
- [Google Scholar]
- Risk assessments and spatial distributions of natural radioactivity and heavy metals in Nasser Lake. Egypt. Enviro. Sci. Pollut. Res.. 2020;27:25475-25493.
- [Google Scholar]
- Protective effect of zinc, selenium, vitamin C, E and epicatechine on cadmium-induced toxicity and disturbances in the kidney, liver, bone, lipid metabolism and oxidative stress in rats. Res. J. Pharm. Biol. Chem. Sci.. 2016;7:647-655.
- [Google Scholar]
- Metal concentrations and risk assessment in water, sediment and economic fish species with various habitat preferences and trophic guilds from Lake Caizi, Southeast China. Ecotoxicol. Environ. Saf.. 2018;157:1-8.
- [Google Scholar]
- Jones, D.S., Sutter, G.W., Hull, R.N., 1997.Toxicological Benchmarks for Screening Contaminants of Potential Concern for Effects on Sediment-associated Biota: 1997 Revision, ES/ER/TM- 95/R4. Oak Ridge National Laboratory, prepared for the US Department of Energy. p.48.
- Trace metal extraction of soils and sediments by nitric acid-hydrogen peroxide. Atom. Absorp. Newsletr. 1976;15:68-70.
- [Google Scholar]
- La CIPEL, 2015. Rapports sur les études et recherches entreprises dans le bassin Lémanique, campagne 2014. Commission internationale pour la protection des eaux du Léman contre la pollution, CIPEL, Nyon Switzerland, pp 27-31.
- Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: A review. Environ. Toxicol. Pharmacol.. 2019;68:101-108.
- [Google Scholar]
- 2018 Fish as a bioindicator of heavy metals pollution in aquatic ecosystem of Pluszne Lake, Poland, and risk assessment for consumer’s health. Ecotoxicol. Environ. Saf.. 2018;153:60-67.
- [Google Scholar]
- Development and evaluation of consensusbased sediment quality guidelines for freshwater ecosystems. Arch. Enviorn. Contam. Toxicol.. 2000;39:20-31.
- [Google Scholar]
- MAFF, 2000. Ministry of Agriculture, Fisheries and Food: Monitoring and surveillance of non-radioactive contaminants in the aquatic environment and activities regulating the disposal of wastes at sea, 1997. In: Aquatic environment monitoring report No. 52. Lowestoft, UK: Center for Environment, Fisheries and Aquaculture Science.
- Determination of heavy metals by inductively coupled plasma-optical emission spectrometry in fish from the Piracicaba River in Southern Brazil. Micro. J.. 2010;94:171-174.
- [Google Scholar]
- Heavy metals in Lake Balaton: water column, suspended matter, sediment and biota. Sci. Total Environ.. 2005;340(1–3):213230
- [Google Scholar]
- Pollution and potential ecological risk assessment of heavy metals in a lake. Pol. J. Environ. Stud.. 2013;22(4):1129-1134.
- [Google Scholar]
- Determination of heavy metal content in Water, Sediment and microalgae from Lake Victoria. East Africa. Open Environ. Eng. J.. 2011;4:156-161.
- [Google Scholar]
- Trace elements in crustaceans, mollusks and fish in the Kenyan part of Lake Victoria: bioaccumulation, bioindication and health risk analysis. Arch. Environ. Contam. Toxicol.. 2020;78:589-603.
- [Google Scholar]
- Guidelines for the Protection and Management of Aquatic Sediment Quality. Ontario, Canada: Ontario Ministry of the Environment; 1992.
- Petri, M., 2006. Water Quality of Lake Constance The Rhine, 127-138.
- Cadmium and lead levels in fish (Tilapia nilotica) tissues as biological indicator for lake water pollution. Environ. Monit. Assess.. 2001;68:75-89.
- [Google Scholar]
- Environmental assessment of physical-chemical features of Lake Nasser. Egypt. Environ. Sci. Pollut. Res.. 2020;27(16):20136-20148.
- [Google Scholar]
- Assessment of heavy metals distribution in Barguzin River aquatic system (Lake Baikal basin) Limnol. Freshw. Biol.. 2020;4:695-696.
- [Google Scholar]
- Distribution and risk assessment of trace metals in multifarious matrices of Vembanad Lake system, Peninsular India. Mar. Pollut. Bull.. 2019;145:490-498.
- [Google Scholar]
- Heavy metal bioaccumulation, oxidative stress and genotoxicity in African catfish Clarias gariepinus from Orontes River. Ecotoxicology. 2020;29:1522-1537.
- [Google Scholar]
- U.S. Environmental Protection Agency. Screening Level Ecological Risk Assessment Protocol for Hazardous Waste Combustion. Facilities. 1999;Vol. 3:Appendix.
- [Google Scholar]
- USGS, 2005. US Geological Survey June. Chapter A8. Bottom-material samples (Version 1.1). National Field Manual for the Collection of Water-Quality Data: Techniques of Water-Resources Investigations (Book 9), 60. Available at: http://water.usgs.gov/owq/FieldManual/ (accessed November 6, 2012).
- USEPA, 2010. United State Environmental Protection Agency; USEPA 3050B, Method 3050B. Acid Digestion of Sediments, Sludges and Soils, http://www.epa.gov/waste/hazard/testmethods/sw846/ pdfs/3050b.pdf, accessed June, 2010
- Metals in the water, sediment, and tissues of two fish species from different trophic levels in a subtropical Brazilian river. Microchem. J.. 2013;106:61-66.
- [Google Scholar]
- WHO, 1989. World Health Organization on Heavy metals -environmental aspects; 1989 Environment health criteria. No. 85. Geneva, Switzerland.
- Role of living environments in the accumulation characteristics of heavy metals in fishes and crabs in the Yangtze River Estuary. China. Mar. Pollut. Bull.. 2012;64(2012):1163-1171.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101748.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







