Translate this page into:
Comparative efficacy of ternary Cu (II) complex and Zn (II)-complex in amelioration of carbon tetrachloride-induced hepatotoxicity in vivo
⁎Corresponding author at: Department of Zoology, College of Science, King Saud University, Building 05, Riyadh 11451, Saudi Arabia.
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cu (II) and Zn (II) are two of the most favored metals in synthesis chemistry, encompassing their therapeutic potentials. The present study aims to evaluate the comparative ameliorative potential of a group B carcinogen, CCl4-induced hepatotoxicity, by our recently synthesized and characterized ternary Cu and Zn-based complexes in vivo. Three groups of rats were treated with CCl4 alone and with the combination of Cu (II) complex and Zn (II) complex beside the control negative group without any treatment. After completion of the treatment, the samples were subjected to biochemical and histological analysis. The analysis demonstrated extensive alteration in redox status, liver markers, and hepatotoxicity markers in the CCl4, treated group compared to the control group. However, the Cu (II) complex showed significant improvement in most of the parameters under the study. Also, histopathological evaluation and comet assay further consolidated the findings. Hence, this investigation reveals that Cu (II) complex has more substantial ameliorative potential in ceasing CCl4-induced hepatotoxic insults. Therefore, the ternary Cu (II) complex act as a more potent chemotherapeutic agent in cancer treatment with milder side effects than the Zn (II) complex.
Keywords
Copper
Zinc
Metal complex
Carbon tetrachloride
Hepatotoxicity
Data Availability
The data relevant to this study are included in the article.
1 Introduction
Contemporary chemotherapeutic-based cancer treatment mostly fails to improve patient mortality because of the drug resistance or accumulation of severe adverse effects as co-morbidities. The oncologists have always favored metal-based drugs since cisplatin was endorsed in 1978 by FDA (Ghosh, 2019). However, the prolonged use of such medications generates many side effects and resistance limiting their efficacy for clinical purposes. In recent years, novel strategies like the usage of chemical compounds with new features, including controlled release, multiple internal triggers, liposome-based delivery, pH and enzyme sensitivity, as well as the incorporation of light and magnetic fields with synthetic, medicinal inorganic, and bio-organic compounds have to widen the therapeutic windows dealing with a diverse range of biomedical applications from diagnosis to cancer therapy (Hassan et al., 2018; Senapati et al., 2018; Khan et al., 2020). Hence, substantial research is focused on newly synthesized metal-based drugs with certain advantages, better efficacy, lower side effects, and cost effectivity (Wang et al., 2019). Copper, a d9 cation with borderline Lewis acid properties, is very active in biological entities for its vigorous hydrolytic and redox activities (Modec et al., 2020). These properties allow complex formation with various coordination numbers and geometries depending on structural versatility, easy synthesis, and a broad range of applications in various fields. The copper ions-rich vicinity of DNA and, along with critical enzymes and vitamins, further biocompatibility and clinical utilization in applied medicines (Hassan et al., 2018). Also, copper complexes affect the tumor's microvascular system and vascular permeability in different forms of cancer. The literature suggests that these complexes trigger cell death by apoptosis induction precisely in cancer cells via the production of ROS, leading to the nuclear deterioration of DNA (Hassan et al., 2019b; Karginova et al., 2019). However, β-carboline is an alkaloid that is chemically 9H-pyrido[3,4-b] indole. It is found in natural products with anticancer activity and is distributed in plants, animals, and humans (Mota et al., 2020; Aaghaz et al., 2021). It is documented that derivatives from β-carboline exert anticancer properties manipulating DNA structure by intercalation, blocking cyclin-dependent kinase, prohibition of topoisomerase I and II and IkK kinase complex (Mota et al., 2020; Alharbi et al., 2021). Recently, we have published the synthesis and characterization of ternary Cu (II) and Zn (II) complexes of Schiff base derived from tryptophan and auxiliary β-carboline as shown in Fig. 1 (Alharbi et al., 2021). In the present study, our goal was to investigate the comparative efficacy of two novel synthesized complexes (Cu and Zn) in alleviating CCl4-induced hepatotoxicity in the rat model. The target organ samples were studied for toxicity profiling, oxidative stress, and macromolecular degradation. Finally, biochemical results were confirmed by histopathology and comet assay.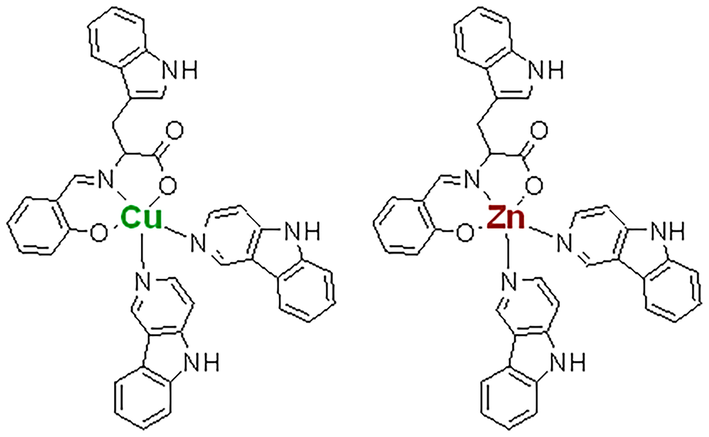
Showing the structure of copper (II) and zinc (II) compounds with amino acid, L-Tryptophan derived ligand along with auxiliary β-carbolines units.
2 Methodology
2.1 Animal treatment
Adult Swiss albino rats (n = 36; 110–130 g) were procured from the Central Animal House, Department of Pharmacy, King Saud University, Riyadh. They were housed in hygienic and humane conditions and maintained temperature and day-night cycles (Alharbi et al., 2021). All the rats were divided into six treatment groups (n = 6) as follows:
Group I: Control without any treatment.
Group II: A single dose of 1 mL/kg of CCl4 treated (Ebaid et al., 2021).
Group III: Cu (II) complex at the dose of 1 mg/kg body/week for one month in CCl4-challenged rats.
Group IV: Zn (II) complex at the dose of 1 mg/kg body/week for one month in CCl4-challenged rats.
All the procedures, including handling and treatment with test chemicals, were conducted per our lab standardized methods (Ebaid et al., 2014). All the animals were sacrificed to retrieve serum and liver samples post-treatment (Hassan et al., 2012). The samples were used for biochemical analysis, histopathological evaluation, and comet assay (Hassan et al., 2019b). The Animal Ethics Committee approved the study at King Saud University, Riyadh (KSA), under KSU-SE-20-38.
The sample preparation and protocols implemented have been detailed in the supplementary file of methodology.
3 Results
3.1 Effect on liver serum markers
3.1.1 Alkaline phosphatase (ALP)
In the present investigation, its activity was elevated by 145.66% in group II compared to the control. In comparison, groups- III and IV showed a decline in its activity by 41.28% and 20.64% concerning group II (Fig. 2).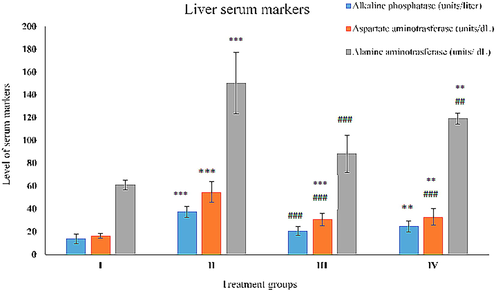
Showing the histogram of standard liver function markers (Alkaline phosphatase, and aspartate alanine aminotransferase) in serum samples from the treated animals. The asterisk mark * indicates values significantly different from the control (group I), while # indicates values significantly different from the control positive (group II). All the data are presented as the mean ± SD. P-values were calculated by Student's t-test, * or # p < 0.05; ** and ## p < 0.01; *** or ### p < 0.001.
3.1.2 Alanine aminotransferase (ALT)
Group II demonstrated rise in its activity by 170.92% as compared to the control, group I confirmed gross live damage. Group III and IV exhibited damage control by 44.40% and 33.64% with respect to group II. (Fig. 2).
3.1.3 Aspartate aminotransferase (AST)
Group II showed an increase in AST activity by 232.34% compared to the control. Group III and IV decreased its activity by 44% and 39.76% with respect to group II (Fig. 2).
3.2 Effect on key antioxidant parameters
3.2.1 Catalase (CAT)
It is one of the primary antioxidant enzymes to regulate oxidative stress. Its activity was compromised by 38.56% in group II compared to group I, whereas its activity was found replenished by 28.12% and 13.84% with respect to group II. (Fig. 3).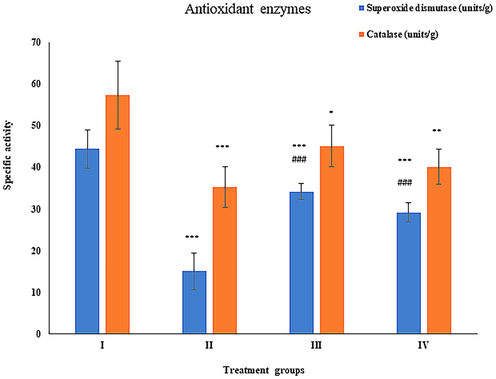
Showing the histogram of key antioxidant enzymes (superoxide dismutase, and catalase) in liver samples from the treated animals. The asterisk mark * indicates values significantly different from the control (group I), while # indicates values significantly different from the control positive (group II). All experiments were conducted in triplicate, and the data are presented as the mean ± SD. P-values were calculated by Student's t-test, * or # p < 0.05; ** and ## p < 0.01; *** or ### p < 0.001.
3.2.2 Superoxide dismutase (SOD)
Its activity was depressed by 66.10% in group II as compared to group I. Hitherto, group III and IV showed elevation in its activity by 127.30% and 93.95% with respect to group II (Fig. 3).
3.2.3 Glutathione reductase (GR)
In the present study, its activity was found a declination by 37.20% in group II as compared to the control. In contrast, group III and IV showed a rise in activity by 45.37% and 36.57% with respect to group II (Fig. 4).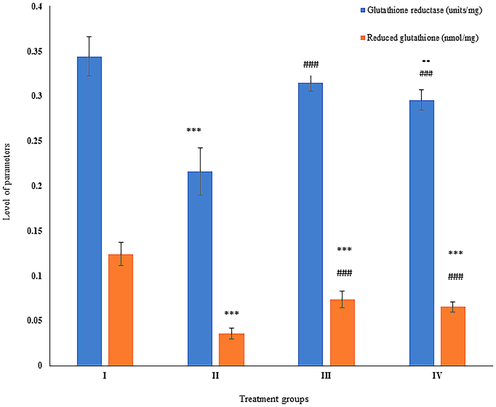
Showing the histogram of standard cellular reducing powers (glutathione reductase and reduced glutathione) in liver samples from the treated animals. The asterisk mark * indicates values significantly different from the control (group I), while # indicates values significantly different from the control positive (group II). All experiments were conducted in triplicate, and the data are presented as the mean ± SD. P-values were calculated by Student's t-test, * or # p < 0.05; ** and ## p < 0.01; *** or ### p < 0.001.
3.2.4 Reduced glutathione level (GSH)
Group II exhibited 71.77% of increase in its level with respect to group I. However, group III and IV showed decrease by 105.76% and 81.89% in comparison to group II (Fig. 4).
3.3 Effect on toxic load over the liver
3.3.1 Gamma-glutamyl transferase (GGT)
Group II showed elevation by 173.59% in its activity compared to control while group III and IV showed a dip in its activity by 41.75% and 34.94% with respect to group II (Fig. 5).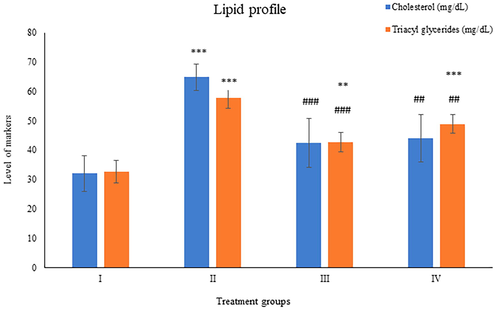
Showing the histogram of liver toxicity markers (gamma-glutamyl transferase and glutathione-S- transferase) in liver samples from the treated animals. The asterisk mark * indicates values significantly different from the control (group I), while # indicates values significantly different from the control positive (group II). All experiments were conducted in triplicate, and the data are presented as the mean ± SD. P-values were calculated by Student's t-test, * or # p < 0.05; ** and ## p < 0.01; *** or ### p < 0.001.
3.3.2 Glutathione-S-transferase (GST)
The activity of this enzyme was observed to be increased by 149.12% in group II while group III and IV demonstrated a dip in its activity by 29.43% and 21.98% in comparison to group II (Fig. 5).
3.4 Effect on lipid profile
3.4.1 Cholesterol
Group II displayed enhancement in its level by 102.62% as compared to the control, whereas groups- III and IV showed a decrease in its level by 34.53% and 31.97% with respect to group II (Fig. 6).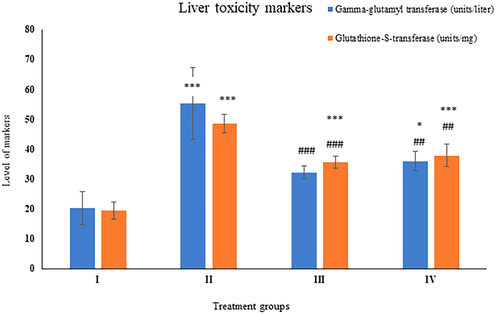
Showing the histogram of standard lipid profile parameters (cholesterol and tri-acyl glycerides) in serum samples from the treated animals. The asterisk mark * indicates values significantly different from the control (group I), while # indicates values significantly different from the control positive (group II). All experiments were conducted in triplicate, and the data are presented as the mean ± SD. P-values were calculated by Student's t-test, * or # p < 0.05; ** and ## p < 0.01; *** or ### p < 0.001.
3.4.2 Triacylglycerides (TAGs)
Group II exhibited a rise in its level by 76.69% as compared to the control, whereas group III and IV showed a dip in its level by 25.91% and 15.25% with respect to group II (Fig. 6).
3.5 Effect on lipid peroxidation
Malondialdehyde (MDA) is a reliable marker for measuring lipid peroxidation in vivo. There was an increase of 260.31% in Group II, while group III and IV showed a decline in their level by 48.01% and 38.32% compared to group II (Fig. 7).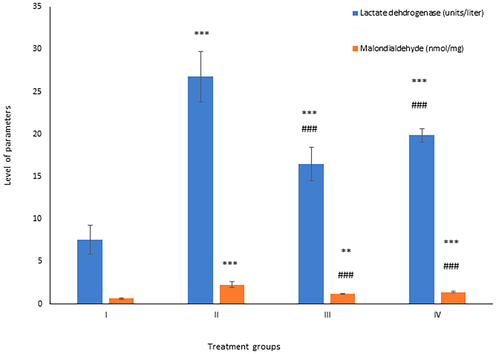
Showing the histogram of lactate dehydrogenase and total malondialdehyde in liver samples from the treated animals. The asterisk mark * indicates values significantly different from the control (group I), while # indicates values significantly different from the control positive (group II). All experiments were conducted in triplicate, and the data are presented as the mean ± SD. P-values were calculated by Student's t-test, * or # p < 0.05; ** and ## p < 0.01; *** or ### p < 0.001.
3.6 Effect of the treatment on lactate dehydrogenase (LDH)
This enzyme is an indirect marker for assessing necrosis (Chan et al., 2013). Its activity was elevated by 253.36% in group II as compared to the control, while group III and IV demonstrated by 38.35% and 25.79% respectively (Fig. 7).
3.7 Albumen
Albumen is a critical indicator of liver damage and dysfunction (Spinella et al., 2016). The level of this parameter increased by 21.4% in group II while group III and IV demonstrated a dip in its activity by 13.75% and 10.05% compared to group II (Fig. 8).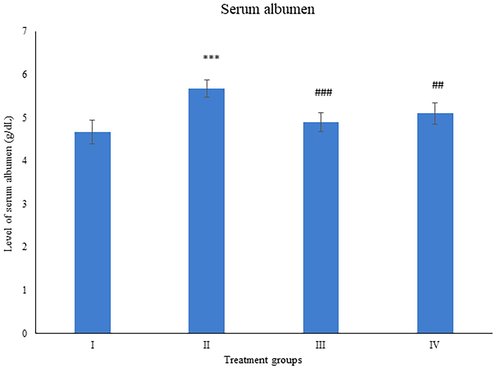
Showing the histogram of serum albumen from the treated animals. The asterisk mark * indicates values significantly different from the control (group I), while # indicates values significantly different from the control positive (group II). All experiments were conducted in triplicate, and the data are presented as the mean ± SD. P-values were calculated by Student's t-test, * or # p < 0.05; ** and ## p < 0.01; *** or ### p < 0.001.
3.8 Comet assay
In the present study, group II showed a rise in comet tail length by 57.25% in the liver samples compared to the control. However, group III and IV decreased the tail length by 28.71% and 23.97% with respect to group II (Fig. 9).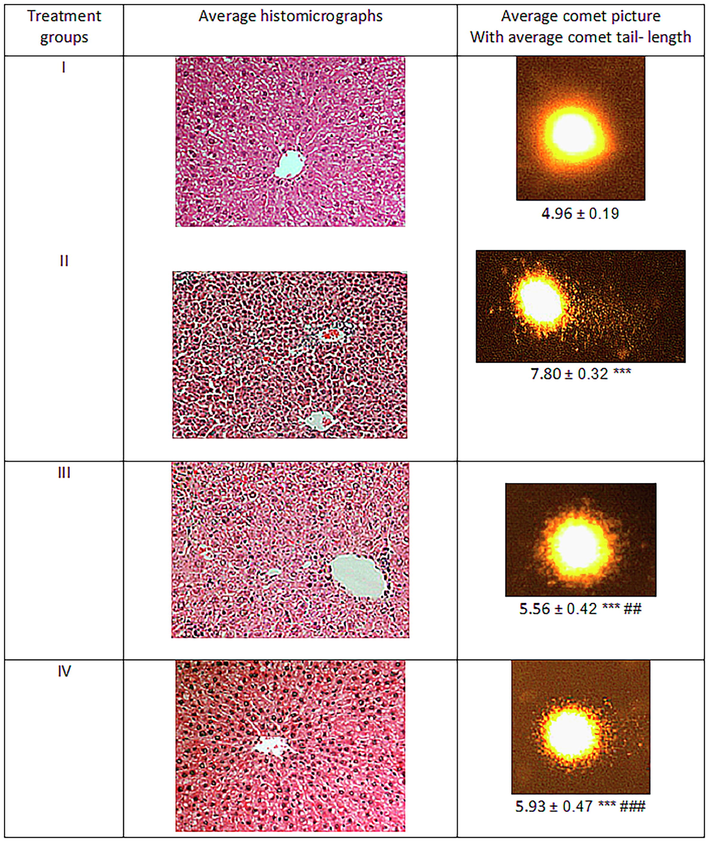
Showing the average comet picture and liver section of the treated animals. The asterisk mark * indicates values significantly different from the control (group I), while # indicates values significantly different from the control positive (group II). All experiments were conducted in triplicate, and the data are presented as the mean ± SD. P-values were calculated by Student's t-test, * or # p < 0.05; ** and ## p < 0.01; *** or ### p < 0.001.
3.9 Effect on histology of the liver tissue
The histology of liver tissue from the treated animals showed prominent alteration post-treatment with test compounds. The histomicrograph of Group II showed clear signs of CCl4- induced toxicity evidenced by extensive inflammation andw distorted tissue microstructure (Fig. 9). Besides, abruptly distributed sinusoids depicting fibrosis and nuclear deterioration were observed in most of the hepatocytes of the same group. Group III exhibited a significant recovery in the liver section as the lineation of the cells found tended to the central vein, and the sinusoids were comparable to the control. A similar pattern but a lower degree of histological improvement was observed in group IV with respect to group III. Hence, the histological evaluation was found in agreement with the biochemical assessment.
4 Discussion
CCl4 is a proven hepatotoxicant and lower-grade carcinogen extensively used for comparative in vivo toxicity assessment (Ebaid et al., 2014; Dutta et al., 2018). The current study entails that CCl4 caused grievous hepatotoxicity in the treated animals. However, the metal complexes alone have shown tolerance in the animals, as reported previously by Alharbi et al., 2021). Previously, both the metals-derived complexes have demonstrated anticancer activity in cell line-based studies (Alharbi et al., 2021).
It is well established that CCl4 exerts hepatotoxicity by eliciting free radicals that invade vital organs, including liver (Al-Tamimi et al., 2021). The radicals deteriorate the membrane and organelles (Golgi bodies, mitochondria, and nucleus) of the cells, altering the nature of macromolecules like proteins, enzymes and nucleic acids. In such circumstances, the antioxidant system gets activated to nullify the same. However, the present study shows that the high dose of CCl4 fails the antioxidant system in checking oxidative stress, as the level of SOD, CAT and GSH was compromised in the liver samples. The Cu and Zn metal complexes significantly ameliorated on CCl4-induced hepatotoxicity, but the amelioration degrees differed.
Contrary to many studies entailing Zn compounds being less toxic than Cu compounds (Khan et al., 2020), the present investigation exhibited the opposite, i.e., stronger amelioration with lesser toxic insults against CCl4 -induced hepatoxicity. Notably, most studies on metal-based drugs induced toxicity are based on cell lines, fishes and microorganisms (Delahaut et al., 2020). In this study, Cu and Zn metal complexes show differential toxicity as Cu- the biological system than Zn better handles complex.
Furthermore, the size of the Cu-complex is smaller than the Zn-complex, which might facilitate better transport in blood circulation followed by its metabolism, assimilation and elimination. Also, the biological systems are well equipped for copper homeostasis at the molecular level backed by a vast repertoire including metallothionein, ceruloplasmin, glutathione, copper chaperones, P-type ATPases, etc. (Tapiero et al., 2003; Barber et al., 2021). The smaller size of the Cu-complex might facilitate its interaction with cellular proteins and nucleic acids, attributing strongly in the ameliorative of the hepatotoxicant. The comet assay results confirm this notion in the present investigation.
According to the meta-studies by Hill and Shannon (2019), excess of Zn leads to lysis of RBC, interfering with the transport of Zn and similar metals like Cu and Fe. Hence, Zn imbalance in the chronic condition is followed by Cu and Fe deficiency in the biological systems (Barber et al., 2021). Besides, the role of Cu at the active site of CuZnSOD, one of the chief antioxidant enzymes, might be dominant over Zn, providing the structural basis for the same (Manieri et al., 2021). In the present study, Cu (II)-complex enhances the activity of SOD significantly different compared to Zn (II)-complex.
On the other hand, Zn has a larger atom with d10 electronic configuration compared to copper's relatively smaller atomic size with d9 configuration. Therefore, the two cations pose a different Lewis acidity degree, favoring their respective complexes' geometries. Further, Cu (II) prefers square planar coordination along with single or double weaker axial ligands attributed to the Jahn–Teller effect of the d 9. Hence, Cu (II), a more vital Lewis acid with Jahn–Teller distortion, facilitates the ion binding to amides at around physiological pH (Schirer et al., 2017).
5 Conclusion
The Cu (II) complex alleviates CCl4-induced hepatotoxicity better than Zn (II) complex by checking oxidative stress and inflammation. Cu is favored by its biocompatibility, smaller size with less toxic burden in vivo. The study attempts to correlate the pharmacological potential of the synthesized metal coordinate compounds that could directly address various diseases of modern times, including cancer.
Authors’ contribution
IH and RAK conceived the research idea and designed the study. RAK, FMH synthesized and characterized the compounds. IH and JA conducted all animal experiments while IH, RAK, HE analyzed the in vivo data with IMA. IH, RAK and FMH drafted the manuscript. All authors read and approved the manuscript.
Data Availability
The data relevant to this study are included in the article.
Acknowledgment
This work was supported by Researchers Supporting Project number (RSP-2021/366), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Samsum ant venom protects against carbon tetrachloride–induced acute spleen toxicity in vivo. Environ. Sci. Pollut. Res. 2021:1-13.
- [Google Scholar]
- Alharbi, W., Hassan, I., Khan, R.A., et al., 2021. Bioactive Tryptophan-Based Copper Complex with Auxiliary β-Carboline Spectacle Potential on Human Breast Cancer Cells: In Vitro and In Vivo Studies. Molecules 26(6), 1606.
- Copper toxicity is not just oxidative damage: Zinc systems and insight from Wilson disease. Biomedicines.. 2021;9(3):316.
- [Google Scholar]
- Chan, F.K.-M., Moriwaki, K., Rosa, M.J.D., 2013. Detection of necrosis by release of lactate dehydrogenase activity. Immune Homeost., Springer, 65–70.
- Toxicity and bioaccumulation of Cadmium, Copper and Zinc in a direct comparison at equitoxic concentrations in common carp (Cyprinus carpio) juveniles. PLoS ONE. 2020;15(4):e0220485.
- [Google Scholar]
- Amelioration of CCl4 induced liver injury in swiss albino mice by antioxidant rich leaf extract of Croton bonplandianus Baill. PLoS ONE. 2018;13(4):e0196411.
- [Google Scholar]
- Neutrophil depletion in the early inflammatory phase delayed cutaneous wound healing in older rats: improvements due to the use of un-denatured camel whey protein. Diagn. Pathol.. 2014;9(1):1-12.
- [Google Scholar]
- Ebaid, H., Al-Tamimi, J., Hassan, I., et al., 2014. Antioxidant bioactivity of Samsum ant (Pachycondyla sennaarensis) venom protects against CCL4-induced nephrotoxicity in mice. Oxidative Med. Cell. Longevity. 2014.
- Curcumin-containing Silver Nanoparticles Prevent Carbon Tetrachloride-induced Hepatotoxicity in Mice. Comb. Chem. High Throughput Screening. 2021;24(10):1609-1617.
- [Google Scholar]
- Riboflavin ameliorates cisplatin induced toxicities under photoillumination. PLoS ONE. 2012;7(5):e36273.
- [Google Scholar]
- Ameliorative effect of zinc oxide nanoparticles against potassium bromate-mediated toxicity in Swiss albino rats. Environ. Sci. Pollut. Res.. 2019;26(10):9966-9980.
- [Google Scholar]
- Restrained management of copper level enhances the antineoplastic activity of imatinib in vitro and in vivo. Sci. Rep.. 2018;8(1):1-17.
- [Google Scholar]
- Copper and zinc nutritional issues for agricultural animal production. Biol. Trace Elem. Res.. 2019;188(1):148-159.
- [Google Scholar]
- Inhibition of Copper Transport Induces Apoptosis in Triple-Negative Breast Cancer Cells and Suppresses Tumor AngiogenesisTargeting Copper Transport in Breast Cancer. Mol. Cancer Ther.. 2019;18(5):873-885.
- [Google Scholar]
- β-Carboline copper complex as a potential mitochondrial-targeted anticancer chemotherapeutic agent: Favorable attenuation of human breast cancer MCF7 cells via apoptosis. Saudi J. Biol. Sci.. 2020;27(8):2164-2173.
- [Google Scholar]
- Structural effects of stabilization and complexation of a zinc-deficient superoxide dismutase. Heliyon.. 2021;7(1):e06100.
- [Google Scholar]
- Beyond the simple copper (II) coordination chemistry with quinaldinate and secondary amines. Molecules. 2020;25(7):1573.
- [Google Scholar]
- β-carboline alkaloid harmine induces DNA damage and triggers apoptosis by a mitochondrial pathway: Study in silico, in vitro and in vivo. Int. J. Funct. Nutr.. 2020;1(1):1.
- [Google Scholar]
- Similarities and differences of copper and zinc cations binding to biologically relevant peptides studied by vibrational spectroscopies. J. Biol. Inorg. Chem.. 2017;22(4):581-589.
- [Google Scholar]
- Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transd. Targeted Ther.. 2018;3(1):1-19.
- [Google Scholar]
- Albumin in chronic liver disease: structure, functions and therapeutic implications. Hep. Intl.. 2016;10(1):124-132.
- [Google Scholar]
- Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother.. 2003;57(9):386-398.
- [Google Scholar]
- Emerging platinum (IV) prodrugs to combat cisplatin resistance: from isolated cancer cells to tumor microenvironment. Dalton Trans.. 2019;48(8):2536-2544.
- [Google Scholar]
Further Reading
- Gao, H., Dai, W., Zhao, L., et al., 2018. The role of zinc and zinc homeostasis in macrophage function. J. Immunol. Res. 2018.
- Hassan, I., Ebaid, H., Alhazza, I.M., et al., 2019. Copper mediates anti-inflammatory and antifibrotic activity of Gleevec in hepatocellular carcinoma-induced male rats. Canadian J. Gastroenterol. Hepatol. 2019.
- Khan, A.A., Jabeen, M., Hassan, I., et al., 2021. Lipid Nanosystems: Targeted Nano-delivery of Therapeutic Agents in Treatment of Cancer. Functional Lipid Nanosystems in Cancer, Jenny Stanford Publishing, pp. 351–377.
- Anticancer potential of biogenic silver nanoparticles: a mechanistic study. Pharmaceutics.. 2021;13(5):707.
- [Google Scholar]
- Research on a Zn-Cu alloy as a biodegradable material for potential vascular stents application. Mater. Sci. Eng., C. 2016;69:407-413.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102420.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







