Translate this page into:
Cholinesterase inhibitory activity and regioselective synthesis of spiropyrrolidinoindole integrated ferrocene hybrid heterocycles via multicomponent cycloaddition reaction
⁎Corresponding author. almansor@ksu.edu.sa (Abdulrahman I. Almansour)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A novel spiroheterocyclic hybrid comprising several privileged structures comprising pyrrolidine, quinoxaline, indole and ferrocene moieties were synthesized in good yields in sustainable fashion using [Bmim]Br augmented four component cycloaddition process. A relatively less explored ylide prepared from quinoxalinone and L-tryptophan with diverse ferrocenyl derivatives in ionic liquids afforded spiropyrrolidinoindole tethered ferrocene hybrids. The reaction provides highly regioselective fashion thus created five new bonds and four adjoining stereocenter in single synthetic transformation, thus created with complete diastereomeric control. The cholinesterase inhibitory potency was performed for synthesized compounds against AChE/BChE enzymes. Among them, compound bearing with fluorine substituted heterocycles showed significant activity which is comparable activity with reference standard, galantamine.
Keywords
Four component reaction
Cycloaddition
Green protocol
Ferrocene fused heterocyclic hybrids
AChE/BChE inhibitory activity
1 Introduction
A highly efficient and sustainable synthetic approach for accessing structural complexity that contains efficient structural fragments with minimal number of preparation steps is highly desirable in modern drug discovery program. Of the various synthetic methods available, multicomponent reactions (MCRs) (Sheldon et al., 2013; Arumugam et al., 2018; Burke et al., 2004) have developed into a prevailing synthetic strategy for the preparation of structurally diverse complex scaffolds in a single transformation and are important to meet the increasing demand for the elaboration of sustainable organic syntheses with maximum molecules diversity (Anusha Rani et al., 2017; Vasudevan Sumesh et al., 2016). MCRs is atom economic efficient straightforward reaction, minimized waste generation, potential to save solvents can avoid time-consuming and costly experimental procedures to purify various intermediate and tedious steps of deprotection and protection of functional groups (Arumugam et al., 2013). Hence, the development of new regio- and stereoselective multicomponent reactions is a constant task at the lead of organic chemistry.
Four-component reactions involving the intermolecular cycloaddition of in situ ylides with activated olefinic dipolarophile facilitate a concise approach into diverse hybrid heterocycles in a regio and stereospecific manner (Ahrendt et al., 2004; Boruah 2007; Pandey et al., 2006). This eco-friendly synthetic protocol (Sheldon, 2012) has attracted much attention and significant advances in the field and providing a rapid access to hybrid spirooxindole-pyrrolidines/pyrrolizidines heterocycles of biological importance (Kobayashi et al., 2002; Kanagaraju et al., 2014; Dhanalakshmi et al., 2015). Intriguingly, spirooxindole heterocycles have intrinsic three-dimensionality and facility to develop compound in all three dimensions. Prominent interactions of a ligand with a three-dimensional binding site are easier to achieve with a spirocompounds than with planer aryl ring systems (Carreira et al., 2014). Apart from that spirooxindolopyrrolidines are an important entrant of many biologically active natural alkaloids and pharmaceutically active synthetic analogues including horsfiline, elacomine, spirotryprostatins A and B, MI-219 and M−888 (Fig. 1). These spiro compounds exhibiting multifarious biological and pharmaceutical properties, for instance, anticonvulsant (Jiang et al., 2006), potential anti-leukaemic (Abou-Gharbia 1979) and antiviral activities (Lundahl et al., 1972). Therefore, the preparation of variety of functionalized spiro unit embedded pyrrolidines/pyrrolizidines is of great value in the field of medicinal chemistry.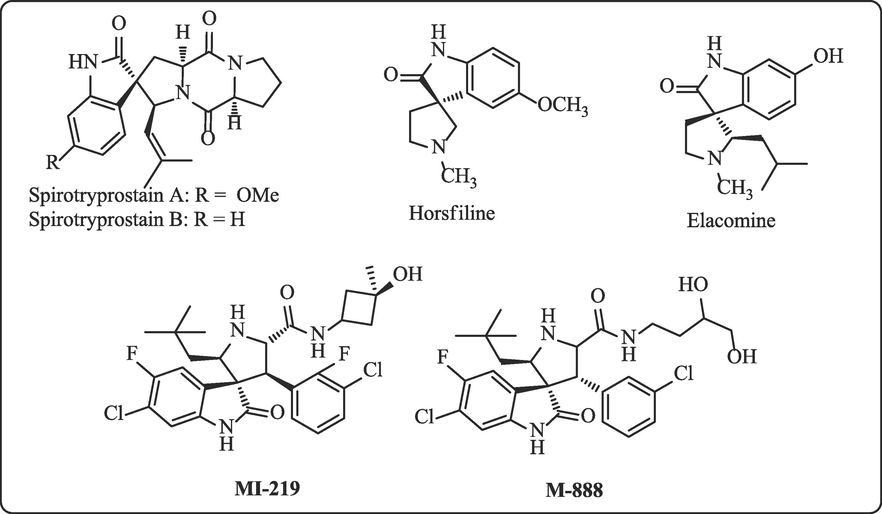
Biological active synthetic spiropyrrolidine derivatives.
Our team mainly engaged in the preparation and biological activities of structurally complex spiroheterocyclic architecture employing single-pot multicomponent cycloaddition methodology (Arumugam et al., 2013 & 2018; Arumugam et al., 2021). These compounds have shown multifarious biological activities (Kornett et al., 1976) viz. antimicrobial activity, anticancer, anti-inflammatory, and cholinesterase inhibitory activities (Arumugam et al., 2019). Some of the spiro heterocycles showed interesting biological activities than the reference standard drug (Arumugam et al., 2018 & 2018). Inspired by these interesting biological precedents and our great interest in the area of cycloaddition (Arumugam et al., 2020), herein we synthesize an easy access to structurally intriguing heterocycles comprising ferrocene grafted tethered spiropyrrolidinoindenoquioxaline via a single-pot, eco-friendly green synthetic transformation using a [3 + 2] cycloaddition reaction with their biological intervention. The synthetic strategy for the formation of spirocompounds has been described in Fig. 2.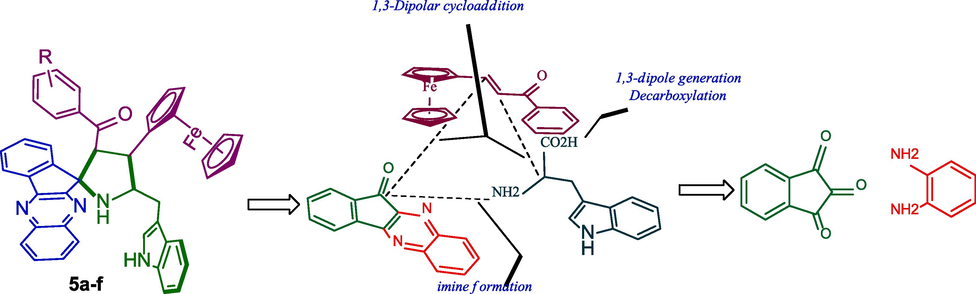
Synthetic strategy for the formation of spiropyrrolidino-indenoquioxalino-indole integrated ferrocene hybrid heterocycles.
2 Experimental section
2.1 Synthesis of spiropyrrolidinoindole integrated ferrocene hybrid heterocycles, 5a-f
An equimolar mixture of substrates 1, 2, L-tryptophan 4 and alkene 5 was stirred 1 h at 100 °C. After an hour, the reaction mixture was diluted with EtOAc and brain water, the organic solvent was dried over Na2SO4 and then solvent was removed under vaccum to give a pure spiro compound in excellent yield.
2.1.1 Spiropyrrolidine, 6b
Yield: 92 %; Brown solid; 1H NMR: δH 3.07–3.11 (1H, dd, J = 13.5, 8.0 Hz), 3.53–3.56 (1H, m), 3.96 (1H, t, J = 10.0 Hz), 4.08–4.23 (7H, m), 4.45–4.50 (2H, m), 5.10 (1H, d, J = 9.5 Hz), 6.86–6.88 (2H, m), 6.97–6.98 (2H, m), 7.44–7.14 (4H, m), 7.22–7.29 (4H, m), 7.64 (1H, d, J = 7.5 Hz), 7.637.74 (3H, m), 8.05–8.16 (2H, m): 13C NMR: δC 30.0, 45.7, 62.9, 66.3, 67.4, 67.7, 68.5, 68.7, 70.4, 89.7, 111.1, 112.8, 119.1, 119.5, 121.5, 122.1, 122.8, 126.7, 127.9, 128.0, 128.9, 129.1, 129.4, 129.7, 129.9, 131.3, 131.7, 135.8, 136.3, 141.9, 142.5, 147.6, 153.2, 166.1, 197.7; LC/MS(ESI): m/z = 766 (M+).
2.1.2 Spiropyrrolidine, 6d
Yield: 90 %; Brown solid; 1H NMR: δH 3.07–3.11 (1H, dd, J = 14.5, 8.0 Hz), 3.55 (1H, d, J = 14.5 Hz), 3.97 (1H, t, J = 10.0 Hz), 4.08–4.23 (7H, m), 4.48–4.50 (1H, m), 5.14 (1H, d, J = 10.0 Hz), 6.48–6.51 (2H, m), 7.01–7.28 (8H, m, ArH), 7.57–7.79 (3H, m, ArH), 8.03–8.23 (3H, m, ArH); 13C NMR: δC 30.0, 45.5, 63.1, 65.2, 66.4, 67.4, 67.7, 68.6, 68.7, 70.5, 89.8, 111.2, 112.7, 115.2, 115.3, 119.1, 119.5, 121.4, 122.0, 122.9, 126.6, 128.0, 129.0, 129.1, 129.3, 129.7, 129.8, 129.9, 130.1, 131.7, 133.6, 136.3, 141.9, 142.5, 147.7, 153.2, 164.2, 166.2, 197.1 LC/MS(ESI): m/z = 706 (M+).
3 Results and discussion
3.1 Chemistry
The starting precursor, ferrocene dipolarophile was prepared according to the literature method [25]. The pre-requisite, ferrocenyl chalcone 4 was prepared by the reaction of ferrocene 2-carboxyaldehyde with appropriate aryl aldehyde in presence of potassium hydroxide. With the highly functionalized dipolarophile 4a in hand, firstly we achieved the one-pot reaction of 4 with in situ ylide synthesized from L-tryptophan (3) and quinoxalinone 7. Thus, a mixture of 1, 2, 3 and 4a in heating MeOH (10 mL, 60 min) affording the spiropyrrolidinoindole grafted ferrocene hybrids 5a as a single compound in 85 % yield. Ultimately, the four-component reaction was also performed in (Bmim)Br at 100 °C (Scheme 1). The desired spirocompound 5a was attained with quantitative yield (94%) compared to conventional method in MeOH.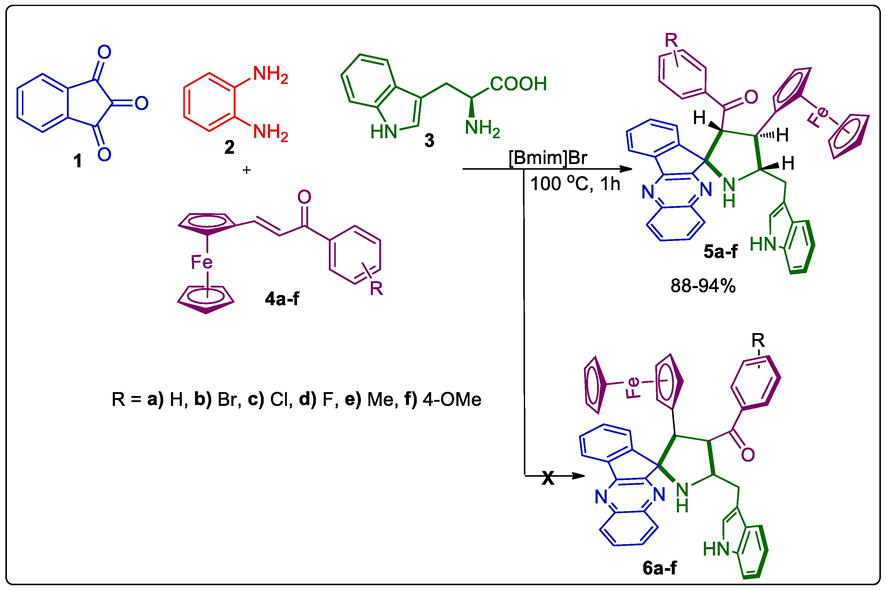
Synthesis of spiropyrrolidinoindole tethered ferrocene hybrid heterocyles, 5a-f.
The regioselective spirohybrid heterocycles 5 was assigned with the help of spectroscopic studies as discussed for a representative case, 5b (Fig. 3). In the 1H NMR spectrum, H-3 hydrogen shows at δ 5.11 as a doublet its showed (i) correlation (proton, proton-COSY) with the triplet at δ 3.97 assigned to H-4 which shows HMBCs (Fig. 4) with the spirocarbon (C-2), benzoylcarbon (C-4), benzoylcarbonyl carbon (C = O) at δ 89.7, 45.7,197.7, respectively. The multiplet at δ 4.47 was assigned to H-5 proton. The doublet of doublet and multiplet at δ 3.07–3.12 and δ 3.54–3.56 were ascribable to H-6, which exhibited HMBCs with C-5 (δ 65.2 ppm).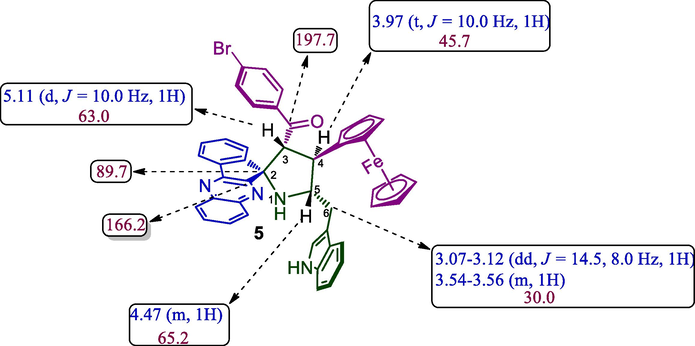
Selected Chemical shift of 5b.
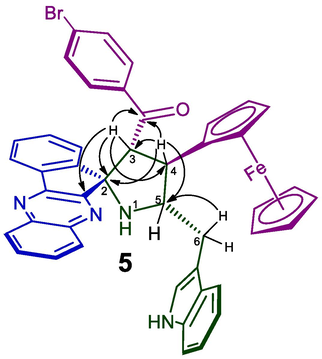
Selected HMBC shift 5b.
Scheme 2 describes a reasonable mechanistic pathway for synthesis of spirocompound 6. The interaction of carbonyl of trione 1 with ionic liquids rises its electrophilicity, permitting attack of the NH of aryldiamine 1 to form quinoxalinone 3 by successive dehydration. Subsequently, quinoxalinone 3 was reacts with L-tryptophan to generate spirooxazolidinone intermediate 8 via intermediate 7 followed by the formation of in situ 1,3-dipole 5 via decarboxylation pathway. Further, the interaction of the C = O group of 5 with [Bmim]Br, stimulates double bond thus allowing the ylide to react with β-carbon ferrocene dipolarophile providing spiropyrrolidine 6. It is important to note that the ionic liquids is play a twin action as catalyst and solvent through the cycloaddition sequence has been well documented in the literature and it has described in Scheme 2. The regioisomer 7 was not detected due to the possible orbital interaction between ylide 11 and ferrocenyl ketone of dipolarophile 4. Besides, the ylide 11 favorably attacks β-carbon of the dipolarophile to afford desired cycloadduct 5. Furthermore, we investigated the stability of compound 5 through theoretical study employing minimization energy calculation (mm2) and found that the ferrocenyl cycloadduct 5 has a lower energy of 39.8022 kcal/mol than the other likely regioisomer 7 with a higher energy of 51.5377 kcal/mol, this shows that the cycloadduct 5 is more preferred than 7 as described in Fig. 5.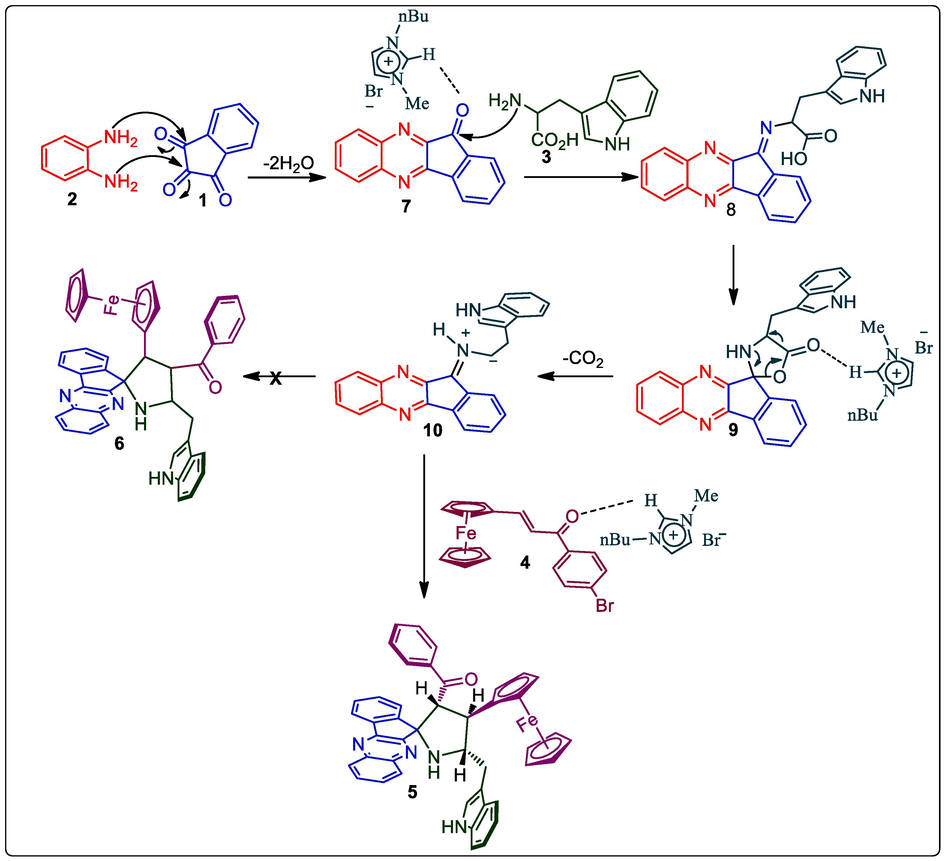
A mechanism for the formation of cycloadduct 5.
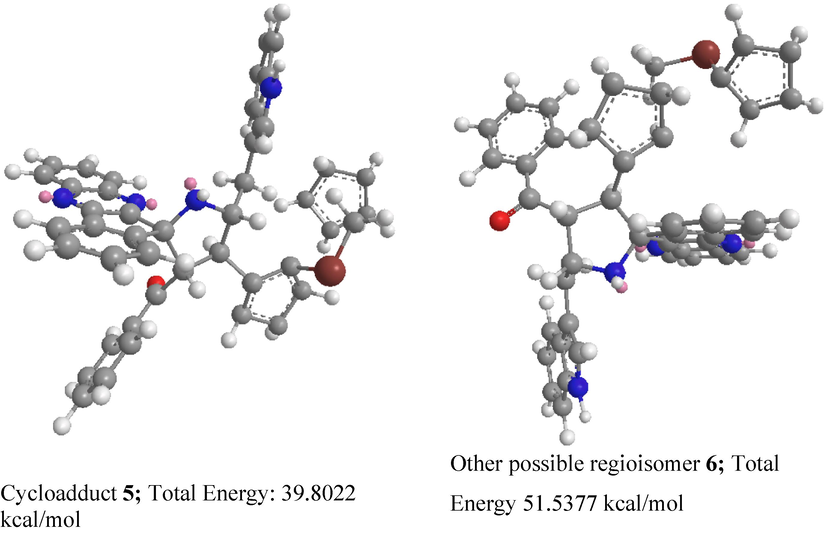
Energy minimization diagram of compound 5.
Cholinesterase inhibitory activity.
3.2 Cholinesterase inhibitory activity
The prepared ferrocene grafted spiroquinoxalinopyrrolidine 5a-j were evaluated cholinesterase inhibitory potency and the results are shown in Table 1. The synthesized compounds 5a-f showed good to moderate AChE inhibitory activity against tested cholinesterase enzymes. Among them, three spiro compounds have showed IC50 values of less than 10 µM; in that compounds IC50 values of 6b (9.50 ± 0.20), 6c (8.81 ± 0.11) and 6d (5.10 ± 0.16 µM) possessing bromo, chloro and fluoro units on the aryl ring exhibited potent activity. Particularly, compound that bearing with fluoro sustituion are most active compound in this series which is a best activity compared to reference standard (IC50 2.09 ± 0.11). Other compounds 5e carrying methyl on the aryl ring exhibited less activity with IC50 value of 22.75 ± 0.18 µM and 26.15 ± 0.10 while other compounds substituted with methoxy unit on the aryl ring displayed very less activity in this series with IC50 value of 26.15 ± 0.10. Likewise, the synthesized spiro cycloadducts exhibited better BChE inhibitory potential with IC50 values from 21.18 ± 0.15 to 30.11 ± 0.15 µM. Compounds 5c (20.02 ± 0.20), 5d (21.18 ± 0.15) and 5b (22.02 ± 0.09) had significant activity against tested BChE activities while compound 5a (28.12 ± 0.25), 5e(29.14 ± 0.17) and 5f (30.11 ± 0.15) have shown moderate to good activity. The most significant activity were observed for the compound 5c (20.02 ± 0.20) bearing chloro on aryl ring. The results revealed that halogenated atoms on the phenyl had significant effect on the inhibitory activities. Over all, the electron withdrawing substituted compounds displayed good activity that has been observed.
Compound
AChE Inhibition
IC50 µM (±SD)
BChE inhibition
IC50 µM (±SD)
AChEa
Selectivity
BChEb
Selectivity
1
24.18 ± 0.20
28.12 ± 0.25
1.16
0.85
2
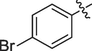
9.50 ± 0.20
22.02 ± 0.09
2.31
0.43
3
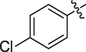
8.81 ± 0.11
20.02 ± 0.20
2.27
0.44
4
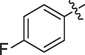
5.10 ± 0.16
21.18 ± 0.15
4.15
0.24
5
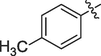
22.75 ± 0.18
29.14 ± 0.17
1.28
0.78
6
26.15 ± 0.10
30.11 ± 0.15
1.15
0.86
11
Galantamine
2.09 ± 0.11
19.34 ± 0.17
9.10
0.23
4 Conclusion
In conclusion, a facile, efficient and eco-friendly protocol for the preparation of spiroquinoxalinopyrrolidine engrafted ferrocene hybrids heterocycles in good to excellent yields. The dipole component generated in situ from the combination of L-tryptophan and quinoxalinone has been comparatively less explored. The formation of Ferrocenyl cycloadduct arose by a [3 + 2] cycloaddition process that created two C–C and three C-N bonds in a single transformation with four adjoining stereogenic bonds that were formed with complete diastereocontrol.The synthesized compounds displayed significant cholinesterase inhibitory activity. Among them, compound thus possessing fluoro on the aryl ring displayed excellent acetyl cholinesterase (5.10 ± 0.16) /butryl cholinesterase (21.18 ± 0.15) inhibitory activity compared to reference standard drug, galatamine.
Acknowledgement
The author extend their appreciation to the Deputyship for Research and Innovation, “Ministry of Education” in Saudi Arabia for funding this research (IFKSUOR3-178-1)
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis of Tricyclic Arylspiro Compounds as Potential Antileukemic and Anticonvulsant Agents. Heterocycles. 1976;12:637-640.
- [Google Scholar]
- A concise asymmetric synthesis of the ADE fragment of nakadomarin A. A Concise Asymmetric Synthesis of the ADE Fragment of Nakadomarin A. Org. Lett.. 2004;6:4539-4541.
- [Google Scholar]
- A One-Pot Multicomponent 1,3-Dipolar Cycloaddition Strategy: Combinatorial Synthesis of Dihydrothiophenone-Engrafted Dispiro Hybrid Heterocycles. ACS Comb. Sci.. 2017;19:308-314.
- [Google Scholar]
- A 1,3-dipolar cycloaddition–annulation protocol for the expedient regio-, stereo- and product-selective construction of novel hybrid heterocycles comprising seven rings and seven contiguous stereocentres. Tetrahedron. Lett.. 2013;54:2515-2519.
- [Google Scholar]
- A 1,3-dipolar cycloaddition–annulation protocol for the expedient regio-, stereo- and product-selective construction of novel hybrid heterocycles comprising seven rings and seven contiguous stereocentres. Tetrahedron. Lett.. 2013;54:2515-2519.
- [Google Scholar]
- Spiropyrrolidine/spiroindolizino[6,7-b]indole heterocyclic hybrids: Stereoselective syn- thesis, cholinesterase inhibitory activity and their molecular docking study. S. Bioorg. Chem.. 2018;79:64-71.
- [Google Scholar]
- Regio- and diastereoselective synthesis of anticancer spirooxindoles derived from tryptophan and histidine via three-component 1,3-dipolar cycloadditions in an ionic liquid. Tetrahedron. 2018;74:5358-5366.
- [Google Scholar]
- Dispiropyrrolidinyl-piperidone embedded indeno[1,2-b]quinoxaline heterocyclic hybrids:Synthesis, cholinesterase inhibitory activity and their molecular docking simulation. Bioorg. Med. Chem.. 2019;27:2621-2628.
- [Google Scholar]
- A facile ionic liquid-accelerated, four-component cascade reaction protocol for the regioselective synthesis of biologically interesting ferrocene engrafted spiropyrrolidine hybrid heterocycles. J. King Saud Univ. Sci.. 2020;32:2500.
- [Google Scholar]
- A stereo, regioselective synthesis and discovery of antimycobaterium tuberculosis activity of novel b-lactam grafted spirooxindolopyrrolidine hybrid heterocycles. Arab. J. Chem.. 2021;14:02938.
- [Google Scholar]
- Arumugam, N., Abdulrahman, A.I., Suresh Kumar, R., Periyasami, G., Dhaifallah M.A., Krishnamoorthy, R., Periasamy, V.S., Ali, A.A., 2018. Mahalingam SM, Shankar T, Menéndez JC. Multicomponent Domino Synthesis, Anticancer Activity and Molecular Modeling Simulation of Complex Dispirooxindolopyrrolidines. Molecules 23,1094.
- Arumugam, N., Abdulrahman, A.I., Suresh Kumar, R., Periyasami, G., Dhaifallah, M.A., Krishnamoorthy, R., Periasamy, V.S., Ali, A.A. Mahalingam SM, Shankar T, Menéndez JC., 2018. Multicomponent Domino Synthesis, Anticancer Activity and Molecular Modeling Simulation of Complex Dispirooxindolopyrrolidines. Molecules 23, 1094.
- KF/Al2O3 mediated 1,3-dipolar cycloaddition of azomethine ylides: A novel and convenient procedure for the synthesis of highly substituted pyrrolidines. Tetrahedron Lett.. 2007;48:4535-4537.
- [Google Scholar]
- Four-membered ring-containing spirocycles: Synthetic strategies and opportunities. Chem. Rev.. 2014;114:8257-8832.
- [Google Scholar]
- One-pot chemo/regio/stereoselective generation of a library of functionalized spiro-oxindoles/pyrrolizines/-pyrrolidines from α-aroylidineketene dithioacetals. RSC Adv.. 2015;5:33705-33719.
- [Google Scholar]
- Stereoselective preparation of 1, 2, 4-oxadiazole derivatives substituted by pentafluorophenyl by 1, 3-dipolar cycloaddition reaction. Tetrahedron. 2006;62(110):08-11011.
- [Google Scholar]
- Design and synthesis of spiro derivatives containing a thiophene ring and evaluation of their anti-microbial activity. Orient. J. Chem.. 2014;30:1619-1630.
- [Google Scholar]
- Cycloaddition Reactions in Organic Synthesis. Weinheim, Germany: Wiley; 2002.
- Oxindole-3-spiropyrrolidines and-piperidines. Synthesis and local anesthetic activity. J. Med. Chem.. 1976;19:892-898.
- [Google Scholar]
- Synthesis and antiviral activities of adamantane spiro compounds. 1. Adamantane and analogous spiro-3’-pyrrolidines. J. Med. Chem.. 1972;15:129-132.
- [Google Scholar]
- Construction of enantiopure pyrrolidine ring system via asymmetric [3+2]-cycloaddition of azomethine ylides. Chem. Rev.. 2006;106:4484-4517.
- [Google Scholar]
- Fundamentals of green chemistry: efficiency in reaction design. Chem. Soc. Rev.. 2012;41:1437-1451.
- [Google Scholar]
- Multicomponent Dipolar Cycloaddition Strategy: Combinatorial Synthesis of Novel Spiro-Tethered Pyrazolo[3,4-b]quinoline Hybrid Heterocycles. ACS Comb. Sci.. 2016;18:262-270.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.103027.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







