Characterization of the cellulolytic enzyme produced by Streptomyces griseorubens (Accession No. AB184139) isolated from Indian soil
*Corresponding author. Tel.: +91 9835412997; fax: +91 22204393 tanujasinghpatna@Yahoo.com (Tanuja Singh), tanujapatnabotany@gmail.com (Tanuja Singh),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 27 March 2013
Abstract
The present study was intended to investigate the effect of different physico-chemical parameters that included pH (3–11), temperature (4–60 °C), incubation period (2–8 days) and NaCl (1–10% w/v) concentration on the growth and cellulase enzyme production of the actinomycete strain St-1, to optimize its enzyme productivity. The strain St-1 isolated locally from soil sample and identified as Streptomyces griseorubens at MTCC and Gene Bank, Chandigarh, India (Accession No. AB184139), showed an optimum growth at pH 7, temperature of 45 °C and 6 days of incubation period. The cellulose hydrolysis by the isolate was also optimum at these parameters when the maximum level of reducing sugar produced due to filter paper activity was 5.6 mg per mL and CMC activity was 4.5 mg per mL. The isolate was moderately halophilic, as it was unable to grow beyond 6% of NaCl (w/v) concentration.
Keywords
Cellulose hydrolysis
CMCase
FPase
Reducing sugar
Cellulose degrading Streptomyces griseorubens
1 Introduction
There has been considerable interest over the past years in the enzymatic degradation of lignocellulosic biomass – a renewable, abundant and inexpensive resource. The reasons for this interest varied and included potential applications in waste treatment, fuel production, oxychemical production, textile industry (Kasana et al., 2008) for biopolishing of fabrics and producing stonewashed look of denims; and more recently, the pulp and paper industry (Noe et al., 1986). Different fungi and bacteria have been used for the production of cellulases using different substrates. Reese and Levinson (1952) after comparing the cellulolytic ability of bacteria and fungi from all the major groups found that fungi have more cellulolytic potential than bacteria. However, for a successful fermentation process, it is necessary to cultivate the microorganism on a large scale for overproduction of the desired metabolite as reported by Gautam et al. (2011). Large-scale cultivation and strain improvement by genetic manipulation in prokaryotes have focused the attention toward isolation of newer lignocellulose-degrading prokaryotes, and the actinomycetes are no exception. Jang and Chen (2003), reported that actinomycetes are potential cellulase-producers and help considerably in recycling nutrients in the biosphere and are thought to be involved in the primary degradation of organic matter in compost and related materials (Goodfellow and Williams, 1983). Since cellulose is the major component of plant biomass and potentially utilizable source of glucose, therefore, the process of microbial degradation of cellulose can be considered as financially viable and seems to be the wise choice. Actinomycete cellulases are inducible extracellular enzymes (Ibrahim and El-diwany, 2007) that can be produced during their growth on cellulosic materials. Thus, introduction of cellulolytic microorganisms is a beneficial microbiological tool for recovery of bioenergy from degraded cellulose (Balamurugan et al., 2011). In this pretext, the objectives of the present study were to isolate some actinomycete strains, screen them for their cellulolytic potential and optimize the physico-chemical parameters to maximize the yield of cellulase enzyme that can be utilized on commercial scale. In the course of screening for industrially important cellulolytic actinomycetes, several aerobic strains were isolated from different localities of Patna (Bihar), India. One promising cellulose degrading actinomycete, designated strain St-1 was selected for the present study to find out the optimum conditions for its growth and enzyme activities.
2 Materials and methods
2.1 Chemicals
Chemicals used for the preparation of the media were of highest purity grade and were obtained from HiMedia, Loba Chemie, Merck and Qualigens.
2.2 Media
Media used during the course of the present investigation, were prepared in distilled water and unless otherwise mentioned, were sterilized by autoclaving at 15 psi for 15 min. Starch Casein Agar (Soluble starch 10 gL−1, Casein 0.3 gL−1, K2HPO4 2 gL−1, CaCO3 0.02 gL−1, FeSO47H2O 0.01 gL−1, KNO3 2 gL−1, MgSO4·7H2O 0.05 gL−1, NaCl 2 gL−1, Agar 18 gL−1) and Nutrient Agar (Peptone 5 gL−1, Beef extract 3 gL−1, Sodium chloride 5 gL−1, Agar 15 gL−1) (Dubey and Maheshwari, 2002) were used for isolation and preservation of the actinomycete strains, respectively. CMC Agar (carboxymethylcellulose 0.5 gL−1, NaNO3 0.1 gL−1, K2HPO4 0.1 gL−1, MgSO4 0.05 gL−1, yeast extract 0.05 gL−1, Agar 15 gL−1) (Kasana et al., 2008) was used for cellulose degrading efficiency test; and Citrate buffer (pH 6), 0.55% CMC in citrate buffer and Dinitrosalicylic acid reagent for cellulase assay.
2.3 Microorganisms
Cellulose degrading actinomycete strain designated as St-1, isolated from municipal wastes from Patna, India, on Starch Casein Agar by direct plating of six fold serial dilutions of the soil samples in sterilized normal saline (0.85%), was selected for the present study. The selection was based on the extent of production of clear zones on CMC Agar when treated with 1% Congo red dye (w/v) followed by 1 N HCl for 15–20 min. The isolate was maintained on slants of Nutrient Agar at 4 °C with periodic sub culturing. For the characterization of the selected isolate, the basic routine laboratory works like morphological, cultural and different biochemical characteristics which included Indole, methyl red, Voges–Proskauer, citrate utilization, catalase, urease, starch hydrolysis, gelatin hydrolysis, sugar fermentation, caseinase, hydrogen sulfide production and nitrate reduction tests were performed (Cappuccino and Sherman, 2005) and compared with Bergey’s Manual of Systematic Bacteriology (Williams et al., 1989). 48 h old cultures were used for all the tests.
2.4 Effect of pH, temperature and NaCl concentration on growth of the isolate
An investigation was carried out to determine the optimum pH and temperature for the isolate for its maximum growth. Five plates of CMC Agar medium each with pH adjusted to 3, 5, 7, 9 and 11, respectively, using 1 N HCl and 1 N NaOH were inoculated with the isolate and incubated at 26 °C for 4 days. Likewise, eight sets of CMC Agar medium each with pH adjusted to 7 were inoculated and incubated at different temperatures that included 4, 15, 26, 37, 45, 50, 55 and 60 °C for 4 days. The effect of NaCl concentrations ranging between 1 and 10% (w/v) on growth of the isolate was investigated by plating the isolate on Nutrient Agar with specified NaCl concentrations and incubating the plates at 26 °C for 4 days. The tests were performed in triplicates and the observations were recorded.
2.5 Enzyme assay
CMC-ase activity and FP-ase activity were assayed using the method described by Wood and Bhat (1998) with slight modifications. For CMC-ase activity, 0.5 mL of enzyme solution was added to 0.5 mL of 0.55% (w/v) of carboxymethylcellulose prepared in sodium citrate buffer (pH 6) and incubated at 50 °C for 60 min. For FP-ase activity, 0.5 mL of enzyme solution was added to 0.5 mL of sodium citrate buffer (pH 6) along with 50 mg Whatman No. 1 filter paper strip (1 × 6 cm) and incubated at 50 °C for 60 min. In both the above mentioned procedures, the reactions were stopped by adding 1.0 mL of 3, 5-dinitro salicylic acid reagent to the mixtures followed by boiling for 10 min and adding 8.0 mL of distilled water. The Optical Density of the mixtures was recorded at 540 nm using a UV/Vis spectrophotometer (Thermo Scientific) and compared with the standard glucose curve to determine the amount of reducing sugar (mg mL−1) produced during cellulose hydrolysis.
2.6 Optimization of temperature for the cellulolytic activity of the isolate
Three 150 mL conical flasks each containing 50 mL of CMC broth were inoculated with the isolate and incubated at 26, 37 and 45 °C for 8 days. After incubation, 10 mL of each culture broth was withdrawn at 2 days interval, centrifuged at 5000 rpm for 20 min at 4 °C and supernatant, serving as the source of crude enzyme, was utilized for the determination of enzyme activities (Immanual et al., 2006).
2.7 Optimization of pH for the cellulolytic activity of the isolate
Four 150 mL conical flasks each containing 50 mL of CMC broth with the pH adjusted to 5, 7, 9 and 11 respectively using 1 N HCl and 1 N NaOH were inoculated with the isolate and incubated for 8 days at the optimum temperature determined at the previous step. After incubation the methods are similar as described above.
3 Results
3.1 Isolation and screening of cellulose degrading actinomycetes
Isolation of strains on plates containing Starch Casein Agar resulted in 42 isolates from seven different soil and compost samples. They were tested for the production of cellulolytic enzyme on CMC Agar plates. Congo red staining of these plates along with 1 N HCl revealed 15 isolates producing clear zones on the plates around the colonies indicating cellulose hydrolysis. Among the 15 isolates, strain St-1 was selected for further investigation on the basis of the area of clear zone.
3.2 Cultural and biochemical characteristics of the selected isolate
St-1 formed a white, powder with radiating margins on the Nutrient Agar medium (Fig. 1). Gram’s staining and microscopic view revealed a gram-positive, filamentous structure (Fig. 2). The specified biochemical tests performed on the isolate showed the positive results for amylase, catalase, sucrose fermentation accompanied with gas formation and Voges–Proskauer tests. Referring to Bergey’s Manual of Systematic Bacteriology (Williams et al., 1989), the strain St-1 was identified up to the genus level as Streptomyces species which was confirmed, on the basis of 16S rRNA sequencing, as Streptomyces griseorubens at Microbial Type Culture Collection and Gene Bank, Institute of Microbial Technology, Chandigarh, India with an Accession No. AB184139. The phylogenetic Neighbor-Joining tree (Fig. 3) was constructed for strain St-1 using an almost complete 16S rRNA gene sequence (1411 bp).
- St-1 (S. griseorubens) on Nutrient Agar.
- Microscopic view of St-1 (S. griseorubens).
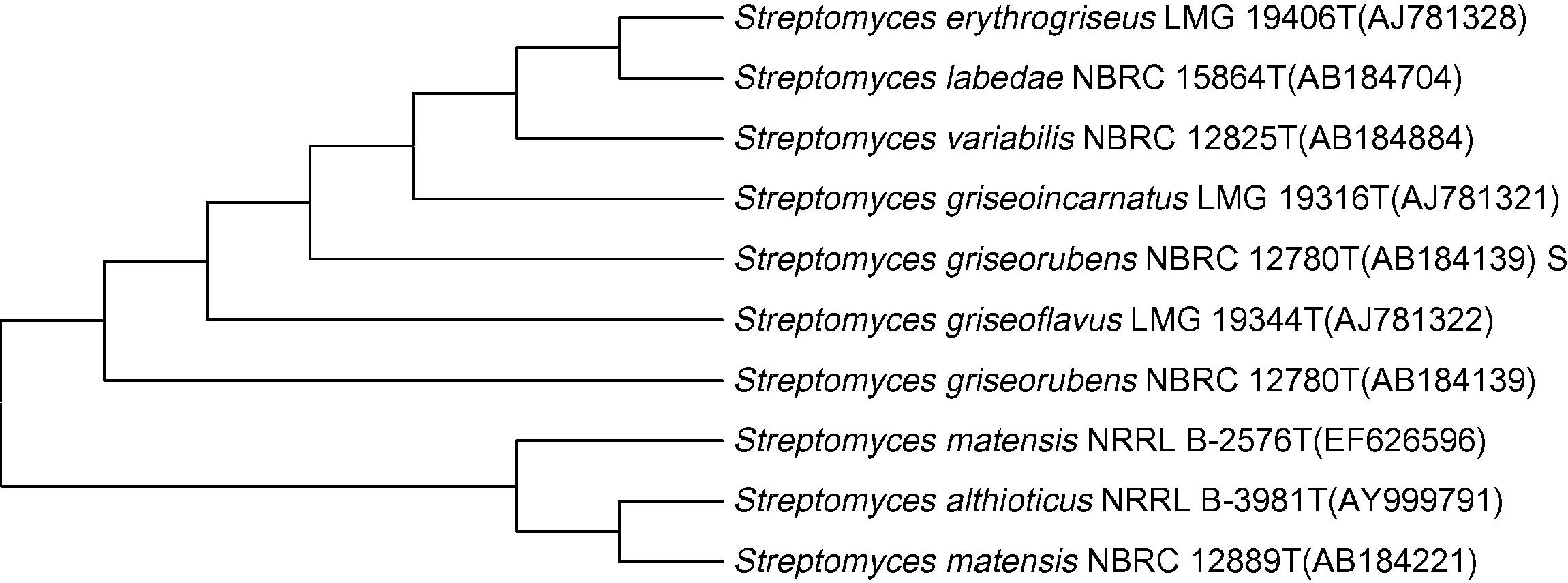
- Evolutionary relationships of taxa. The evolutionary history was inferred using the Neighbor-Joining method (Saitou and Nei, 1987). The optimal tree with the sum of branch length = 0.60185941 is shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method (Tamura et al., 2004) and are in the units of the number of base substitutions per site. The analysis involved 40 nucleotide sequences. Codon positions included were 1st + 2nd + 3rd + Noncoding. All positions containing gaps and missing data were eliminated. There were a total of 1274 positions in the final dataset. Evolutionary analyses were conducted in MEGA5 (Tamura et al., 2011).
3.3 Effect of pH, temperature and NaCl concentration on growth of the isolate
To characterize the growth conditions, the selected isolate was allowed to grow at the specified pH (3–11), temperature (4–60 °C) and NaCl concentrations (1–10%). The observations revealed that St-1 (S. griseorubens) could grow well in the slightly acidic to highly alkaline medium indicating its wide pH tolerant adaptability. It was also found that the strain was able to survive at a wide range of temperatures (4–45 °C), though the growth was restricted at higher temperatures from 50 °C onward. Regarding the effect of different NaCl concentrations, the isolate was found to be moderately halophilic as it did not respond very positively to increasing salinity after 6% NaCl concentration. The Indian soil varies from acidic to alkaline through neutral. The investigation on the salt tolerance of the strain would definitely help in finding out its adaptability to various kinds of soil, prevalent in India, as its habitat. The data are presented in Table 1.
| pH | Growth | Temp. (°C) | Growth | NaCl (%) | Growth |
|---|---|---|---|---|---|
| 3 | − | 4 | +++ | 1 | +++ |
| 5 | +++ | 15 | +++ | 2 | ++ |
| 7 | +++ | 26 | +++ | 3 | ++ |
| 9 | +++ | 37 | +++ | 4 | ++ |
| 11 | +++ | 45 | +++ | 5 | ++ |
| 50 | − | 6 | ++ | ||
| 55 | − | 7 | − | ||
| 60 | − | 8 | − | ||
| 9 | − | ||||
| 10 | − |
− = No growth, ++=moderate growth, +++=luxuriant growth, initial medium pH 7.
3.4 Optimization of temperature for the cellulolytic activity of the isolate
In the present investigation the Optical Density of the strain St-1 (S. griseorubens) was recorded at temperatures 26, 37 and 45 °C on 2nd, 4th, 6th and 8th day of incubation for the CMC and the filter paper activities. The observations obtained were compared with the standard glucose curve to determine the amount of reducing sugar (mg mL−1) released. The data are provided in Figs. 4 and 5. Highest enzymatic activity was obtained at 45 °C and on 6th day of incubation (Figs. 4 and 5).
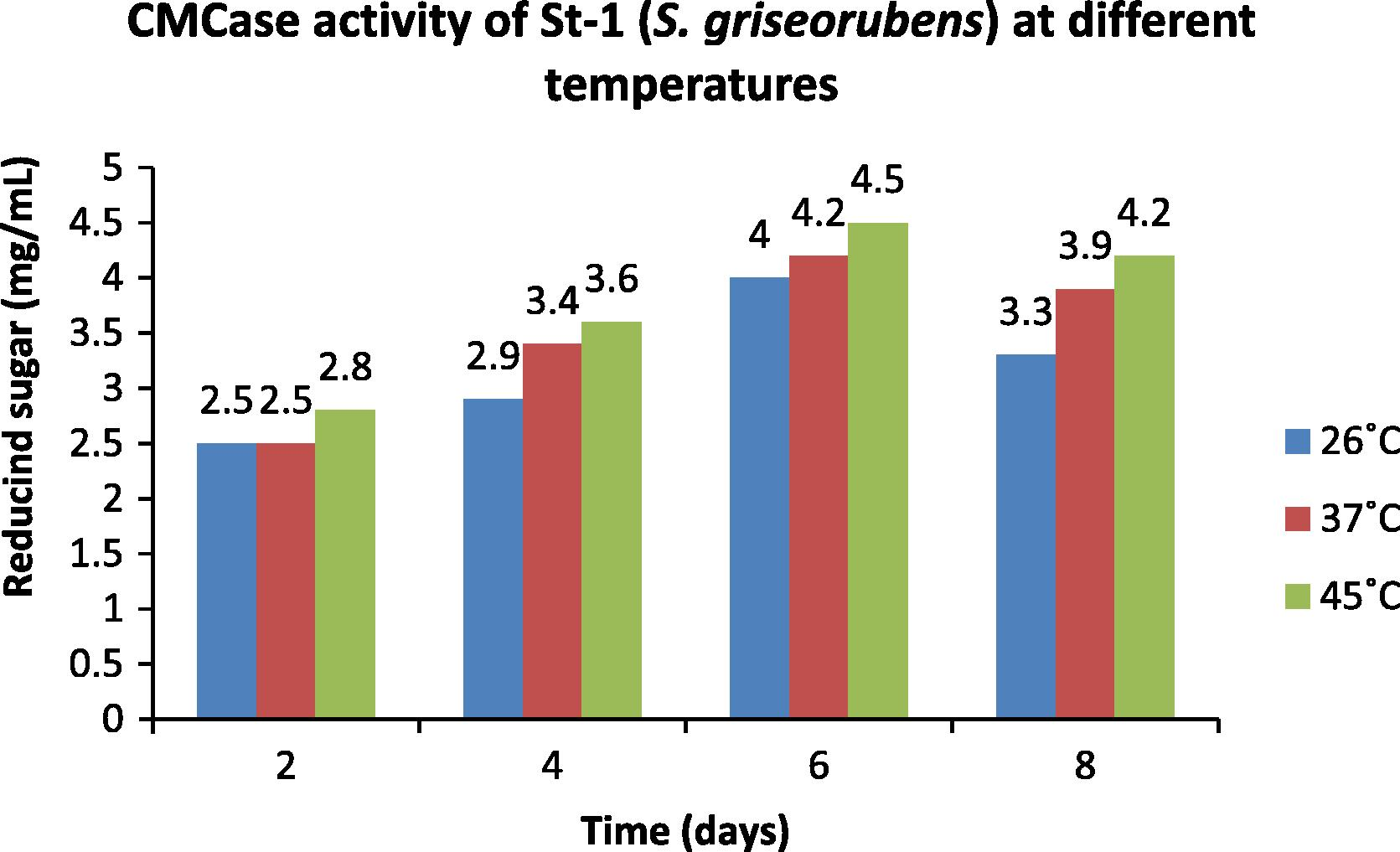
- Optimization of temperature for the CMCase activity of St-1 (S. griseorubens).
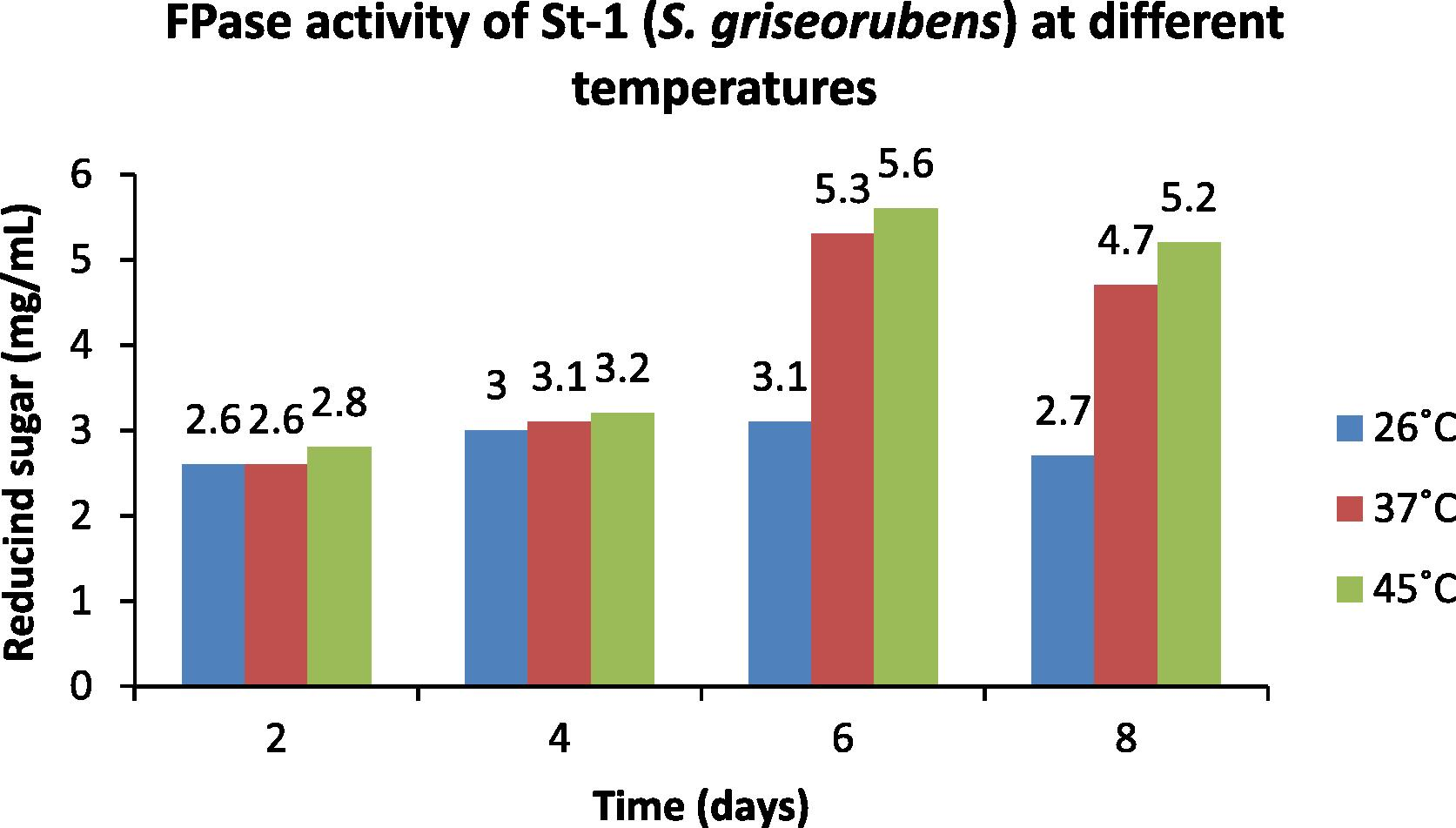
- Optimization of temperature for the FPase activity of St-1 (S. griseorubens).
3.5 Optimization of pH for the cellulolytic activity of the isolate
After incubating at 45 °C, the crude enzymes produced at different pH (5, 7, 9 and 11) were collected at an interval of two days and were assayed for their CMC and filter paper activities. The data obtained were compared with the standard glucose curve and the amount of reducing sugar (mg mL−1) was estimated for the respective pH (Figs. 6 and 7). Highest enzymatic activity was obtained at pH 7 and on the 6th day of incubation (Figs. 6 and 7).
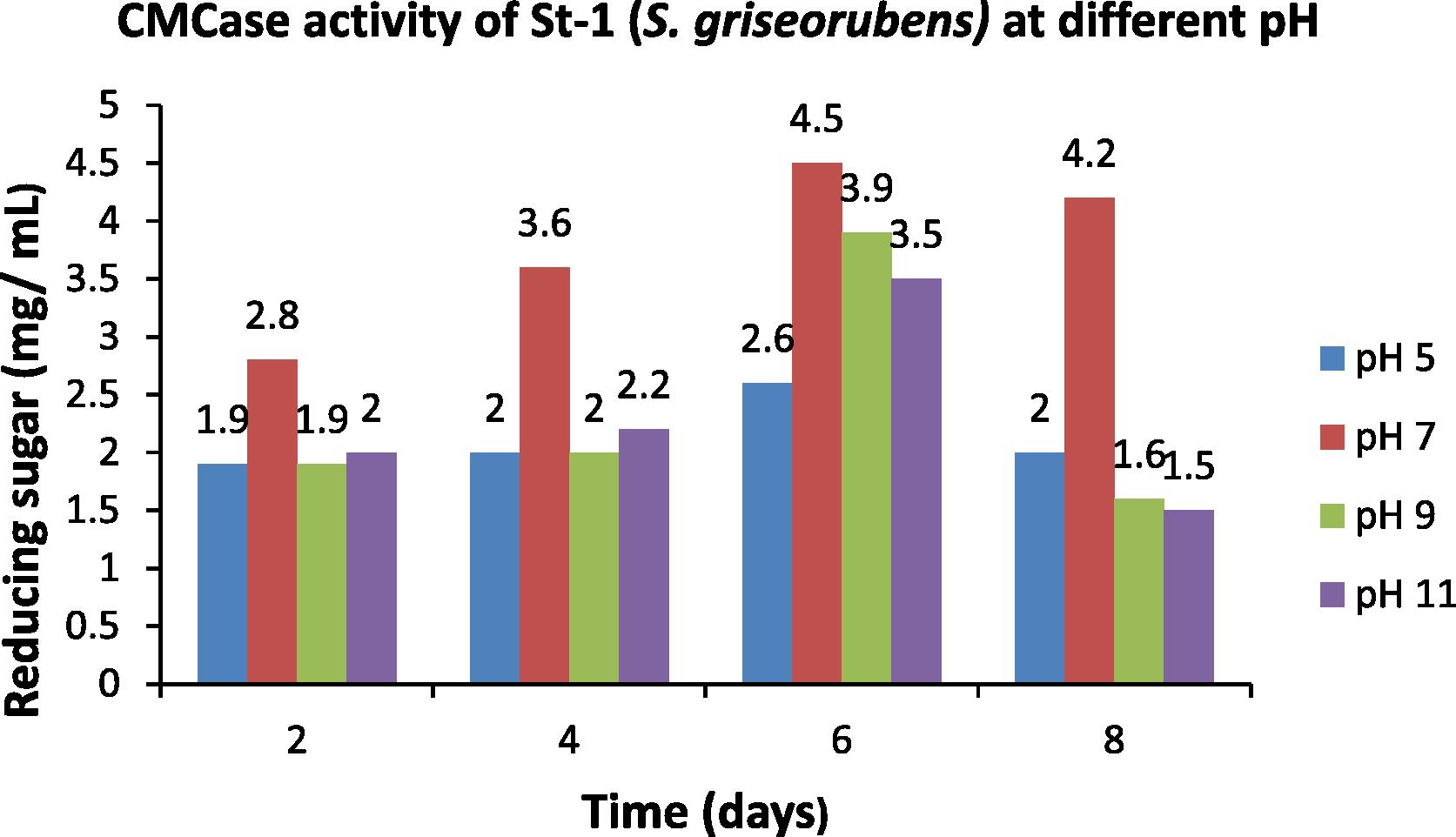
- Optimization of pH for the CMCase activity of St-1 (S. griseorubens).
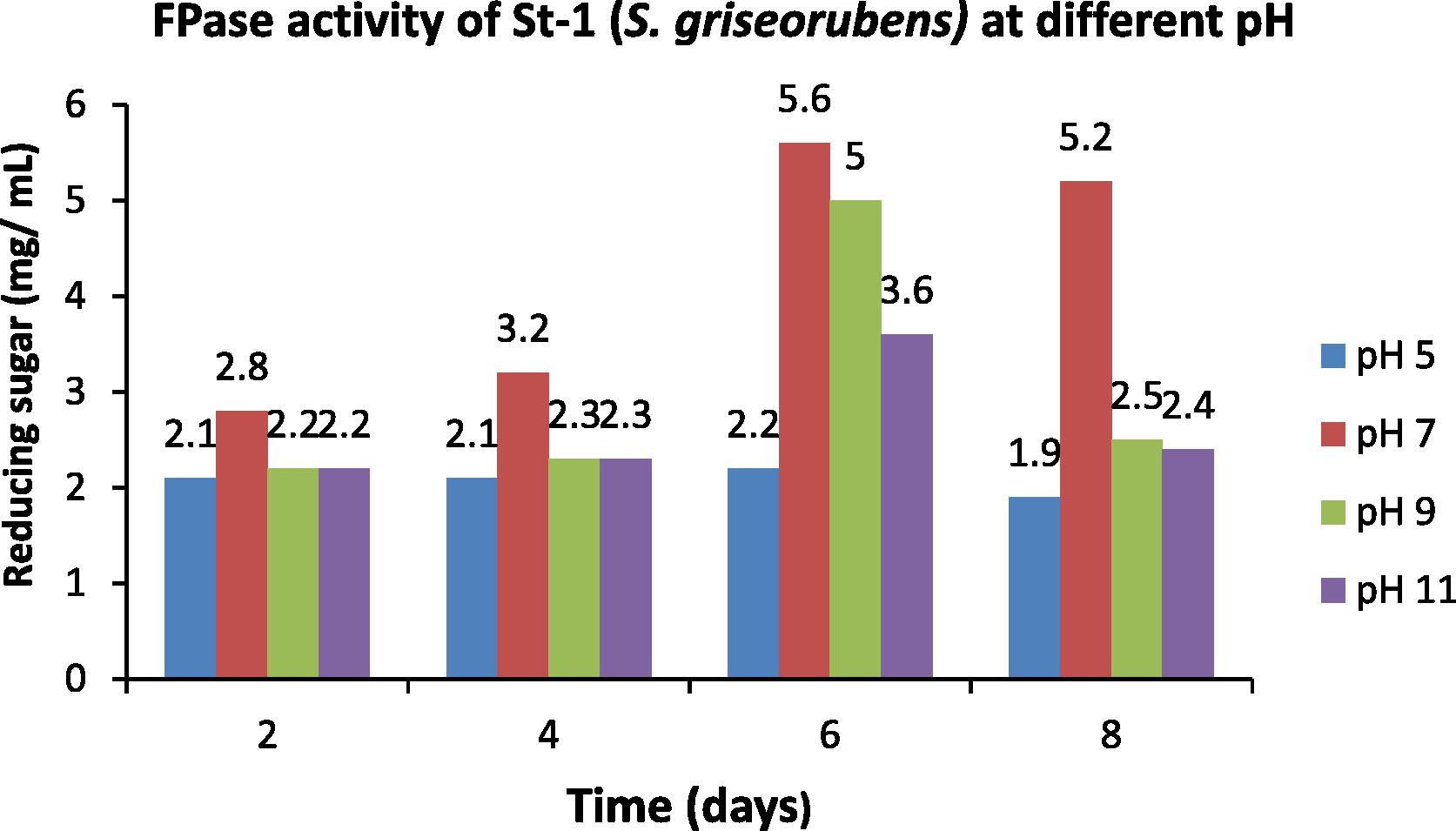
- Optimization of pH for the FPase activity of St-1 (S. griseorubens).
4 Discussion
As stated by Vinogradova and Kushnir (2003), the actinomycetes are one of the important microbial communities responsible for cellulose degradation found abundantly in the plant cell walls. The effect of environmental factors such as temperature, pH and salinity are found to be important parameters that influence enzyme activities. According to Tholudur et al. (1999), cellulase production in cultures is growth associated and is influenced by various factors and their interactions can affect cellulase productivity. The present study indicated that the isolate was capable of growing on a wide range of temperature (4–45 °C) and pH (5–11) as shown in Table 1. However, the isolate was intolerant to high salinity and the growth was restricted at 7% salinity of the growth medium (Table 1). It was also investigated that the maximum cellulase activity of St-1 (S.griseorubens) occurred at 45 °C (Figs. 4 and 5) which is in accordance with the findings of McCarthy (1987) who reported that an optimal temperature for cellulase activity is in the range of 40–55 °C for several Streptomyces species including Streptomyces lividans, Streptomyces flavogrisus, and Streptomyces nitrosporus. Cellulase enzyme from St-1 (S. griseorubens) was found active over a pH range of 5–11 with maximum activity at pH 7 (Figs. 6 and 7). This result differed from that of Theberge et al. (1992) who showed that the optimum pH for endoglucanase from a strain of S. lividans was 5.5. However, Jaradat et al. (2008) reported that cellulase enzyme from Streptomyces sp. (strain J2) was active over a pH range of 4–7 with maximum activity at pH 6. It was also found that for the first six days there was a gradual increase in the production of cellulase enzyme by the isolate and thereafter a decrease was registered in cellulase production (Figs. 4–7). This result was supported by the findings of Mandels and Weber (1969); and Howell and Mangat (1978). Van Dycke (1972) also reported that the end product acts as inhibitor to the process of cellulose hydrolysis and the decline in hydrolysis are the early removal of more assessable amorphous cellulose, resulting in an increase in the proportion of more resistant crystalline cellulose. The present study is a preliminary characterization of cellulase activities of the strain among the 42 cellulose degrading microorganisms isolated from soil samples of different locations. Once the screening of strains with potential cellulolytic activities is completed and the efficient ones have been selected, then a detailed study is intended to be conducted on endoglucanase, exoglucanase and betaglucosidase activities of the cellulase enzyme.
5 Conclusion
It may be concluded that the adaptability of the isolate St-1 (S. griseorubens) to a wide range of temperatures and pH for its growth and cellulolytic activity, strongly indicates that this actinomycete may be grown in various habitats with different environmental conditions of pH and temperature and can play an important role in cellulose degradation for sustainable development. It will not only help in the production of useful end products including bioethanol from the biodegradation of the low cost enormous stock of cellulose but also help in the disposal of cellulosic wastes which is continuously added to the environment through the process of photosynthesis.
Acknowledgements
We are grateful to the DST, Ministry of Science and Technology, Govt. of India for special allocation under the WOS-A scheme (File no.: SR/WOS- A/LS-290/2011). This study was carried out at the Department of Industrial Microbiology, Patna Women’s College, Patna University, Patna, India and the authors thank Dr. (Sister) Doris D’Souza, A.C., Principal, Patna Women’s College for providing necessary infrastructural facilities.
References
- Studies on cellulose degrading bacteria in tea garden soils. Afr. J. Plant Sci.. 2011;5(1):22-27.
- [Google Scholar]
- Microbiology A Laboratory Manual (sixth ed.). Pearson Education; 2005.
- Practical Microbiology (first ed.). New Delhi: S. Chand & Company Ltd.; 2002.
- Optimization for the production of cellulase enzyme from municipal solid waste residue by two novel cellulolytic fungi. Biotechnol. Res. Int. 2011:810425.
- [CrossRef] [Google Scholar]
- Enzyme deactivation during cellulose hydrolysis. Biotechnol. Bioeng.. 1978;20:847-863.
- [Google Scholar]
- Isolation and identification of new cellulase producing thermophilic bacteria from an Egyptian hot spring and some properties of the crude enzyme. Aust. J. Basic Appl. Sci.. 2007;1:473-478.
- [Google Scholar]
- Effect of different growth parameters on endoglucanase enzyme activity by bacteria isolated from coir retting effluents of estuarine environment. Int. J. Environ. Sci. Technol.. 2006;3:25-34.
- [Google Scholar]
- Production and characterization of thermostable cellulases from Streptomyces transformant T3–1. World J. Microbiol. Biotechnol.. 2003;19:263-268.
- [Google Scholar]
- Influence of culture conditions on cellulose production by Streptomyces sp. (Strain J2) Jordan J. Biol. Sci.. 2008;1:141-146.
- [Google Scholar]
- A rapid and easy method for the detection of microbial cellulases on Agar plates using gram’s iodine. Curr. Microbiol.. 2008;55:503-507.
- [Google Scholar]
- Adv. Chem. Ser.. 1969;95:391-414.
- Action of xylanases on chemical pulp fibers. Part II enzymatic beating. J. Wood Chem. Technol.. 1986;6:167-184.
- [Google Scholar]
- A comparative study of the breakdown of cellulose by microorganisms. Physiol. Plant. 1952;5:345-366.
- [Google Scholar]
- The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mole. Biol. Evol.. 1987;4:406-425.
- [Google Scholar]
- Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. (USA). 2004;101:11030-11035.
- [Google Scholar]
- Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., Kumar, S., In Press MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. molecular biology and evolution.
- Purification and characterization of an endoglucanase from Streptomyces lavidans 66 and DNA sequence of the gene. Appl. Microbiol.. 1992;58:815-820.
- [Google Scholar]
- Mathematical modeling and optimization of cellulase protein production using Trichoderma reesei RL-P37. Biotechnol. Bioeng.. 1999;66:1-16.
- [Google Scholar]
- Van Dycke, 1972. Enzymatic hydrolysis of cellulose – a kinetic study. Ph.D. Thesis. MIT. Cambridge. MA.
- Biosynthesis of hydrolytic enzymes during cocultivation of macro- and micromycetes. Appl. Biochem. Microbiol.. 2003;39:573-575.
- [Google Scholar]
- Williams, S. T., Sharpe, E. M., Holt, J. G., 1989. Bergey’s Manual of Systematic Bacteriology, first ed. vol. 4. Springer, New York.
- Method for Measuring Cellulase Activities. In: Wood W.A., Kellogg J.A., eds. Methods in Enzymology Cellulose and Hemicellulose. New York: Academic Press; 1998. p. :87-112.
- [Google Scholar]







