Translate this page into:
Characterization of fenugreek and its natural compounds targeting AKT-1 protein in cancer: Pharmacophore, virtual screening, and MD simulation techniques
⁎Corresponding author. azkhan@ksu.edu.sa (Azmat Ali Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Cancer is the second biggest cause of death in humans. Rapid advancements in clinical bioinformatics and high-throughput proteomics technology are essential tools and methodologies for cancer research. Medicinal plants are considered operative therapeutic agents that are utilized for novel drug discovery and development, using computer-aided drug design (CAAD). The AKT-1 Serine/Threonine Kinase family was chosen as the target protein because it is implicated in the mediation of the cancer process. In this work, phytocompounds that target AKT-1 in cancer were recognized and were used to identify their binding relationship with the nominated receptor.
Methods
A dried extraction of Fenugreek (Trigonella foenum-graecum) seeds in solid form was obtained using an evaporation technique and phytochemicals present in it were tested using qualitative tests. Fenugreek and its natural components were evaluated against AKT-1 because chemical agents acquired from natural sources appear to be particularly appealing alternatives to synthetic compounds. Different bioinformatics computational methods were used for protein structural analysis, interactive comparison, alignment, and superposition of AKT-1 protein structures in 3D. By using molecular docking and molecular dynamic (MD) simulation experiments, the binding interaction of the selected compounds with AKT-1 was investigated.
Results
Fenugreek seeds yielded by phytochemicals screening and crude extractions of tannins, saponin, steroids, and cardiac glycosides were found to have very high antioxidant activity. After virtual screening hits, the top three phytocompounds (Compound-1 tannins, Compound-2 saponins, and Compound-3 steroids) were selected against AKT-1 on the basis of their strong binding energy (−10.9 to −9.2 Kcal/mol). Compound-1 showed the highest pharmacophore fit score (43.91) among all compounds. In molecular interactions, some crucial residues (Gln-79, Trp-80, Thr-82, Val-270, Tyr-272, Asp-274, and Asp-292) were observed at binding pockets of AKT-1. MD simulations at 100 ns revealed that the ligand was bound to the protein with some major conformational changes in the protein structure.
Conclusions
In-silico studies report new dimensions for novel drug design targeting AKT-1. The three leading hits inhibit the cancer cell's inflammation activity in the AKT-1 pathway. In the future, these three compounds might be tested for the drug discovery of inflammatory disorders.
Keywords
Docking analyses
AutoDock Vina
AKT serine/threonine kinase
Virtual screening
Pharmacophore modeling
Anticancer
1 Introduction
Cancer is a group of conditions characterized by uncontrolled cell divisions that can invade or spread to other parts of the body (Chaudhuri et al., 2018). When compared to the proliferation of normal cells, malignant cells have the potential to infect other tissues, disrupt the cell's normal function, and grow in an erratic pattern (Chen et al., 2020a). Cancer is the second leading cause of death in the biosphere. It is on the rise in developing countries due to greater usage of carcinogenic activities, particularly smoking (Thun et al., 2010).
AKT-1 (serine/threonine-protein kinase) is a sub-clade of the AKT family that is frequently involved in the modulation of tumor angiogenesis and cancer metastasis (Bellacosa et al., 2005). It plays a vital role in the cancer pathway organized by the P13K gene (Hoxhaj and Manning, 2020). AKT-1 proteins regulate several functions related to cell growth, proliferation, metabolism, cell survival, and angiogenesis (Hoxhaj and Manning, 2020). In recent studies, some somatic mutations in AKT-1 (E17K) have been discovered in different types of cancers, including lung, colorectal and ovarian cancer (Chen et al., 2020c). These mutations in AKT-1 are reported to occur in a tissue-specific region having general and clinical implications (Schneider et al., 2017). AKT-1 mutations (E17K) have been reported in 1.4–8% of breast cancer patients. Mutation studies in different cancers are important in the inhibition of cancerous cells (Rashid et al., 2017). AKT-1 is a prominent protein, and mutations of this sort are strongly regarded as the therapeutic target (Kumar et al., 2019). Inhibition of AKT-1 activity can be used as a therapeutic target to reduce carcinogenesis in the early stages of cancer (Crowell et al., 2007). Despite the notion that targeting AKT-1 would be a useful tool for treating cancer, the inconsistent effect of AKT-1 in advanced cancer stages must also be addressed in cancer therapy (Collins and Workman, 2006).
Herbs are natural substances that work well with our biological systems, while modern medicine converts them to create synthetic and man-made molecules (Topliss et al., 2002). People who practice herbal medicine often tell us that the right combination of medicinal ingredients, not just one component, makes the combination of herbs work (Krishnaiah et al., 2007). For people who can't take medications or have bad reactions to them, herbal treatments may be easier to accept (Ige et al., 2012). Natural treatments address the source of the symptomatology in certain disorders, while medications only treat the symptoms. Synthetic treatments are considered to have a negative effects, which can range from minor to severe (Liu et al., 2022; Wojcikowski et al., 2006). They can be excessively harsh on a person's skin, hair, teeth, and other internal organs, and can then damage other parts of the body (Fietta et al., 2009). Earlier researcher (Tihelka, 2018), find that improvement is slower but healthier if individuals use natural compounds and treatment. One of the main reasons is that; unlike other medicine-making drugs, they do not consist of a single highly refined ingredient. Big Pharma cannot patent plants or natural substances, but it can, if it can find the active ingredient in these plants, purify it and test its efficacy for FDA approval (Firenzuoli and Gori, 2007). Toxicity is sometimes an issue when a natural substance is artificially processed, and the dose is frequently important to safety. Medicinal herbs are employed with the utmost care, frequently extracting not just one active molecule but a slew of additional synergistic chemicals that tend to increase the impact, and the main compound is less concentrated than what pharma firms aim to generate (Kanda et al., 1998). When compared to using merely a refined medicine, one might frequently end up with a considerably less toxic pharmaceutical that is even more effective.
Plants and their compounds are used extensively in traditional medicine. According to current studies, two-thirds to three-quarters of the global population is dependent on medicinal plants for the therapy of various disorders, including cancer. The interest of the global population is increasing day by day in studying phytomedicines and their active biological functions and properties. Phytochemicals, which are bioactive non-nutrient molecules found in fruits, vegetables, and other plant-based foods, have been linked to a lower risk of major chronic diseases. Plants contain approximately 25,000 terpenoids, 12,000 alkaloids, and 8,000 phenolics, according to estimates (Radulović et al., 2013). To better understand the role of phytochemicals on health effects, a variety of critical phytochemicals such as flavonoids, chlorogenic acids, alkaloids, carotenoids, minerals, and hazardous compounds may be investigated (Grabacka et al., 2014). New evidence suggests that the benefits of phytochemicals in vegetables and fruit may be even greater than previously thought, because antioxidants can help to reduce oxidative stress caused by free radicals, which are implicated in the etiology of a wide range of chronic illnesses (Khan et al., 2021; Khizar et al., 2020). However, a significant portion remains unidentified and has to be identified and measured before their health risks can be assessed. Fenugreek (Trigonella foenum-graecum Linn.) is a short-lived medicinal plant that is widely used as a herb, food, flavoring, and conventional medicine all over the world. It is an ancient medicinal herb, and its health benefits have been described in Ayurveda and traditional Chinese medicine. The anticancer potential of fenugreek has been examined in both in-vivo and in-vitro investigations (El Bairi et al., 2017; Kaviarasan et al., 2006; Mohamadi et al., 2018).
Bioinformatics approaches are being used to tackle biological problems (Li et al., 2020; Zafar et al., 2021), and many inhibitors against cancer and neurological illnesses have been reported using these approaches (Fellner et al., 2018). Computational methods are also used to determine the efficacy of plant extracts in the treatment of rheumatoid arthritis, inflammation, liver injury, and diabetes (Wang et al., 2020). In-silico approaches are being used to resolve the natural complexities of biological systems, and have been found to be effective in solving biological challenges related to cancer (Lavecchia and Cerchia, 2016). These techniques have led to the discovery of many cancer inhibitors (Dali et al., 2019; Zafar et al., 2020) and have produced a number of novel anticancer drugs (Dincel and Guzeldemirci, 2020). These approaches may deliver a dependable research framework that could support to develop a novel drug targeting cancer.
The 3D structure of AKT-1 has not been disclosed in the PDB library as a 3D model. The research began with the prediction of a three-dimensional structure for structural and functional assessments of the target protein. We wanted to recognize the binding interaction of the selected compounds with the nominated receptor viz., AKT-1. Present work identifies novel phytocompounds against cancer-targeting AKT-1. 3D structure of AKT-1 was generated by different structure prediction tools. We aimed to study the binding interaction of the selected compounds against the AKT-1 by utilizing the structure modeling, molecular docking analyses, plant compounds, ADMET properties, and simulation studies.
2 Materials and methods
2.1 Plant materials
The fenugreek seeds were collected at random and were washed and dried at room temperature for the best results. The 75 g powder was mixed with a methanol solution and shaken on an orbital shaker for seven days before being filtered to get an aqueous filtrate. Using an evaporation technique, the methanol solution was evaporated to produce a dried extraction of fenugreek seeds in solid form for quantitative measurements.
2.2 Phytochemical screening
The content of tannins, flobatannins, saponins, steroids, and terpenoids in fenugreek seeds was determined using traditional methods. Tannins were isolated from powdered dried fenugreek seeds that were then cooked in 20 ml of water before being filtered. Following filtration, a few drops of 0.1 percent FeCl3 were added, resulting in a brown-green color. A 0.5 g sample was cooked with 1 percent aqueous HCl to identify flobatonins, with a crimson precipitate indicating the presence of flobatonins. A water bath was used to collect 2 g of the sample, which was then heated in distilled water to detect saponin (20 ml). After that, 5 ml of distilled water was added to 10 ml of filtrate and the mixture was shaken vigorously to generate a stable foam. The olive oil drops were then added to the foaming mixture and mixed quickly to form an emulsion. To the steroid screening test tube was added 0.5 g of ethanol extract of powder together with 2 ml of H2SO4 and 2 ml of acetic anhydride. The change of color from violet to green, indicates the presence of steroids. To determine terpenoids, 5 ml of each extract was placed in a test tube and mixed gently with 2 ml of chloroform and 3 ml of concentrated sulfuric acid to produce a layer. Reddish-brown staining of the interface suggests the presence of terpenoids.
2.3 DPPH analyses
The antioxidant ability of fenugreek seeds to scavenge free radicals was determined using the DPPH radical scavenging assay, with slight modifications (Rahman et al., 2015). Briefly, 0.5 mM of 2,2-diphenyl-1-picrylhydrazyl (DPPH) in methanol was mixed with 0.5 ml extract and 3 ml methanol. The reaction mixture was vortexed and incubated at RT for 30 min. The absorbance of the reaction mixture was measured at 517 nm. Butylated hydroxytoluene (BHT), a synthetic antioxidant, was used as a positive control. The antioxidant activity was calculated as a percentage of DPPH radical scavenging activity.
2.4 Structure prediction
The amino acid sequence of the targeted human protein AKT-1 was obtained from the UniProt database in FASTA format. The protein was subjected to BLASTp against the Protein DataBank (PDB) (www.wwpdb.org), to recognize the suitable templates. The SWISS model was utilized to predict the 3D structures of proteins. ITASSER was also used to predict the 3D structures and cross-validate them. Numerous online structure validation tools were used, such as ERRAT, Verify3D, and Rampage, to evaluate the 3D structures of proteins. The 3D structures of the final selected compounds with targeted protein were minimized by using Chimera. Protein secondary structure elements (PSSE) were determined during simulation studies.
2.5 Docking analysis
A library of natural compounds from the Asinex database (https://www.asinex.com) was used for in-silico studies. Based on AutoDock Vina's pharmacophore fit score, plant-based phytocompounds were employed for pharmacophore modeling (Dixon et al., 2006). The compounds were then used in LigandScout software for virtual screening (VS). AutoDock Vina was used to perform the targeted molecular dockings, and UCSF Chimera v1.12 and Discovery Studio were used to analyze and visualize the docking investigations. Databases like PubChem were used to identify 2D structures. ChemDraw and Chimera were used to create plant-based molecules. For each docking experiment, 100 docking runs were established, and polar hydrogen atoms were added to all targeted proteins. AKT-1 was chosen as the grid size for docking investigations. The Lipinski's Rule of Five (RO5) was analyzed from the online mCule server and the drug characteristics of all specified compounds were calculated. The ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) properties were evaluated with AdmetSAR tool utilizing the admetSAR online server to study the bioavailability of the selected compounds.
2.6 Molecular dynamic simulation
Molecular Dynamic (MD) simulations have advanced to the point that they can be used to efficiently investigate biomolecule configuration interactions (Salo-Ahen et al., 2020). MD simulations were performed for the top docked complex by using different modules of the Schrodinger suite. A basic simulation environment for the docked systems was prepared using an NPT ensemble at a temperature of 300 K during 100 ns (ns). After, protein-ligand docking, the docked conformers were undergone for identification of root mean square deviation (RMSD) and root mean square fluctuation (RMSF) plot analysis.
3 Results and discussion
3.1 Phytochemical screening of fenugreek seeds
The pharmaceutical society's comfortability in plant-based products has grown in recent years, thanks to the growing benefits of phytomedicines (Malik et al., 2019). In the present study, the presence of phytochemicals in fenugreek seeds was investigated using a variety of qualitative tests. The qualitative phytochemical analysis revealed the presence of tannins, saponin, steroids, and cardiac glycosides in fenugreek seeds while the phlobatannins, terpenoids, and alkaloids were absent in fenugreek seeds. Phytochemicals such as saponin and tannins are extensively distributed in plants as natural antibiotics and are utilized to treat common pathogenic strains. Alkaloids, which are intermediate nitrogen-containing substances with confirmed antioxidant activities, have found uses in ethnomedicine (Mou et al., 2021). It has been considered a therapeutic agent towards oxidative stress-induced illnesses since it is mostly terpenoid. Saponin is a powerful antioxidant that really causes tumorigenesis (Ahmad et al., 2013; Liu et al., 2016). Tannin has already been employed as an active compound in pharmaceuticals and beverages due to its antioxidant effects (McCune and Johns, 2002). It inhibits the precipitation method by transferring hydrogen and alloying metallic ions such as Fe (II), Zn (II), and Cu (II). Glycosides, such as flavonol glycosides, were also found to be potent lipid peroxidation inhibitors (Plumb et al., 1999a; Plumb et al., 1999b; Ratty and Das, 1988). Steroids are potential candidates that work on free radicals to convert them into a more stable molecule, bringing the terminal chain reaction to an end (De Grey, 1999). By blocking the Na+/K+ pump and boosting ca++ concentrations, cardiac glycosides were also used to treat moderate to severe myocardial infarctions (Souza et al., 2021). These phytochemicals have many other effective health benefits. They are anticancer agents that reduce blood cholesterol levels, make bones healthy, and improve a man's immune system. From these phytoconstituents, saponins have been reported to exhibit antifungal, anti-inflammatory, fungistatic, molluscicidal, hemolytic, and foaming activity. The discovered results were comparable to those previously described by Edeoga et al. (2005). The results thus confirms that the fenugreek seeds have some phytochemical constituents that were proposed to be responsible for the antioxidant and anticancer activities of food from plants as reported in earlier studies (Mohamadi et al., 2018).
3.2 DPPH analysis of fenugreek seeds
Antioxidant properties can affect DPPH due to its electron-donating ability (Patel Rajesh and Patel Natvar, 2011). Radical scavenging is crucial in combating the harmful effects of free radicals (Kunwar and Priyadarsini, 2011). The DPPH test is also used to detect phenolic and flavonoid components in natural substances and is a typical approach to investigate the antioxidant properties in crude extracts (Gul et al., 2017). The antioxidant potential is generally analyzed with the DPPH free radical assay. DPPH is a stable radical that can be easily removed with antioxidants. It has been widely used to evaluate the antioxidant activity of plant extracts and flours, as well as to investigate the ability of substances to act as free radicals or hydrogen donors (Stankovic, 2011).
In this study, our findings showed that both extraction and positive controls had significant antioxidant activity among values and concentration. The different solvent extracts of fenugreek seeds exhibited different antioxidant actions on DPPH as listed in Table 1. The percentage scavenging rate of these extracts was n-hexane (71% ± 0.07), chloroform (71.52% ± 0.07), ethyl acetate (74.12% ± 0.073), n-butanol (75.16% ± 0.074) and methanol (75.58% ± 0.0751) respectively also compared with earlier research baseline (Puntel et al., 2009; Shalaby and Shanab, 2013). The 80% methanol extract showed the maximum value of total phenol content, total flavonoid content, radical scavenging activity, and percentage inhibition of linoleic acid oxidation.
Extracts
DPPH percentage scavenging
n-hexane
71 ± 0.07
Chloroform
71.52 ± 0.07
Ethyl acetate
74.12 ± 0.073
n-butanol
75.16 ± 0.074
Methanol
75.58 ± 0.0751
BHT
75.16 ± 0.074
Phytoconstituents containing DPPH free radical scavenging antioxidants may contribute hydrogen to lipid peroxidase or hydroperoxidase free radicals, which are major propagators of lipid chain autoxidation. Non-radicals are also produced, which interrupt the lipid peroxidation chain reaction (Sowndhararajan and Kang, 2013).
The n-hexane extraction exhibits the minimum value due to its low polarity (Table 1, Supplementary Fig. 1) as compared with earlier research (Mogole et al., 2020). The extract's reducing power may have a significant influence on the antioxidant activity. The ability to scavenge the free radical of the extracts was raised since it depends on the concentration as many authors (Canadanovic-Brunet et al., 2005; Singh and Rajini, 2004) relay on same results for numerous different plants. The absorption and rate of hydroxylation and DPPH scavenging activity were increased at a directly proportional rate. The antioxidant character was boosted and DPPH showed very high sensitivity to dynamic ingredients, even at minor changes or low concentrations. It was concluded that the seeds of fenugreek possessed potent antioxidant activity due to their ability to act as a source of antioxidant agents.
3.3 Virtual screening analyses
Computer-aided drug design (CADD) approaches for novel drug discovery are shown to be an emergent discipline with the potential to considerably improve how innovative plant-based drugs are found for cancer disorders. Computational drug designing is a specific field that relates computational methods to making innovative drugs from plant-based natural compound libraries. Anticancer natural compounds could be used as healing agents for drug design studies. The AKT-1 protein, which is primarily effective in cancer, is the target of therapeutic attempts. The sequence of the AKT-1 protein was studied against PDB and the best protein templates with maximum identity and query coverage were selected for homology-based modeling. Herein, the selected compounds were used for pharmacophore modeling. Fig. 1 shows a 3D representation of pharmacophore modeling. The selected compounds showed promising scores from the pharmacophore fit results (Supplementary Table 1). Compound-1 showed the highest pharmacophore fit score (43.91) among all compounds.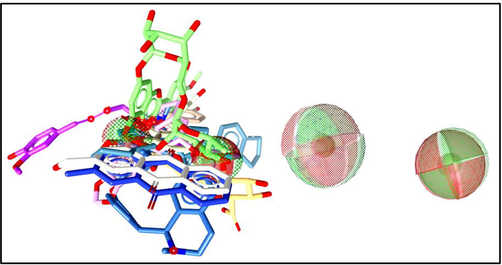
3D representation of pharmacophore modeling.
3.4 Molecular docking analyses
The top three anticancer compounds from the screen were analyzed analytically based on their drug-likeness properties, binding affinities, and energies. The binding affinities of all top 10 ranked compounds against targeted protein AKT-1 were obtained in the range of −10.9 to −5.8 Kcal/mol. Among all the generated docked complexes, the selected top three hits showed good binding energies, and the least binding affinities. All top 3 leading hits were bound to the same active regions of the targeted protein (Fig. 2). Docking results displayed that these top 3 natural compounds (Compound-1, Compound-2, and Compound-3) displayed key interactive residues and the highest binding energies (−10.9 to −9.2 Kcal/mol) as compared to other compounds (Table 2). The least binding affinity of Compound-1 was observed to be −10.9 Kcal/mol in AKT-1 docked complex. The 3D docked possess of leading hits is displayed in Fig. 3. Furthermore, residues, binding affinities, and common molecular interactions of top hits were calculated in docked complexes of all ligands. The utilized docking tool showed effective binding regions and common molecular interactions among most docked complexes (Table 3). The three leading compounds were the most active as compared to other studied compounds and bound at the active sites of AKT-1. Overall, the interactive residues and binding affinities proved that the selected compounds are effective against targeted proteins.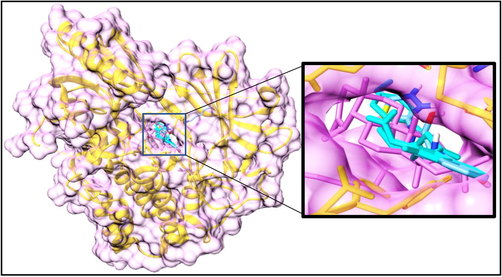
All 3 ligands are attached to the same binding pockets.
Compounds
Binding affinities (kcal/mol)
Compound-1
−10.9
Compound-2
−9.6
Compound-3
−9.2
Compound-4
−7.3
Compound-5
−5.8
Compound-6
−6.6
Compound-7
−6.5
Compound-8
−6.9
Compound-9
−6.5
Compound-10
−6.6
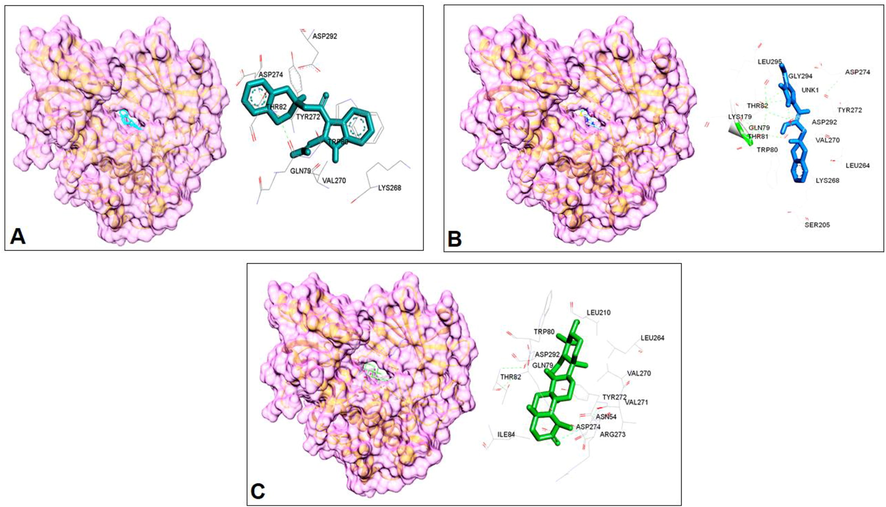
3D interaction of Compound-1 (A), Compound-2 (B) and Compound-3 (C) with AKT-1.
Compounds
Interactive residues in receptor-ligand interactions
Compound-1
Gln-79, Trp-80, Thr-82, Lys-268, Val-270, Tyr-272, Asp-274, Asp-292
Compound-2
Gln-79, Trp-80, Thr-81, Thr-82, Lys-179, Ser-205, Leu-264, Lys-268, Val-270, Tyr-272, Asp-274, Asp-292, Gly-294, Leu-295
Compound-3
Asn-54, Gln-79, Trp-80, Thr-82, ILE-84, Leu-210, Leu-264, Val-270, Val-271, Tyr-272, Arg-273, Asp-274, Asp-292
The chemical structures of phytochemicals are assessed to be effective drug-like compounds according to Lipinski’s rule of five (Chen et al., 2020b). According to Lipinski’s rule of five, small molecules that can cross the membrane have ≤500 molecular weight, ≤10 hydrogen acceptors, ≤5 hydrogen donors and ≤5 LogP values. The molecular properties of compounds always depend on LogP such as bioavailability and membrane permeability. The inhibitors having cyclic rings show important biological characteristics. ADMET properties characterize the drug-like activity of phytocompounds. The analyses of physicochemical properties of the top 3 hits are shown in Table 4. Almost the predicted properties of the selected compounds were satisfactory and the compounds were non-carcinogenic. The ADMET analyzed results showed that the compounds can be easily absorbed by the intestine. These physicochemical properties and drug-likeness properties proved that these compounds are strong candidates as an anticancer drug.
Properties
Compound-1
Compound-2
Compound-3
Molecular weight (g/mol)
320.3877
303.4048
426.7162
Logp (o/w)
1.0649
2.8989
8.0248
H-bond acceptors
5
5
1
H-bonds donors
2
2
1
Rotatable bonds
4
4
0
PSA
64.9200
91.0500
20.2300
Atoms
44
40
81
Rings
4
3
5
3.5 MD simulation
MD simulation has become an essential tool for understanding the physical basis of the structure of proteins and their biological utilities. The simulation process timelines are equivalent to biologically relevant timescales. The amount of data acquired on macromolecule dynamic properties is sufficient to shift structural bioinformatics towards examining individual structures and more towards analyzing structural ensembles. To examine the stability and conformational changes of AKT-1 in its innate state and in the presence of ligand, we did MD simulation studies. The RMSD plot are significant to transfer out the calculation of structural stability on protein (Dhandare et al., 2020; Jing et al., 2019). The changes in different conformations in the protein–ligand complex were done during 100 ns. About 100 ns of simulation time used is suitable time for the side chain reorganizations in native as well as protein–ligand complexes in order to facilitate the most stable binding conformation. As shown in Fig. 4, RMSD plot represented the major increase in the C-α backbone at one ns till 25 ns. There was a gradual decrease in the C-α backbone during 26th ns to 30th ns, and then the peaks became higher from 35 ns to 40 ns. During 65 ns to 100 ns, there was a gradual decrease in the C-α backbone. The ligand remained consistent with the RMSD deviations of the C-α backbone. The average RMSD of the docked complex during the 100 ns was 3.2 Å (Fig. 4A). The average RMSF for the docked complex was 4 Å. There was a rise in RMSF at residues 1–95 with a maximum value of 5 Å. From their residues, minor fluctuations were observed with an average RMSF of 3 Å. There was a rise in peaks 3 and 4 (Fig. 4B) among residues with a value of 4.8 Å.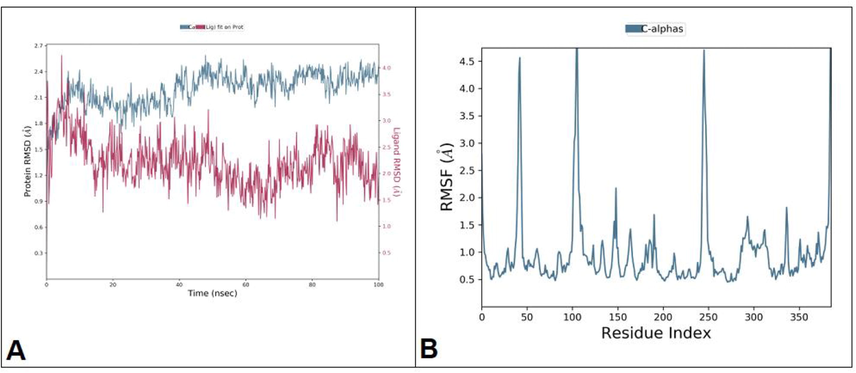
(A) The RMSD plot for ligand–protein complex during 100 ns of MD simulation. (B) The RMSF plot representing the major fluctuations at peak 1, 2, 3, and 4.
A radius of gyration is well-defined as the dissemination of atoms of a protein around its axis. In case of protein structure radius of gyration is an indicator of compactness. It is largely concerned with secondary structures that are tightly packed within the protein's three-dimensional structure. In comparison to the lowermost radius of gyration, a protein's maximum radius of gyration has less tight packing (Rather et al., 2020). Our results indicate tighter bound protein–ligand bound complex that is in accordance with other studies (Rather et al., 2017) The RMSD/RMSF analysis of the phytochemicals alone also verified no significant structural changes.
3.6 Protein structure prediction analyses
PSSE were examined during MD simulation. As shown in Fig. 5, there was a prominent conformational change in helices (31.95%), strands (11.83%), and a total of 43.78% of protein SSE have been depicted at 100 ns of simulation. Protein-ligand contacts are an essential factor in representing that the ligand fits with the protein during the whole duration of MD simulations and the interaction is responsible for binding the ligand with the protein. Ligand properties such as Radius of Gyration, Intra-molecular Hydrogen Bonds, Molecular Surface Area, Solvent Accessible Surface Area, and Polar Surface Area (PSA) were examined during simulations (Fig. 6).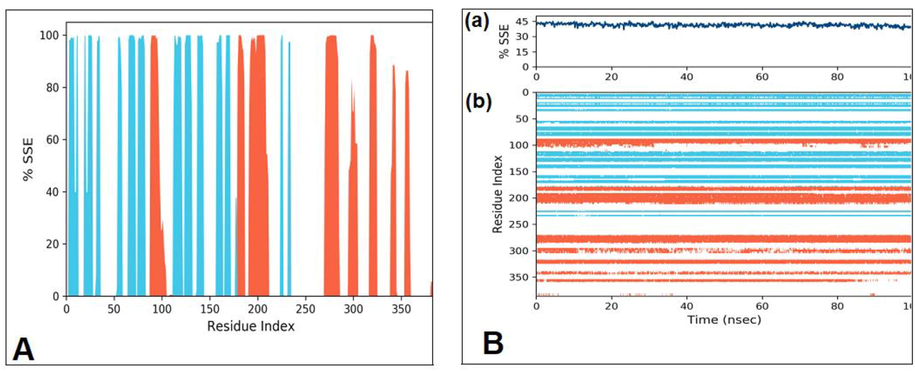
The protein SSE depiction. (A) The SSE distribution by residue index throughout the protein structure. The red peaks represent helices and blue peaks represent beta-strands. (B) The plot “a” summarizes the SSE composition for each trajectory frame over the course of the simulation, and the plot at “b” monitors each residue and its SSE assignment over time.
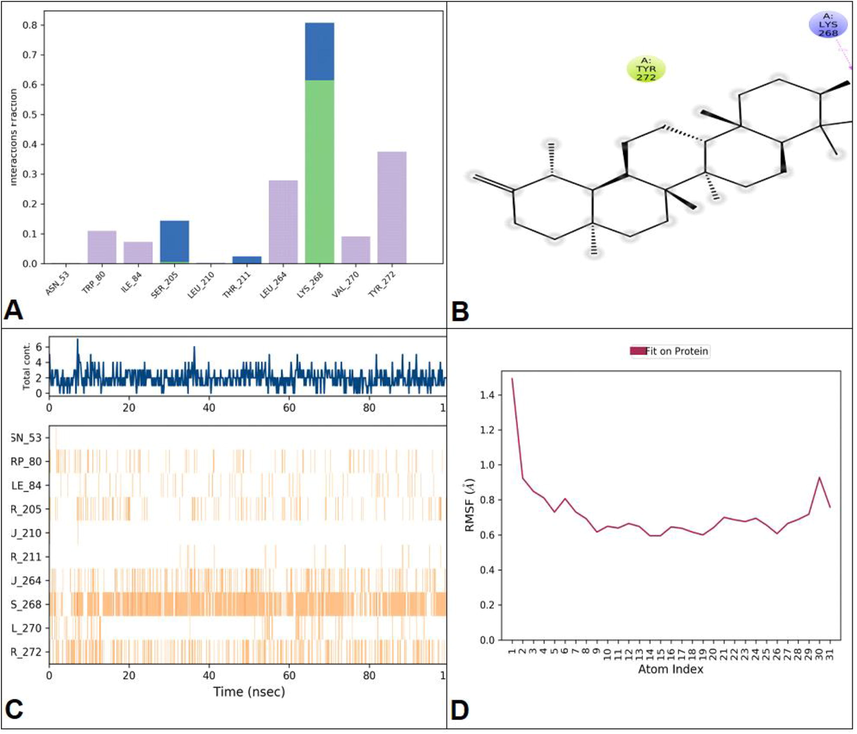
The representation of protein–ligand contacts. (A) The interactions involved in protein–ligand contacts categorized by type. (B) A timeline representation of the interactions and contacts (H-bonds, Hydrophobic) summarized in the previous page. The top panel shows the total number of specific contacts the protein makes with the ligand over the course of the trajectory. The bottom panel shows which residues interact with the ligand in each trajectory frame. Some residues make more than one specific contact with the ligand, which is represented by a darker shade of orange, according to the scale to the right of the plot. (C) A schematic of detailed ligand atom interactions with the protein residues. (D) The ligand properties with respect to Radius of Gyration (rGyr), Intramolecular Hydrogen Bonds (intraHB), Molecular Surface Area (MolSA), Solvent Accessible Surface Area (SASA), and Polar Surface Area (PSA).
4 Conclusion
The current study suggests that the selected compounds are effective against cancer cells. The present research on fenugreek to determine the biological activities, including phytochemical, anti-inflammatory, antioxidant, and cytotoxic studies, concluded from its noticeable results that it can be used medicinally in the treatment of cancer. The present study concluded that the three leading hits show prominent effects on cancer. The CADD results indicate marked effects against the targeted AKT-1 protein. It not only reduces cancerous cells but also inhibits the AKT pathway. To summarize, the computational models of selected compounds may be used to better understand the protein–ligand interactions and binding affinities. These compounds will be an excellent model for the development of innovative, less toxic, and highly effective drugs for the treatment of cancer.
Acknowledgment
This work was funded by the Researchers Supporting Project Number (RSP-2021/339) King Saud University, Riyadh, Saudi Arabia. Authors want to acknowledge their institutes for general support, such as helping in doing some bioinformatics analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- In vitro antioxidant potential of dicliptera roxburghiana. BMC Complement. Altern. Med.. 2013;13(1):1-10.
- [Google Scholar]
- Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv. Cancer Res.. 2005;94:29-86.
- [Google Scholar]
- Free-radical scavenging activity of wormwood (Artemisia absinthium L) extracts. J. Sci. Food Agric.. 2005;85(2):265-272.
- [Google Scholar]
- Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ.. 2020;27(1):242-254.
- [Google Scholar]
- Chen, Y., Huang, L., Dong, Y., Tao, C., Zhang, R., Shao, H., Shen, H., 2020. Effect of AKT1 (p. E17K) Hotspot Mutation on Malignant Tumorigenesis and Prognosis, Front. Cell Dev. Biol., 8, 996.
- Analysis of the physicochemical properties of acaricides based on Lipinski's rule of five. J. Comput. Biol.. 2020;27(9):1397-1406.
- [Google Scholar]
- New approaches to molecular cancer therapeutics. Nat. Chem. Biol.. 2006;2(12):689-700.
- [Google Scholar]
- Targeting the AKT protein kinase for cancer chemoprevention. Mol. Cancer Therap.. 2007;6(8):2139-2148.
- [Google Scholar]
- Computational drug design and exploration of potent phytochemicals against cancer through in silico approaches. Biomed. Lett.. 2019;5(1):21-26.
- [Google Scholar]
- De Grey, A.D., 1999. The mitochondrial free radical theory of aging: Citeseer.
- Molecular modeling, docking and dynamic simulations of growth hormone receptor (GHR) of Labeo rohita. J. Biomol. Struct. Dyn. 2020:1-14.
- [Google Scholar]
- Synthesis and computer-aided drug design studies of novel thiosemicarbazide derivatives as potent and target-oriented anti-cancer agents. Medicine. 2020;9(2):305-313.
- [Google Scholar]
- PHASE: a novel approach to pharmacophore modeling and 3D database searching. Chem. Biol. Drug Des.. 2006;67(5):370-372.
- [Google Scholar]
- Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol.. 2005;4(7):685-688.
- [Google Scholar]
- Anticancer potential of Trigonella foenum graecum: Cellular and molecular targets. Biomed. Pharmacother.. 2017;90:479-491.
- [CrossRef] [Google Scholar]
- Neurologic complications of immune checkpoint inhibitors. J. Neurooncol.. 2018;137(3):601-609.
- [Google Scholar]
- Central nervous system effects of natural and synthetic glucocorticoids. Psychiatry Clin. Neurosci.. 2009;63(5):613-622.
- [Google Scholar]
- Herbal medicine today: clinical and research issues. Evid. Based Complement. Altern. Med.. 2007;4(S1):37-40.
- [Google Scholar]
- Phytochemical modulators of mitochondria: the search for chemopreventive agents and supportive therapeutics. Pharmaceuticals. 2014;7(9):913-942.
- [Google Scholar]
- Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from ephedra intermedia indigenous to balochistan. Sci. World J.. 2017;2017:1-7.
- [Google Scholar]
- The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer. 2020;20(2):74-88.
- [Google Scholar]
- Polarizable force fields for biomolecular simulations: Recent advances and applications. Annu. Rev. Biophys.. 2019;48(1):371-394.
- [Google Scholar]
- Use of herbal medicine for treating psychiatric disorders in Japan. Psychiatry Clin. Neurosci.. 1998;52(S6):S331-S333.
- [Google Scholar]
- Fenugreek (Trigonella foenum graecum) seed extract prevents ethanol-induced toxicity and apoptosis in Chang liver cells. Alcohol Alcohol. 2006;41(3):267-273.
- [CrossRef] [Google Scholar]
- Pomegranate peel induced biogenic synthesis of silver nanoparticles and their multifaceted potential against intracellular pathogen and cancer. Saudi J Biol Sci. 2021;28(8):4191-4200.
- [CrossRef] [Google Scholar]
- Aminodextran coated CoFe2O4 nanoparticles for combined magnetic resonance imaging and hyperthermia. Nanomaterials. 2020;10(11):2182.
- [Google Scholar]
- Phytochemical antioxidants for health and medicine a move towards nature. Biotechnol. Mol. Biol. Rev.. 2007;2(4):97-104.
- [Google Scholar]
- A computational approach for investigating the mutational landscape of RAC-alpha serine/threonine-protein kinase (AKT1) and screening inhibitors against the oncogenic E17K mutation causing breast cancer. Comput. Biol. Med.. 2019;115:103513
- [Google Scholar]
- Free radicals, oxidative stress and importance of antioxidants in human health. J. Med. Allied Sci.. 2011;1(2):53.
- [Google Scholar]
- In silico methods to address polypharmacology: current status, applications and future perspectives. Drug Discovery Today. 2016;21(2):288-298.
- [Google Scholar]
- Bioinformatics approaches for anti-cancer drug discovery. Curr. Drug Targets. 2020;21(1):3-17.
- [Google Scholar]
- Inhibition of urethane-induced lung carcinogenesis in mice by a Rhizoma paridis saponin involved EGFR/PI3K/Akt pathway. RSC Adv.. 2016;6(95):92330-92334.
- [Google Scholar]
- Natural substances derived from herbs or plants are promising sources of anticancer agents against colorectal cancer via triggering apoptosis. J. Pharm. Pharmacol.. 2022;74(2):162-178.
- [Google Scholar]
- In silico and in vitro studies of lupeol and iso-orientin as potential antidiabetic agents in a rat model. Drug Design, Dev. Ther.. 2019;13:1501.
- [Google Scholar]
- Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North American boreal forest. J. Ethnopharmacol.. 2002;82(2–3):197-205.
- [Google Scholar]
- Phytochemical screening, anti-oxidant activity and α-amylase inhibition study using different extracts of loquat (Eriobotrya japonica) leaves. Heliyon. 2020;6(8):e04736.
- [Google Scholar]
- A review on biosynthesis, analytical techniques, and pharmacological activities of trigonelline as a plant alkaloid. J Diet Suppl. 2018;15(2):207-222.
- [CrossRef] [Google Scholar]
- Identification, biological activities and biosynthetic pathway of Dendrobium alkaloids. Front. Pharmacol.. 2021;12
- [Google Scholar]
- In vitro antioxidant activity of coumarin compounds by DPPH, Super oxide and nitric oxide free radical scavenging methods. J. Adv. Pharm. Educ. Res.. 2011;1:52-68.
- [Google Scholar]
- Antioxidant properties of flavonol glycosides from tea. Redox Rep.. 1999;4(1–2):13-16.
- [Google Scholar]
- Antioxidant properties of flavonol glycosides from green beans. Redox Rep.. 1999;4(3):123-127.
- [Google Scholar]
- Butane-2, 3-dionethiosemicarbazone: an oxime with antioxidant properties. Chem. Biol. Interact.. 2009;177(2):153-160.
- [Google Scholar]
- Antimicrobial plant metabolites: structural diversity and mechanism of action. Curr. Med. Chem.. 2013;20(7):932-952.
- [Google Scholar]
- In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes. 2015;8(1)
- [Google Scholar]
- PI3K signaling pathway targeting by using different molecular approaches to treat cancer. 中国药学 (英文版). 2017;26(9):621.
- [Google Scholar]
- Characterization, molecular docking, dynamics simulation and metadynamics of kisspeptin receptor with kisspeptin. Int. J. Biol. Macromol.. 2017;101:241-253.
- [Google Scholar]
- Structural analysis, molecular docking and molecular dynamics simulations of G-protein-coupled receptor (kisspeptin) in fish. J. Biomol. Struct. Dyn.. 2020;38(8):2422-2439.
- [Google Scholar]
- Effects of flavonoids on nonenzymatic lipid peroxidation: structure-activity relationship. Biochem. Med. Metab. Biol.. 1988;39(1):69-79.
- [Google Scholar]
- Molecular dynamics simulations in drug discovery and pharmaceutical development. Processes. 2020;9(1):71.
- [Google Scholar]
- Tissue-specific tumorigenesis: context matters. Nat. Rev. Cancer. 2017;17(4):239-253.
- [Google Scholar]
- Shalaby, E.A., Shanab, S.M., 2013. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis.
- Free radical scavenging activity of an aqueous extract of potato peel. Food Chem.. 2004;85(4):611-616.
- [Google Scholar]
- Na+/K+-ATPase as a target of cardiac glycosides for the treatment of SARS-CoV-2 infection. Front. Pharmacol.. 2021;12
- [Google Scholar]
- Free radical scavenging activity from different extracts of leaves of Bauhinia vahlii Wight & Arn. Saudi J. Biol. Sci.. 2013;20(4):319-325.
- [Google Scholar]
- Total phenolic content, flavonoid concentration and antioxidant activity of Marrubium peregrinum L. extracts. Kragujevac J. Sci.. 2011;33(2011):63-72.
- [Google Scholar]
- The global burden of cancer: priorities for prevention. Carcinogenesis. 2010;31(1):100-110.
- [Google Scholar]
- Effects of synthetic and organic acaricides on honey bee health: a review. Slovenian Vet. Res.. 2018;55(3):114-140.
- [Google Scholar]
- Natural and synthetic substances related to human health (IUPAC Technical Report) Pure Appl. Chem.. 2002;74(10):1957-1985.
- [Google Scholar]
- Target recognition and network pharmacology for revealing anti-diabetes mechanisms of natural product. J. Comput. Sci.. 2020;45:101186.
- [Google Scholar]
- Herbs or natural substances as complementary therapies for chronic kidney disease: ideas for future studies. J. Lab. Clin. Med.. 2006;147(4):160-166.
- [Google Scholar]
- Genome-wide identification and expression analysis of PPOs and POX gene families in the selected plant species. Biosci. Biotechnol. Res. Asia. 2020;17(2):301-318.
- [Google Scholar]
- Genome and Gene Editing by Artificial Intelligence Programs Advanced AI Techniques and Applications in Bioinformatics. CRC Press; 2021. p. :165-188.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102186.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







