Translate this page into:
Characterization of an Ascochyta disease of the invasive vine Araujia hortorum E. Fourn. (Apocynaceae)
⁎Corresponding author at: CERZOS-CONICET CCT Bahía Blanca, Camino La Carrindanga Km 7. 8000, Bahía Blanca, Argentina. anderson@criba.edu.ar (Freda Elizabeth Anderson)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Araujia hortorum E. Fourn. is native to South America, where it is a valued species. In contrast, in other parts of the world where it was introduced as an ornamental, it has become an invasive weed. In New Zealand, it has been targeted for biological control, and this motivated field surveys for fungal pathogens in its native range in Argentina. The etiology of a frequently encountered disease was studied. Affected plants showed round necrotic leaf spots, and sometimes defoliation. Pathogenicity tests were conducted, and Koch’s postulates were completed. Ascochyta araujiae Speg. was identified as the causal agent. Through inoculation tests conducted on other species within the Apocynaceae it was shown that this pathogen is not highly specific. All five non-target species tested were found to be susceptible to the disease.
Keywords
Ascochyta araujiae
Ascochyta blight
Araujia hortorum
Moth plant
- PDA
-
potato dextrose agar
- RH
-
relative humidity
Abbreviations
1 Introduction
The scientific name of the plant species known by the common name “tasi” in the pampean region in Argentina and “moth plant” in New Zealand is a subject of controversy. Many authors refer to it as Araujia sericifera Brot. following Forster and Bruyns (1992) who considered A. hortorum to be a synonym of the former but in most floristic works in Argentina they are treated as two separate species (Villamil and Barton, 2009). The name A. sericifera Brot. has been used for moth plant in New Zealand (Winks and Fowler, 2000), but A. hortorum is now the preferred scientific name (Champion et al., 2010) and is the one we have adopted here. This species is native to South America. It is a perennial, fast-growing, broad-leaved herbaceous climber with twining stems that exhibits, as do the other members within the Apocynaceae, elaborate floral features, milky latex and seeds with an apical tuft of long silky hairs (Bucciarelli et al., 2008; Endress and Bruyns, 2000). In its native range this plant is appreciated for its medicinal properties; its edible fruits; and its ornamental value (Bayón and Arambarri, 1999). Because of the latter, it has been introduced into many countries where it has since become cause of concern owing to its high invasive potential and capacity to generate serious environmental problems (Csurhes and Edwards, 1998; Waipara et al., 2006). Vines can grow over other plants, even shrubs and trees (Fig. 1A), sometimes getting to completely cover and smother them or breaking branches because of their weight. They are also strong competitors for water and nutrients. Their caustic milky sap can cause skin or eye irritation (Elorza et al., 2004).
Araujia hortorum. A. Plant growing in its natural habitat. B, C. Disease symptoms on leaves (B) and a stem (C).
It is an important weed in plantations of citrus in California (Spellman and Gunn, 1976) and of kiwi-fruit in New Zealand (Kiwifruit Vine Health, 2016), but it is mainly considered an environmental weed in some countries of Europe, Israel, South Africa, Australia, and New Zealand (Coombs and Peter, 2010; Csurhes and Edwards, 1998; Elorza et al., 2004). In New Zealand A. hortorum has been targeted for classical biological control (Waipara et al., 2006; Winks and Fowler, 2000). The rust Puccinia araujiae has been proposed as a biological control agent and permission to import and release it in this country has been granted (Anderson et al., 2016).
Fairly little is known about other fungal pathogens associated with the target species in its native range in Argentina. There are only a few old records of causal agents of spots on leaves, stems and fruits in the literature (Spegazzini, 1880, 1882; Viégas, 1961) and other more recent ones that have resulted from surveys carried out with the aim of identifying suitable biocontrol agents to be applied in New Zealand (Waipara et al., 2006). The purpose of this investigation was to study in detail one of the diseases encountered during such surveys selected because it was frequently encountered and because it was observed to be particularly damaging to its host at some collection sites.
2 Materials and methods
2.1 Field surveys
Field surveys were conducted to collect diseased plant material from Araujia hortorum populations between autumn 2012 and spring 2013 in Buenos Aires province, Argentina. Leaves and stems were collected and placed in a plant press while fruits were placed in paper bags for further study in the laboratory. A GPS reading was taken at each collection site.
2.2 Isolation and identification of the pathogen
The pathogen was first found in association with large necrotic leaf spots showing concentric growth rings affecting plants growing near the town of Carlos Casares (−35.578°S, −61,126°W). This site is arrowed in Fig. 2. The fungus was isolated by transference of fruiting bodies (pycnidia) found on the spots to potato dextrose agar (PDA) with a sterile needle. Pycnidia were subjected to superficial disinfection prior to transference. To this end they were submerged in pure ethanol for one minute, rinsed in sterile distilled water, then submerged in sodium hypochlorite for two minutes and finally rinsed again in sterile distilled water (Yuan and Mohammed, 2002). In order to achieve the identification of the pathogen, pycnidia obtained from diseased plant material were cut by hand with a razor blade, and the resulting slices were mounted in water for microscopic observation and measurements. Some sections were stained with Phloxine B to facilitate the study of the conidiogenous cells.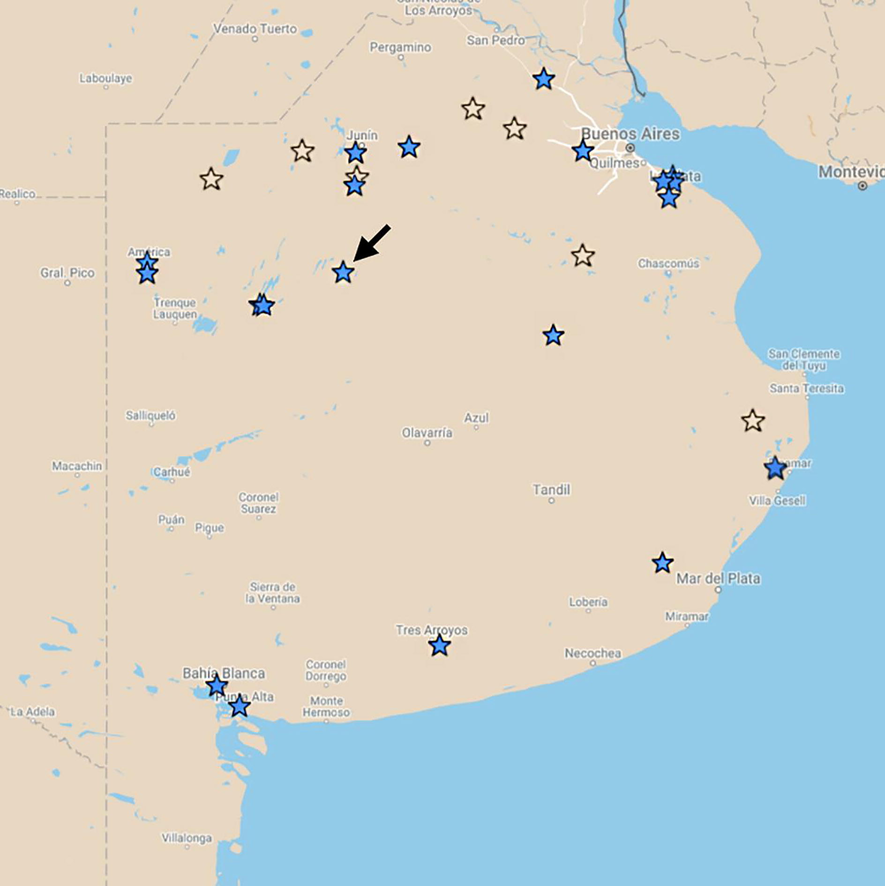
Populations visited during surveys in Buenos Aires province. Sites where Ascochyta araujiae was/was not found are indicated by full/empty stars respectively. The arrow indicates site 10.
2.3 Sourcing and maintenance of plants
Araujia hortorum plants from several different locations were grown from seed collected during field trips. Seeds were superficially disinfected by immersion in sodium hypochlorite in water (3:10) for 2 mins followed by a short rinse in sterile distilled water. To promote germination, seeds were then placed in Petri dishes lined with wet cotton wool and filter paper and incubated at room temperature for 7–10 days. At hypocotyl emergence, seedlings were planted individually into potting mix in plastic seedling trays. At the four-leaf stage, plantlets were transferred to 10-cm-diameter plastic pots containing a 1:1 mixture of potting mix and local soil. Plants were kept until needed on a glasshouse bench (temperature range 16–26 °C).
2.4 Artificial inoculation experiments
Experiments were conducted to: (1) Confirm the pathogenicity of the fungus isolated from the spots; (2) Investigate its penetration mode and (3) Study its level of specificity.
2.4.1 Inoculum preparation
Inoculum was prepared from sporulating fungal colonies growing on PDA at room temperature. Fruiting bodies were removed from the colonies with the aid of a fine pair of forceps and transferred to a mortar in which they were squashed for a complete release of conidia. Sterile distilled water was added while beating with a glass rod to obtain a suspension of the released spores. The suspension was poured into a glass beaker, to which more distilled water was added until the desired volume was reached. Final spore concentration was estimated with a haemocytometer.
2.4.2 Plant inoculation
Inoculum was applied by means of either an atomizer or a fine brush to young, healthy, recently pruned plants. When an atomizer was used, care was taken to wet the whole plant to runoff. In contrast, when a brush was used, either the adaxial or abaxial sides of selected leaf blades were inoculated. Inoculated plants were kept inside inoculation chambers, which consisted of cube-shaped polyethylene boxes with the floor lined with water-soaked newspaper to provide around 100% relative humidity (RH). After six days, they were removed and kept in controlled environment cabinets at around 40% RH, 18–20 °C and a 12-h light regime (fluorescent, 1400 l) until the end of the experiments.
2.4.3 Pathogenicity confirmation tests (Koch’s postulates)
According to Koch’s postulates, the following steps should be followed to confirm that a certain pathogen is the causal agent of a disease: 1) The pathogen must be found associated with the disease in all the diseased plants examined, 2) the pathogen must be isolated and grown in pure culture and its characteristics studied, 3) the pathogen must be inoculated on healthy plants of the same species on which the disease appeared in the field and produce the same disease, 4) the pathogen must be isolated again in pure culture and show the same characteristics as those observed in step 2. In order to complete the third step, 40 ml of a spore suspension (1 × 106 spores/ml) were applied to six A. hortorum plants with an atomizer. Inoculated plants were incubated as explained above until the development of disease symptoms. Finally, to complete the fourth postulate, the pathogen was isolated from these symptoms and compared with that isolated from the original ones collected in the field.
2.4.4 Mode of penetration of the pathogen
Leaves of A. hortorum have stomata only on the abaxial side (Bucciarelli et al., 2008). To investigate whether the pathogen depended on these natural openings for penetration or if, alternatively, it was able to enter the host directly, a spore suspension was applied with a brush onto the adaxial side only of two leaves per plant of two plants and onto the abaxial side only of two leaves per plant of another two. Plants were kept in inoculation chambers for the first six days and then transferred to a controlled environment cabinet until the development of symptoms of disease as explained above.
2.4.5 Specificity tests
An experiment was conducted to investigate whether the pathogen is able to infect other species within the Apocynaceae. The tested species were Araujia angustifolia, Morrenia brachystephana, M. odorata, Asclepias curassavica and Gomphocarpus physocarpus. Twenty-four plants (four per tested species plus four A. hortorum ones included as positive controls) were inoculated to runoff with 100 ml of a spore suspension in water (1 × 106) with an atomizer. Plants were incubated in two separate incubation chambers, two individuals per species in each, for the first week. After that, plants were kept in controlled environment cabinets under the same conditions but with humidity lowered to ca. 40% for four weeks (more than double the time needed for symptoms to develop on A. hortorum) to allow enough time for disease symptoms to develop on the tested species. The experiment was repeated once.
3 Results
3.1 Field surveys
The disease was found at most of the twenty-eight sites visited throughout the province of Buenos Aires (Fig. 2). The most severely affected plants were observed at the site at which the disease was first encountered (Site 10, arrowed). There foliage had an overall unhealthy appearance and premature defoliation was obvious, with lots of fallen leaves on the soil under the plants. Upon closer examination in the laboratory, individual leaf spots were observed to be big (3–7 mm diam., Fig. 1B), circular to ellipsoidal target-shaped necrotic spots, on which black pycnidia were formed in concentric circles. Spots can coalesce, thus affecting large areas of the leaf blades and causing premature foliage senescence and fall. Symptoms on leaf blades are the most conspicuous but the disease also affects other organs such as petioles, stems and fruits. Symptoms on stems are dark purple elliptic or circular spots (Fig. 1C) of which the older ones show typical concentric growth rings. Symptoms on fruits are big necrotic areas with concentric rings of pycnidia. These symptoms were observed to be mostly superficial, affecting only the pericarp. Only once was the mycelium observed inside a fruit reaching and affecting the seed. Infection of seeds resulted in a reduced germination and an early death of seedlings.
3.2 Identification and description of the pathogen
The pathogen was identified by the authors as Ascochyta araujiae Speg. based on the original description by Spegazzini (1882) as A. araujae.
This description is now amended and completed as follows: On PDA colonies show scarce, white to ochre aerial mycelium. On these, conidiomata with oozes of dark brown conidial masses, are formed in more or less regular concentric circles. On host tissue, conidiomata pycnidial (Fig. 3-A,B), mostly solitary, rarely confluent, semi-immersed, globose to subglobose, 80–180 µm diam. (mean = 19820 µm; n = 60), glabrous, ostiolate. Ostiole single, only slightly or not papillate, 16–25 µm diam. Pycnidial wall pseudoparenchymatous, composed of 3–6 layers of isodiametric cells disposed in textura angularis, 14–23 µm thick, outer 2–3 layers dark brown. Conidiophores absent. Conidiogenous cells enteroblastic, phialidic, hyaline, smooth, ampulliform, lageniform or doliiform, 5–9 × 3–7 µm (mean = 7 × 5.4 µm; n = 58) formed from the internal cells of the pycnidial wall (Fig. 3-C). Conidia subhyaline, with a faint green tinge, smooth, cylindrical to elliptical with rounded ends, 6.5–11 × 3–5 µm (mean = 8.6 × 3.5; n = 90), mainly 1-septate but sometimes 0 or 2 septate, not or only slightly constricted, egutullate (Fig. 3-D).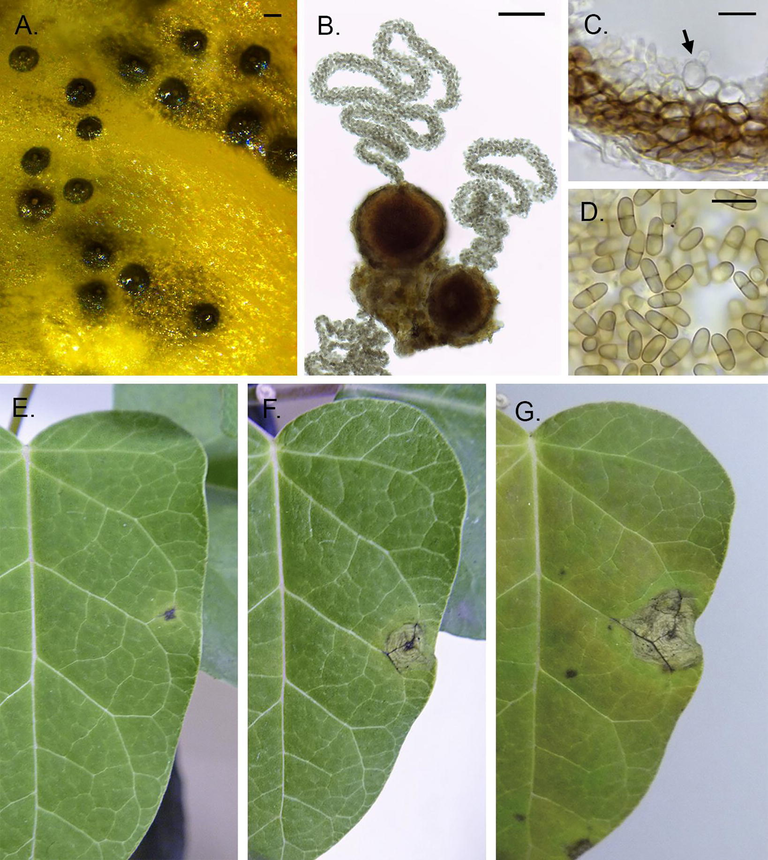
Ascochyta araujiae. A. Pycnidia developed on affected tissue. B. Microscopic detail of pycnidia and cirri. C. Pycnidial wall and fertile layer; the arrow indicates a conidiogenous cell. D. Conidia. E,F,G. Symptom development after week one (A), two (B) and three (C). Scale bars: A and B: 50 µm; C and D: 10 µm.
3.3 Artificial inoculation experiments
3.3.1 Pathogenicity confirmation tests (Koch’s postulates)
Disease developed on all the inoculated plants. The first noticeable symptoms to develop were small purple spots, mostly located on leaf blades but also on petioles and stems. Spots were initially circular, somewhat sunken, surrounded by a chlorotic halo (Fig. 3-E). After a few days they became bigger, necrotic, and started to show concentric growth rings (Fig. 3-F,G). No pycnidia formed on the spots during the experiment while plants were subjected to low RH, but were formed readily when spotted plant material was subjected to 100% RH (Fig. 3-A). The pathogen re-isolated on PDA from these symptoms showed the same characteristics as the one isolated originally from field site 10, allowing for the completion of Koch’s postulates.
3.3.2 Mode of penetration of the pathogen
Symptoms were formed equally on all but one of the leaves inoculated on the stomata-free adaxial side of leaf blades and on all but one of those inoculated on the stomata-bearing abaxial sides.
3.3.3 Specificity tests
Symptoms of disease developed on plants of all the tested species and the presence of the pathogen Ascochyta araujiae was confirmed in all cases (Table 1). Spots continued to appear for up to 18 days after the inoculation date. In the case of Araujia and Morrenia species, leaves were the most affected organs while in the case of both Asclepias curassavica and Gomphocarpus physocarpus spots were formed mostly on stems. When spots were formed on petioles, the affected leaves were shed a couple of days later.
Subtribe
Plant species
N° Tested Plants
Percentage diseased plants
Affected organs
Oxypetalinae
Araujia hortorum E. Fourn.
8
100%
Leaves, petioles, stems
A. angustifolia (Hook & Arn.) Steud
8
87.5%
Leaves
Morrenia odorata Hort. ex Kunze
8
87.5%
Leaves, stems
M. brachystephana Griseb.
8
100%
Leaves, petioles, stems
Asclepiadinae
Asclepias curassavica L.
8
100%
Leaves, stems
Gomphocarpus physocarpus E. Mey.
8
75%
Leaves, stems
4 Discussion
It was proven that the causal agent of the target-shaped leaf spot of A. hortorum is Ascochyta araujiae, a pathogen described by Spegazzini in 1882 as the causal agent of a leaf spot on A. albentis, a plant species that has since been synonymized with A. hortorum. A full description of both the pathogen and the disease is provided. The characteristics of the pathogen found in association with diseased material are in accordance with those in Spegazzini’s original description, but for the fact that pycnidia are twice as large. Such differences are to be expected since, according to Mel'nik (2000), the difference in size of pycnidia between specimens of a single Ascochyta species can be significant. The typical zonation of the cultures growing on artificial media in the laboratory is also a common feature in some Ascochyta species when exposed to alternating light and dark periods (Kaiser, 1973). The development of the disease was followed on artificially inoculated plants and this allowed for the association between the initial purple spots and the fully developed target-shaped spots to be made. This in turn led to the realization that the disease is more widespread than had been anticipated, and by far the most frequent one encountered during our field surveys on this host.
There are many reported studies on Ascochyta diseases in the literature, especially on those affecting crops in the Fabaceae (Hernandez-Bello et al., 2006; Mel'nik, 2000). The diseases they cause are known as ascochyta blights and represent serious limitations to legume production worldwide (Peever, 2007; Tivoli and Banniza, 2007). Ascochytoses can affect crops, forage and ornamentals. The development of resistant cultivars has shown to be the best long-term strategy for the control of ascochyta blights in crops. The use of fungicides for treatment of large areas is often not practical or even feasible, but for the control of ascochytoses on ornamental plants it is the most widely used method. Broad-spectrum fungicides with systemic activity, especially the ones in the benzimidazole group, have shown to be fairly effective to control ascochyta blights (Bretag et al., 2006).
The main adverse effect of diseases caused by Ascochyta spp. is a reduction in photosynthetic area due to necrosis (Mel'nik, 2000) and a decrease of photosynthetic efficiency of the green leaf area (Garry et al., 1998). The ability of many fungi to produce disease is strictly related to their ability to produce phytotoxins (Yoder, 1980). Although the pathogenesis mechanism has not been studied for A. araujiae, studies carried out on the related species A. rabiei, the causal agent of chickpea blight, indicate necrosis is produced prior to hyphal invasion of tissues, suggesting the participation of fungal toxins in pathogenesis (Kaur, 1995; Pande et al., 2005). The involvement of phytotoxins in pathogenesis has also been reported for A. pisi, the causal agent of leaf spot disease of pea (Abouzeid and El-Tarabily, 2003) and several other Ascochyta species (Andolfi et al., 2013; Masi et al., 2018). This kind of pathogenesis is considered characteristic of necrotrophs (Tivoli and Banniza, 2007). Necrotrophic plant pathogens kill host cells by means of toxic molecules and lytic enzymes and they subsequently decompose the plant tissue and consume it for their own growth (van Kan, 2006).
In accordance with knowledge on other Ascochyta species (Peever, 2007) it was found, through both artificial inoculation experiments and field observations, that foliage, petioles, stems and fruits of A. hortorum can all be affected by the disease. Pande et al. (2005) observed that in chickpea plants affected by A. rabiei, parts above girdled stems are killed and break off. Stem lesions did not girdle in our experiments, but petiole ones did and caused premature fall. The disease is widespread throughout the natural distribution of its host in the province of Buenos Aires, albeit causing significantly different levels of damage between locations. This widespread distribution might be explained by the disease being dispersed with the seed, as is known to occur for other Ascochyta diseases, for which infected seed can be the most important source of inoculum for long distance spread (Tivoli and Banniza, 2007). Notwithstanding, more studies are needed to determine the role of seed in the dispersion of the disease studied here.
Legume-associated Ascochyta species are host-specific. It has been reported that certain species can only cause disease on their respective hosts (Peever, 2007). In contrast, other Ascochyta species have shown a lower degree of specialization in host range tests. For example, Chiu and Walker (1949) found that A. cucumeris is able to produce disease to several host species within the Cucurbitaceae. This result is more in accordance with our findings since the isolate from A. hortorum was able to infect and produce disease on plants belonging to five other species within the Apocynaceae, two of them, Asclepias curassavica and Gomphocarpus physocarpus from a different subtribe than that of the preferred host. Further trials are planned to better assess the circumscription of the host range of A. araujiae.
Moth plant has become a cause of worry because of its invasive potential in New Zealand and other parts of the world where it has been introduced. As control using traditional methods is not always possible or successful, the feasibility of applying biological control is being investigated. In this regard, although the main interest as far as pathogens are concerned, is centered on the use of the rust Puccinia araujiae (Anderson et al., 2016) other complementary agents, such as A. araujiae, are being investigated. In this context, the results obtained from the specificity tests are relevant as it was shown that A. araujiae has the capacity of infecting and causing disease to G. physocarpus, known as “giant swan plant” and A. curassavica as “milkweed” or “bandera española”. Although both these species are exotic to New Zealand they are very much appreciated because they are the main hosts of larvae of the monarch butterfly Danaus plexippus (Elliott et al., 2009; Zalucki and Kitching, 1982) that has become an iconic species in New Zealand. In consequence, Ascochyta araujiae would most likely be disregarded as a potential biocontrol agent in this country.
5 Disclosure
The funding source had no involvement in the performance of this research. There is no conflict of interest.
6 Declarations of interest
None.
Author contributions
G.H.R.: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing original draft.
F.E.A.: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing, review & editing.
Acknowledgments
Jane Barton is warmly thanked for critically reading the manuscript. This research was performed in the frame of a Memorandum of Understanding signed between CERZOS-CONICET and Landcare Research New Zealand.
References
- Production of phytotoxins by Ascochyta pisi Lib., the causal agent of leaf spot disease of pea. Int. J. Agric. Biol.. 2003;5:145-149.
- [Google Scholar]
- Puccinia araujiae, a promising classical biocontrol agent for moth plant in New Zealand: biology, host range and hyperparasitism by Cladosporium uredinicola. Biol. Control. 2016;95:23-30.
- [CrossRef] [Google Scholar]
- Lentisone, a new phytotoxic anthraquinone produced by Ascochyta lentis, the causal agent of ascochyta blight in Lens culinaris. J. Agric. Food Chem.. 2013;61:7301-7308.
- [CrossRef] [Google Scholar]
- Anatomía y etnobotánica de las especies medicinales de la provincia pampeana: Asclepiadaceae [Anatomy and ethnobotany of the medicinal species of the Pampean province: Asclepiadaceae] Acta Farm. Bonaer.. 1999;18:23-31.
- [Google Scholar]
- The epidemiology and control of ascochyta blight in field peas: a review. Aust. J. Agric. Res.. 2006;57:883-902.
- [CrossRef] [Google Scholar]
- Morfoanatomía de Araujia hortorum E. Fourn. (Asclepiadaceae), especie nativa de interés medicinal [Morphoanatomy of Araujia hortorum E. Fourn. (Asclepiadaceae), native species with medicinal interest] Phyton (Buenos Aires). 2008;77:283-295.
- [Google Scholar]
- Physiology and pathogenicity of the cucurbit black rot fungus. J. Agric. Res.. 1949;78:589-615.
- [Google Scholar]
- The invasive ‘mothcatcher’ (Araujia sericifera Brot.; Asclepiadoideae) co-opts native honeybees as its primary pollinator in South Africa. AoB Plants. 2010;2010:1-14.
- [CrossRef] [Google Scholar]
- Potential Environmental Weeds in Australia: Candidate Species for Preventative Control. Canberra: Queensland Department of Natural Resources; 1998. p. :208.
- Supplemental host range of Araujia mosaic virus, a potential biological control agent of moth plant in New Zealand. Australas. Plant Pathol.. 2009;38:603-607.
- [CrossRef] [Google Scholar]
- Atlas de las plantas alóctonas invasoras en España. Madrid: Dirección General para la Biodiversidad; 2004.
- A revised classification of the Apocynaceae sl. Bot. Rev.. 2000;66:1-56.
- [CrossRef] [Google Scholar]
- Clarification of synonymy for the common moth-vine Araujia sericifera (Asclepiadaceae) Taxon. 1992;41:746-749.
- [CrossRef] [Google Scholar]
- Effects of Ascochyta blight (Mycosphaerella pinodes) on the photosynthesizing leaf area and the photosynthetic efficiency of the green leaf area of dried-pea (Pisum sativum) Plant. Pathol.. 1998;47:473-479.
- [CrossRef] [Google Scholar]
- Host specificity of Ascochyta spp. infecting legumes of the Viciae and Cicerae tribes and pathogenicity of an interspecific hybrid. Phytopathology. 2006;96:1148-1156.
- [CrossRef] [Google Scholar]
- Factors affecting growth, sporulation, pathogenicity, and survival of Ascochyta rabiei. Mycologia. 1973;65:444-457.
- [CrossRef] [Google Scholar]
- Phytotoxicity of solanapyrones produced by the fungus Ascochyta rabiei and their possible role in blight of chickpea (Cicer arietinum) Plant Sci.. 1995;109:23-29.
- [CrossRef] [Google Scholar]
- Kiwifruit Vine Health, 2016. Keep on top of moth plant. http://www.kvh.org.nz/newsroom/id/932 (Accessed 2 May 2018).
- Lathyroxins A and B, phytotoxic monosubstituted phenols isolated from Ascochyta lentis var. lathyri, a fungal pathogen of grass pea (Lathyrus sativus) J. Nat. Prod.. 2018;81:1093-1097.
- [CrossRef] [Google Scholar]
- Mel'nik, V.A., 2000. Key to the fungi of the genus Ascochyta Lib. (Coelomycetes). Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft, Parey.
- Ascochyta blight of chickpea (Cicer arietinum L.): a review of biology, pathogenicity, and disease management. Aust. J. Agric. Res.. 2005;56:317-332.
- [CrossRef] [Google Scholar]
- Role of host specificity in the speciation of Ascochyta pathogens of cool season food legumes. Eur. J. Plant Pathol.. 2007;119:119-126.
- [CrossRef] [Google Scholar]
- Fungi argentini additis nonnullis brasiliensibus montevideensibusque. Pugillus quartus. Anales Soc. Ci. Argent.. 1882;13:1-35.
- [Google Scholar]
- Morrenia odorata and Araujia sericofera (Asclepiadaceae): weeds in citrus groves. Castanea. 1976;41:139-148.
- [Google Scholar]
- Comparison of the epidemiology of ascochyta blights on grain legumes. Eur. J. Plant Pathol.. 2007;119:59-76.
- [CrossRef] [Google Scholar]
- Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci.. 2006;11:247-253.
- [CrossRef] [Google Scholar]
- Índice de fungos da América do Sul. Seção de Fitopatologia. Campinas: Instituto Agronômico; 1961. p. :921.
- La identidad sistemática del “tasi” (Araujia sp., Apocynaceae), especie sudamericana invasora en Nueva Zelanda [The systematic identity of the “moth plant” (Araujia sp., Apocynaceae), a South American species, invasive in New Zealand] In: XXXII Jornadas Argentinas de Botánica. Córdoba: Sociedad Argentina de Botánica; 2009. p. :195.
- [Google Scholar]
- Surveys for potential biocontrol agents for moth plant in New Zealand and Argentina. N.Z Plant Prot.. 2006;59:18-22.
- [Google Scholar]
- Prospects for biological control of moth plant, Araujia sericifera (Asclepiadaceae). Auckland: Landcare Research; 2000.
- Ceratocystis moniliformopsis sp. nov., an early coloniser of Eucalyptus obliqua logs in Tasmania, Australia. Austral. Syst. Bot.. 2002;15:125-133.
- [CrossRef] [Google Scholar]
- Dynamics of oviposition in Danaus plexippus (Insecta: Lepidoptera) on milkweed, Asclepias spp. J. Zool.. 1982;198:103-116.
- [CrossRef] [Google Scholar]







