Translate this page into:
Changes in antioxidants and nutritional properties during germination of soybean
⁎Corresponding author at: Institute of Specific Prophylaxis and Tropical Medicine [ISPTM], Center for Pathophysiology, Infectiology and Immunology [CePII], Medical University of Vienna, Vienna, Austria. ezzat.awad@meduniwien.ac.at (Ezzat M. Awad),
⁎⁎Corresponding author at: Department of Zoology, College of Science, King Saud University, P.O. Box 2455, Riyadh 11451, Saudi Arabia. jhattamimi@gmail.com (Jameel Al-Tamimi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Soybeans are a valuable source of nutrients and bioactive compounds. However, their nutritional quality can be limited by the presence of antinutrients, such as phytic acid and trypsin inhibitors. Germination is a natural process that can enhance the nutritional value of legumes by reducing antinutrients and increasing the bioavailability of nutrients. The objective of this study was to evaluate the effect of soaking and germination on the antioxidant and nutritional attributes of soybean. Results showed that the germination process led to a significant increase (p ≤ 0.05) in the content of phenolic, flavonoids, and antioxidat activity (%) compounds following the 72-hour germination compared to samples from ungerminated soybeans. Strong correlations were concluded between antioxidant activity and total phenolic (R2 0.92), flavonoids (R2 0.82), and ascorbic acid (R2 0.99). The germination process also enhanced proteases activities, lipase activity, and protein digestibility. Concurrently, there was a significant reduction in phytic acid from 99.20 ± 2.56 to 73.39 ± 0.85, mg/100 g as well as trypsin inhibitors from 5.73 ± 0.116 to 2.91 ± 0.126 mg/g. Germination led to these alterations contributed to an enhancement in the antioxidant and nutritional characteristics of soybean.
Keywords
Soybean
Germination
Phytic acid
Protein digestibility
Phenols
Antioxidants
1 Introduction
Despite the widely recognized nutritional and physiological benefits of soybean products, their consumption is still limited in many countries due to disagreeable beany tastes. Asian countries, however, have embraced soybean products more readily. To create products that appeal to global consumers, researchers are exploring advanced methods to enhance the properties of traditional soy foods (Gong et al., 2020).
Germination is a conventional strategy for improving the nutritional and sensory quality of soybeans (Moumita et al., 2018). During germination, soaked soybeans sprout, activating enzymes that break down complex nutrients into simpler, more bioavailable forms. This process increases levels of beneficial compounds, including vitamins (especially vitamin C), minerals, antioxidants, and phytochemicals (Kumari et al., 2015, Silva et al., 2020, Wu et al., 2023). Germinated soybeans also contain higher levels of free amino acids, making them a valuable plant-based protein source, especially for vegetarians. Additionally, germination reduces the levels of antinutritional factors like trypsin inhibitors and phytic acid, improving nutrient absorption (Bueno et al., 2020, Wu et al., 2023). This enhanced digestibility ensures better utilization of the nutrients in germinated soybeans.
Germinated soybeans exhibit higher antioxidant activity than ungerminated soybeans (Kumari et al., 2015, Bueno et al., 2020). Moreover, studies have shown that consuming germinated soybeans can lower cholesterol levels, improve blood lipid profiles (Qi et al., 2022, Zinia et al., 2022).
Germinated soybeans can be used in various food preparations, adding texture, flavor, and nutritional value. Their fresh, crunchy texture and mild, nutty taste make them popular in salads, stir-fries, soups, and sandwiches.
Germination is a cost-effective and efficient method for improving soybean quality. This study aimed to investigate the effects of the germination process on the chemical (proximate) and functional components of soybeans at ambient temperature. By analyzing changes in total phenolic content, flavonoids, antioxidant capacities, L-ascorbic acid, antinutritional factors, and digestibility, this research will provide insights into the variations in functional components and guide manufacturers in producing germinated soybeans with high nutritional value.
2 Materials and methods
2.1 Soaking and germination of soybeans
To sterilize the seeds, they were steeped in a 1 % sodium hypochlorite solution for around 15 min and then rinsed in distilled water. The sterilized seeds were soaked for 16 h in water (1:10 (w/v), drained, spread on try, and dried in a hot air oven at 45 °C for 24 h. For germination, the soaked seeds were spread on try and covered with a wet muslin cloth at 25 ± 2◦C in the dark for 72 h. After 72 h, the germinated soybeans were harvested and then dried at a temperature of 45 °C in a hot air oven for a period of 24 h.
2.2 Determination of antioxidant properties
2.2.1 Sample extraction
The soybean powder of soymilk samples were extracted according to (Guzmán-Ortiz et al., 2017) and (Leksono et al., 2022) with some modification using an acidified methanol solution (methanol: Hydrochloric acid, 99:1.0 v/v). A 2 g sample of soybean powder was agitated in 10 mL of acidified methanol on a platform shaker at room temperature for 16 h in complete darkness. The mixture was centrifuged at 10,000 g for 10 min at 4 °C (Hettich centrifuge EBA 8, Zentrifugen D-78532 Tuttlingen, Germany). The supernatant was filtered through Whatman No. 42 filter paper and kept in the dark at 5 °C to determine antioxidant activity, TP content, and TF concentration in soybean samples.
2.2.2 Total phenol content (TPC)
The total phenolic content (TPC) was quantified using a Folin-Ciocalteu method as per reference (Uddin et al., 2015). The TPC were computed and represented as milligrams of gallic acid equivalents per gram of soybean (mg of GAE/100 g).
2.2.3 Total flavonoid content (TFC)
The TFC was calculated using a colorimetric technique described in (Josipović et al., 2016). The TFC was determined and represented as milligrams of quercetin equivalents per gram of soybean (mg QE/100 g).
2.2.4 Ascorbic acid content
The determination of ascorbic acid content was conducted using the 2,6-dichlorophenol indophenol (DCPIP) dye method (Nielsen and Nielsen 2017). This colorimetric approach involves reducing DCPIP with ascorbic acid, which results in a color shift that may be measured spectrophotometrically. The ascorbic acid content was then reported as milligrams per 100 g of sample (mg/100 g).
2.2.5 Antioxidant activity
The overall potential antioxidant activity of the soybean seed sample extract was evaluated by its ability to scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals, using a modified version of the DPPH assay (Al-Saleh et al., 2014). To conduct the test, 250 µl of the sample extract was mixed with 4 ml of the DPPH working solution. After allowing the mixture to stand for 30 min, its absorbance was measured at 517 nm against a blank that contained absolute methanol instead of the sample. The activity is expressed as a percentage relative to the control.
2.3 Nutritional methods
2.3.1 Protease activity
The protease activity of soybean samples was assessed using the O-phthaldialdehyde (OPA) method, with modifications based on (Church et al., 1983). The protease activity was expressed as a degree of hydrolysis according to the following equation:
Att is the optical density (OD) of the hydrolyzed sample at time t, Bt0 is OD of the unhydrolyzed sample at time zero (control sample), and C24 is OD of the hydrolyzed sample after 24 h.
2.3.2 Lipase activity
The lipase activity was measured using the titrimetric technique described in (Mandepudi et al., 2012). Olive oil with Arabic gum in a pH 8.0 phosphate buffer served as the substrate. Lipase activity is the percentage of free fatty acids released after a 90-minute incubation, and ultimately expressed as U/g/min.
*0.67 because time conversion from 90 min to 60 min (1 h)
2.3.3 Digestibility
The study evaluated soybean protein digestibility in vitro using gastric and intestinal enzymes (pepsin and trypsin respectively) accordance with the procedure outlined by (Aboubacar et al., 2001). Samples were dissolved in phosphate buffer solution and mixed with trypsin and pepsin solutions. The mixture was incubated at 37 degrees Celsius for 90 min. After removing portions (after 0, 30, 60 and 90-min incubation), digestion was stopped with 24 % trichloroacetic acid. Samples were filtered and total nitrogen was determined using the Kjeldahl method (Kirk 1950). %digestibility was calculated using the equation.
2.3.4 Total phytic acid
The total phytic acid content in soybeans was determined using the Wade reagent method. The reagent was FeCl3ˑ6H2O and sulfosalicylic acid in distilled water at a ratio of 1:10. Soybean samples (0.5 g) were mixed with 10 mL of 2.4 % (0.64 N) HCl in Falcon tubes and shaken for 16 h at 300 rpm and room temperature. After centrifugation, the supernatant was mixed with NaCl, and a portion was further diluted and mixed with Wade reagent. After centrifugation, absorbance was measured at 500 nm against water. Phytic acid content was calculated as g/100g soybean (Darambazar et al., 2019).
2.3.5 Trypsin inhibitor activity
The modified method outlined by (Van Eys et al., 2004) was employed to assess trypsin inhibitor activity. Casein solution (1 % in PBS, pH 7.0, 0.02 M CaCl2) served as the substrate. Finely ground soybean samples (100 mesh) were dissolved in 0.01 mol/L NaOH to achieve a pH of 8.4–10. Duplicate sets of test tubes were prepared, one for the standard and the other for the sample. Sample extract and trypsin solution were added to sample tubes, while phosphate buffer was added to standard tubes. After a 15-minute incubation at 37 °C, substrate solution was added and incubated for an additional 10 min. The reaction was stopped with 30 % acetic acid, and the mixture was filtered for absorbance measurement at 410 nm using a spectrophotometer. For blank preparation, trypsin solution was added after acetic acid. The trypsin inhibitor activity, measured in Trypsin Inhibitor Units per milliliter (TIU/mL), can be calculated using the following formula:
Ar: represents the absorbance reading of the standard solution. Abr: is the absorbance of the standard blank solution. As: denotes the absorbance of the sample solution. Abs: indicates the absorbance of the sample blank solution. F: is the dilution factor applied. A 0.04 corresponds to the sample weight utilized in the analysis. This equation allows for the determination of trypsin inhibitor activity by comparing the absorbance values of standard and sample solutions, while accounting for their respective blanks and any dilution performed.
2.4 Statistical analysis
The differences in the antioxidants and nutritional attributes of raw, soaked and germinated soybeans were analyzed using the one-way analysis of variance (ANOVA) procedure of MINITAB vision 19 statistical software for windows (2010). Dunken test was applied to compare the mean values of the different compounds. Probability less than 0.05 was considered significant (p ≤ 0.05). Mean values with standard deviation media (SD) are reported. All data were analyzed using vision 19 MINITAB statistical software for windows (2010).
3 Results
3.1 Antioxidant properties
3.1.1 Total phenol
Data in Table 1, reveal that phenol contents in soybean seeds increased from 108.80 ± 20.50 mg GAE/100 g (milligrams equivalents of gallic acid) in raw soybean to 111.78 ± 4.45 and 161.41 ± 11.67 mg/100 g in soaked and germinated seeds, respectively. No significant differences were observed between raw and soaked beans, but germination significantly increased phenol content. Values are mean ± SD; means carrying the same superscript letters in the same column are for significantly different P ≤ 0.05 using Minitab.
Treatments
Total Phenol (mg/100 g)
Flavonoid (mg/100 g)
Ascorbic acid (mg/100 g)
Antioxidation activity (%)
Raw
108.80 ± 20.50b
14.64 ± 1.02c
3.47 ± 0.50c
54.76 ± 1.29b
Soaked
111.78 ± 4.45b
67.95 ± 0.45b
5.62 ± 0.76b
58.48 ± 2.57b
Germinated
161.41 ± 11.67a
87.18 ± 7.10a
11.08 ± 0.29a
65.60 ± 1.24a
3.1.2 Total flavonoids
The impact of soaking and germination on flavonoid contents were shown in Table 1. The total flavonoids in soybean seed extracts significantly increased from 14.64 ± 1.02 mg QE/100 g (quercetin equivalents), in raw seeds to 67.95 ± 0.45 mg QE/100 g in soaked seeds, and further to 87.18 ± 7.10 mg QE/100 g in germinated seeds. The total isoflavone content in whole soybean usually ranges from 50 to 450 mg/100 g raw weight (Kim et al., 2011).
3.1.3 Ascorbic acid
Ascorbic acid is an antioxidant that protects body cells against the effects of free radicals. The results in Table 1 showed the concentration of ascorbic acid in raw, soaked, and germinated soybean. The results clearly demonstrated that the soaking and germination process significantly increased the levels of ascorbic acid from 3.47 ± 0.50 mg/100 g to 5.62 ± 0.76 and 11.08 ± 0.29 mg/100 g respectively. These results in a good agreement with those obtained by (Kumari et al., 2015) where, they reported values, 3.5; 5.28 and 14.3 mg/100 g in yellow soybean in the same order. A study to (Villares et al., 2011) reported ascorbic acid values in soybean varies from 0.03 to 35 mg/100 g raw weight. However, (Xue et al., 2016, Saini and Morya 2021) did not detect ascorbic acid in raw soybean.
3.1.4 Antioxidants activity
The results presented in Table 1 illustrate the effect of soaking and germination on the antioxidant capacity of soybeans, as measured by their ability to scavenge DPPH free radicals. While no significant difference was observed between raw and soaked beans (54.76 ± 1.29 % vs. 58.48 ± 2.57 %), a notable increase in antioxidant activity was seen during germination, reaching 65.60 ± 1.24 %. Previous studies (Lien et al., 2015), reported varying levels of free radical scavenging activity in raw soybeans, with values ranging from approximately 57 % to 79 % depending on the extraction method used. The current study showed higher antioxidant activity compared to those reported by (Xue et al., 2016).
3.1.5 Correlations
The relations between antioxidant activity and total phenolic compounds, total flavonoids and ascorbic acid were statistically assessed as shown in Fig. 1. Positive and high correlations were observed (p ≤ 0.05) between antioxidant and ascorbic acid (R2 = 0.996), flavonoids (R2 = 0.816) and phenolic compounds (R2 = 0.916) respectively. The most significant correlation was observed with ascorbic acid, which showed a substantial increase (319 %, rising from 3.47 ± 0.50 to 5.62 ± 0.76 and 11.08 ± 0.29 mg/100 g) following the soaking and germination processes.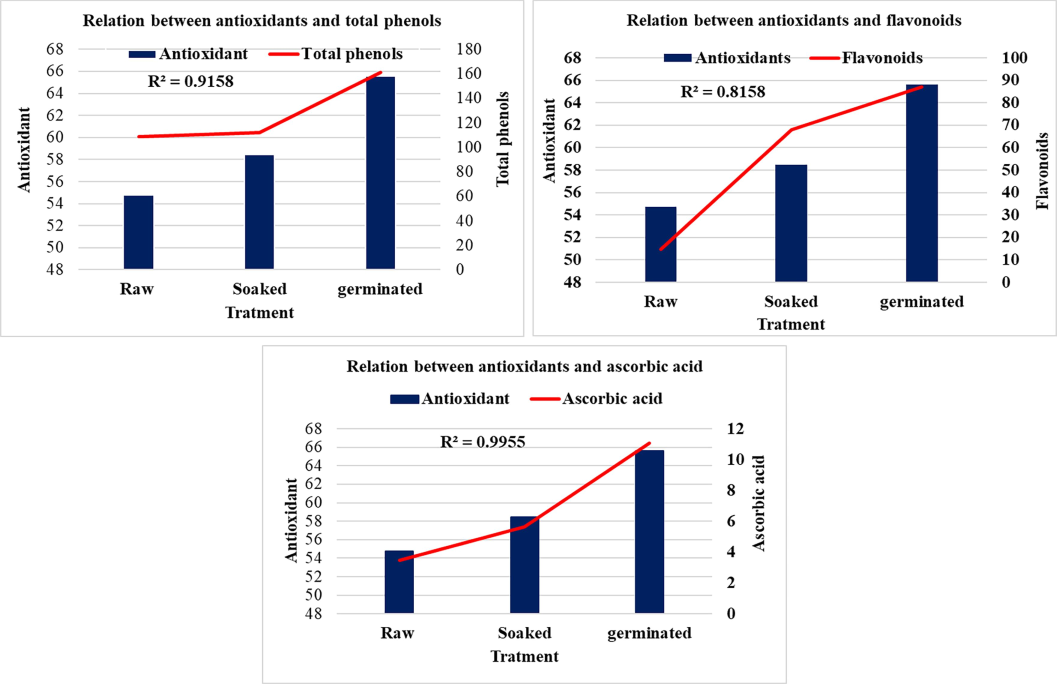
The present study revealed increase of total phenolic compound, flavonoids and ascorbic acid during germination process. The same trend was obtained by (Guzmán-Ortiz et al., 2017).
3.2 Nutritional composition
3.2.1 Protease activity
During a three-day germination process, the degree of protein hydrolysis (DH) in soybeans increased significantly. Germinated soybean has the highest protease activity. The DH in raw soybeans was 29.71 %, rising to 36.65 % after soaking and further to 59.82 % after germination (Table 2). Values mean ± SD; means carrying the same superscript letters in the same column are for significantly different P ≤ 0.05 using Minitab.
Treatments
protease activity (DH %)
lipase activity (U/g/min)
Raw
29.71 ± 0.35c
6.75 ± 0.05c
Soaked
36.65 ± 0.45b
8.45 ± 0.05b
Germinated
59.82 ± 0.33a
12.27 ± 0.98a
3.2.2 Lipase activity
The specific activity is a ratio of lipase activity (Unit/g) and protein content (mg/g), being expressed as units per mg of total proteins. Table 2 indicates the effect of germination treatment on lipase activity measured by titration method and expressed as U/g/min. A significant change in the lipase activity, due to the soaking and germination processes was observed. In soaked and germinated soybean, activities of lipase were increased by about 25 % (8.45 ± 0.05 U/g/min) and 87.46 % (12.27 ± 0.98 U/g/min) higher than control (6.75 ± 0.05 U/g/min).
3.2.3 Digestibility
According to the data in Fig. 2 a significant increase in protein digestibility is observed soon after the first 30 h of germination reaching its maximum at 90 h. The protein digestibility expressed as NPN (Fig. 2) showed a rise of 48 %, 59 %, and 62 % for raw, soaked, and germinated samples respectively when assessed with trypsin after a 90-minute incubation. Similarly, these percentages were 50 %, 53 %, and 56 % in the same order when tested with pepsin.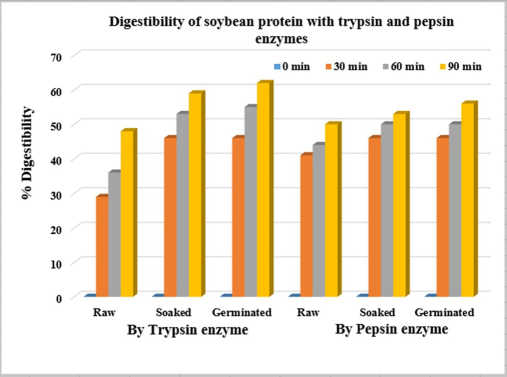
3.2.4 Phytic acid
Table 3 displays the phytic acid content in raw, soaked, and germinated soybeans. The data indicate a significant (P < 0.5) reduction in phytic acid in soaked (1.50 ± 0.04 mg/100 g) and germinated (1.24 ± 0.01 mg/100 g) soybeans compared to raw beans (1.68 ± 0.04 mg/100 g). Phytic acid decreased by 10.51 % and 26.02 % in soaked and germinated beans, respectively. Values mean ± SD; means carrying the same superscript letters in the same column are for significantly different P ≤ 0.05 using Minitab.
Treatments
Phytic Acid g/100 g
Phytic Acid (%)
Trypsin Inhibitor mg/g
Trypsin Inhibitor %
Raw
1.68 ± 0.04a
100
5.73 ± 0.12a
100
Soaked
1.50 ± 0.04b
89.49
5.36 ± 0.04b
93.45
Germinated
1.24 ± 0.01c
73.98
2.91 ± 0.13c
50.85
3.2.5 Trypsin inhibitor
Cámara et al. reported lower trypsin inhibitor values of 1.91 mg/g for Argentina, 2.83 mg/g for Brazil, and 3.0 mg/g for USA soybeans (Cámara et al., 2017), which are significantly lower than the average concentration (5.73 ± 0.12 mg/g) observed in the present study. As evident from Table 3, here, significant (p < 0.05) differences were observed in trypsin inhibitor levels among raw, soaked, and germinated soybeans. The concentration of trypsin inhibitor in raw soybeans was 5.73 ± 0.12 mg/g. Pesic et al. determined the Kunitz trypsin inhibitor (KTI) type, the most represented part of soybean trypsin inhibitor in 12 genotypes and reported values ranging from 4.28 to 6.85 mg/g, with an average of 4.94 mg/g (Pesic et al., 2007). However, Khan et al. found KTI values ranging from 0.07 to 15.9 mg/g in full-fat soy flour, with an average value of 6.81 mg/g in 102 soybean genotypes (Khan et al., 2019).
4 Discussion
Current investigation revealed that the germinated soybean possessed higher benefit compounds, Phenol. Flavonoid, Ascorbic acid and Antioxidation activity. The total phenol contents in soybean cultivars ranged from 59.61 ± 1.70 (Chen et al., 2023) to 548.0 ± 21.0 mg GAE/100 g (Guzmán-Ortiz et al., 2017). Differences in reported values among studies may be due to differences in soybean genotypes, planting areas, and analysis methods. Ma et al. attributed the increase in phenolic compounds of soybean during germination to the activity increase of the enzymes involved in the biosynthesis of phenolic acids such as of phenylalanine ammonia-lyase (PAL), cinnamic acid 4-hydroxylase (C4H) and 4-coumarate coenzyme A ligase (4CL) (Ma et al., 2023). This increase in total Flavonoids in soaked and germinated soybean in this study was reported by (Xue et al., 2016, Yang et al., 2019). Among the studied cultivars, yellow seed coats exhibited the highest flavonoid levels, whereas the Conquista cultivar, which also has a yellow seed coat but a black hilum, displayed the lowest flavonoid content (Ciabotti et al., 2016). A study (Guzmán-Ortiz et al., 2017) found that the flavonoid content increased by 314.59 % after germination for 6 days. The activation of enzymes involved in the manufacture of flavonoids from large molecular weight polyphenols, or glucosidase enzymes that create aglycones (Ribeiro et al., 2006) resulted in the increase in flavonoid concentrations during germination (Yang et al., 2019).
Ascorbic acid as presented in Table 1 increased significantly during both soaking (61.96 %) and the germination process (219 %). These findings align with the previous outcomes (Xue et al., 2016, Yang et al., 2019). A similar increase in ascorbic acid levels was observed during soaking and germination. Previous studies demonstrated found that ascorbic acid was undetectable in the raw material but increased to 1.93 mg/g after germination and reached 93.33 mg/kg after 4 days of germination (Xue et al., 2016, Yang et al., 2019). The increase in ascorbic acid during germination could be attributed to the activity increase of glutamic dehydrogenase which is the key enzyme in ascorbic acid biosynthesis (Yang et al., 2019).
A study by Guzmán-Ortiz et al. found that the antioxidant activity of soybeans germinated for 2 days was not significantly, but it significantly increased during germination (Guzmán-Ortiz et al., 2017). Similarly, Xua et al. observed a significant increase in antioxidant activity from approximately 10 % in ungerminated soybeans to around 30 % after 3 days of germination (Xue et al., 2016). Furthermore, several studies have observed substantial increases in antioxidant activity during germination, using various assays including ABTS and FRAP (Guzmán-Ortiz et al., 2017).
However, the relationship between isoflavones and antioxidant capacity was noted to depend on the structure of the isoflavones (Cvejić et al., 2009). In all cases, the germinated soybeans have the highest levels of these beneficial compounds. This suggests that germination process increases the levels of total phenol.
Germinated soybean increased the nutritional values of beans. Germination, as expected, led to an increase in DH. This aligns with previous research by Zahir et al. who found higher protease activity in germinated soybeans (Zahir et al., 2021). Similar findings were reported in other studies Xue et al. 2016 and Bueno et al. 2020, where germinated soybeans had a higher DH compared to ungerminated controls (Xue et al., 2016, Bueno et al., 2020). These studies suggest that multiple protease types are involved in the germination process. Research by Bueno et al. points towards a significant increase in trypsin-like proteases during germination (Bueno et al., 2020). Additionally, Bueno et al. identified specific soybean proteins like β-conglycinin and glycinin as targets for degradation by these proteases (Bueno et al., 2020). Germination leads to the breakdown of these proteins into smaller polypeptides. For example, β-conglycinin degrades into at least six polypeptides, while the acidic component of glycinin is more readily hydrolyzed compared to the basic component. Overall, the results highlight the dynamic changes in protein composition and structure that occur during soybean germination. The activation of proteases plays a crucial role in mobilizing stored protein reserves within the soybean seed, providing essential nutrients for seedling growth during the early stages of germination. Similarly, germinated soybean has the highest lipase activity, these findings are in accordance with those obtained by Gadge et al. where they reported values 10.37 U/ml/min for germinated soybean (Gadge et al., 2011). However, these values are higher than those reported by Paul and Rakshit, they reported values 0.053 – 0.105 and 0.064 U /g respectively (Paul and Rakshit 2023). Moreover, Paul and Rakshit reported an increase in lipase activity of germinated soybean 1.75- and 2.2 times after 3 and 5 days compared with ungerminated beans (Paul and Rakshit 2023). Chen et al. reported an increase in lipase activity with concomitant increase of free fatty acid, glycerol, and phospholipids during soybean germination (Chen et al., 2023). This may be due to the increase of lipase activities during germination process. The activation of the lipase enzyme may be caused by the involvement of the Ca+2and Mg2+ions at lower concentration as catalysts in the reaction mixture (Gadge et al., 2011). Tan et al. attributed the activity of lipase enzyme during germination to the inhibition of lipase inhibitors such as tannin and phytic acid (Tan et al., 2017). Based on the results shown in Fig. 2, it's evident that the protein digestibility using trypsin significantly increased in soaked and germinated samples compared to those using pepsin. This improvement could be attributed to the reduction in trypsin inhibitors present in soaked and germinated samples (Table 3).
This reduction in phytic acid during soaking may be due to the fact that phytic acid in raw legumes and cereals is mostly present as a water-soluble salt (likely potassium, magnesium, and manganese phytate), which is often lost in the steeping medium and rinsing during soaking (Gupta et al., 2015). Several authors have reported an inverse relationship between the decrease in phytic acid content and the increase in phytase activity during germination. Murugkar specifically noted a threefold to fivefold increase in phytase activity during germination, leading to an increase in inorganic phosphate and lower phosphoric esters (Murugkar 2014). Wu et al. documented a reduction of approximately 52.4 % in phytic acid levels during the germination of soybeans (Wu et al., 2023). They also observed a phytic acid content of about 0.15 g/100 g in germinated soybeans, compared to approximately 0.31 g/100 g in ungerminated beans, representing a decrease of roughly 48.4 %. Moreover, Pesic et al. reported a decrease in phytic acid content during germination from 740 mg/100 g in ungerminated beans to 216 mg/100 g in germinated beans (Pesic et al., 2007).
Soaking and germination can significantly reduce trypsin inhibitor levels in soybeans. These findings are in agreement with those obtained by (Kumari et al., 2015), who found a 47.24 % decrease in trypsin inhibitor levels in germinated (72 h) soybeans compared to ungerminated beans, and a decrease of 69.72 % in black soybeans over the same period. Furthermore, Wu et al. found that the trypsin inhibitor content was reduced by 22.3 % through germination (Wu et al., 2023). The decrease in trypsin inhibitors during germination can be attributed to their hydrolysis into essential amino acids to support seedling growth (Saini and Morya 2021) or to protein denaturation and their involvement in complex physiological metabolism at the early stage of germination.
5 Conclusion
Here, we found that soaking and germination significantly enhanced the antioxidant and nutritional properties of soybeans. Notably, these processes led to a substantial increase in essential components such as ascorbic acid (Vitamin C), total phenolic and flavonoid compounds, and DPPH radical scavenging capacity. Correlation analyses revealed a strong association between total phenolics, total flavonoids, and ascorbic acid levels, which collectively influenced antioxidant activity, with ascorbic acid demonstrating the most significant impact. Moreover, soaking and germination improved protease and lipase activities, protein digestibility, while simultaneously reducing phytic acid and trypsin inhibitor levels. These findings underscore the effectiveness of these methods in enhancing the overall nutritional quality of soybeans.
CRediT authorship contribution statement
Nouh M. Saleh: Writing – original draft, Methodology, Formal analysis. Ahmed S. Zahran: Writing – original draft, Methodology, Investigation. Ali A. Metwalli: Writing – review & editing, Supervision, Methodology. Ezzat M. Awad: Supervision. Hossam Ebiad: Writing – review & editing, Supervision, Project administration. Jameel Al-Tamimi: Writing – review & editing, Visualization, Project administration.
Acknowledgments
Researchers Supporting Project number (RSP2024R366), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A rapid protein digestibility assay for identifying highly digestible sorghum lines. Cereal Chem.. 2001;78(2):160-165.
- [Google Scholar]
- Antioxidative activity of camel milk casein hydrolysates. J. Camel Pract. Res.. 2014;21(2):229-237.
- [Google Scholar]
- The germination of soybeans increases the water-soluble components and could generate innovations in soy-based foods. Lwt. 2020;117:108599
- [Google Scholar]
- Chemical composition of commercial soybean meals according to the origin of the beans. Asociación Española De Ciencia Avícola (WPSA Branch) AECA. 2017;54:178-182.
- [Google Scholar]
- Ultrasound treatments improve germinability of soybean seeds: The key role of working frequency. Ultrason. Sonochem.. 2023;96:106434
- [Google Scholar]
- Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci.. 1983;66(6):1219-1227.
- [Google Scholar]
- Ciabotti, S., A. Silva, A. Juhasz, et al., 2016. Chemical composition, protein profile, and isoflavones content in soybean genotypes with different seed coat colors.
- Determination of phytoestrogen composition in soybean cultivars in Serbia. Nat. Prod. Commun.. 2009;4(8):1934578X0900400810
- [Google Scholar]
- Effect of hydrothermal, phytase, or organic acid pretreatments of canola meal on phytate level of the meal. Sustainable Agric. Res.. 2019;8(4):35-47.
- [Google Scholar]
- Gadge, P., S. Madhikar, J. Yewle, et al., 2011. Biochemical studies of lipase from germinating oil seeds (Glycine max).
- Effects of phytochemicals from plant-based functional foods on hyperlipidemia and their underpinning mechanisms. Trends Food Sci. Technol.. 2020;103:304-320.
- [Google Scholar]
- Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol.. 2015;52:676-684.
- [Google Scholar]
- Profile analysis and correlation across phenolic compounds, isoflavones and antioxidant capacity during germination of soybeans (Glycine max L.) CyTA-J. Food. 2017;15(4):516-524.
- [Google Scholar]
- Total phenolic and total flavonoid content variability of soybean genotypes in eastern Croatia. Croatian J. Food Sci. Technol.. 2016;8(2):60-65.
- [Google Scholar]
- Rapid green synthesis and characterization of silver nanoparticles arbitrated by curcumin in an alkaline medium. Molecules. 2019;24(4):719.
- [Google Scholar]
- Mobilization of storage proteins in soybean seed (Glycine max L.) during germination and seedling growth. Biochim. Biophys. Acta (BBA)-Proteins Proteomics. 2011;1814(9):1178-1187.
- [Google Scholar]
- Impact of soaking and germination durations on antioxidants and anti-nutrients of black and yellow soybean (Glycine max. L) varieties. J. Plant Biochem. Biotechnol.. 2015;24:355-358.
- [Google Scholar]
- Enhancement of antioxidant activities in black soy milk through isoflavone aglycone production during indigenous lactic acid bacteria fermentation. Fermentation. 2022;8(7):326.
- [Google Scholar]
- Effects of extraction process on phenolic content and antioxidant activity of soybean. J. Food Nutr. Sci.. 2015;3(1–2):33-38.
- [Google Scholar]
- Zinc ameliorates tripterygium glycosides-induced reproductive impairment in male rats by regulating zinc homeostasis and expression of oxidative stress-related genes. Biol. Trace Elem. Res. 2023:1-13.
- [Google Scholar]
- Optimization of media parameters for the enhanced production and activity of lipase by bacterial lipase isolates. Int. J. Biol. Sci. Technol.. 2012;4(4):23.
- [Google Scholar]
- Study of soy-fortified green tea curd formulated using potential hypocholesterolemic and hypotensive probiotics isolated from locally made curd. Food Chem.. 2018;268:558-566.
- [Google Scholar]
- Effect of sprouting of soybean on the chemical composition and quality of soymilk and tofu. J. Food Sci. Technol.. 2014;51:915-921.
- [Google Scholar]
- Bio-priming with Trichoderma enhanced faster reserve mobilization in germinating soybean cotyledons under graded macronutrients. J. Plant Growth Regul.. 2023;42(9):5461-5475.
- [Google Scholar]
- Influence of different genotypes on trypsin inhibitor levels and activity in soybeans. Sensors. 2007;7(1):67-74.
- [Google Scholar]
- Preparation and antioxidant properties of germinated soybean protein hydrolysates. Front. Nutr.. 2022;9:866239
- [Google Scholar]
- β-glucosidase activity and isoflavone content in germinated soybean radicles and cotyledons. J. Food Biochem.. 2006;30(4):453-465.
- [Google Scholar]
- Review based study on Soymilk: Focuses on production technology, prospects and progress scenario in last decade. Pharma Innov.. 2021;5(10)
- [Google Scholar]
- Silva, M. B. R., R. S. Leite, M. Á. d. Oliveira, et al., 2020. Germination conditions influence the physical characteristics, isoflavones, and vitamin C of soybean sprouts. Pesquisa Agropecuária Brasileira. 55 e01409.
- Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem.. 2017;214:259-268.
- [Google Scholar]
- Vanda roxburghii chloroform extract as a potential source of polyphenols with antioxidant and cholinesterase inhibitory activities: identification of a strong phenolic antioxidant. BMC Complement. Altern. Med.. 2015;15:1-9.
- [Google Scholar]
- Manual of quality analyses for soybean products in the feed industry. USA: American Soyabean Association; 2004.
- The influence of high pressure processing and germination on anti-nutrients contents, in vitro amino acid release and mineral digestibility of soybeans. J. Food Compos. Anal.. 2023;115:104953
- [Google Scholar]
- Bioactive compounds and antioxidant activity of mung bean (Vigna radiata L.), soybean (Glycine max L.) and black bean (Phaseolus vulgaris L.) during the germination process. Czech J. Food Sci.. 2016;34(1)
- [Google Scholar]
- Effect of chitosan pre-soaking on the growth and quality of yellow soybean sprouts. J. Sci. Food Agric.. 2019;99(4):1596-1603.
- [Google Scholar]
- Soybean germination limits the role of cell wall integrity in controlling protein physicochemical changes during cooking and improves protein digestibility. Food Res. Int.. 2021;143:110254
- [Google Scholar]
- Effects of sprouting of soybean on the anti-nutritional, nutritional, textural and sensory quality of tofu. Heliyon. 2022;8(10)
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103527.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







