Cellular network of damage caused by exposure to high ambient temperature in Wistar rats: The role of Hsp70
⁎Corresponding author at: Faculty of Medicine, University of Sarajevo, Čekaluša 90, Bosnia and Herzegovina. emina.dervisevic@mf.unsa.ba (Emina Dervišević),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
As a universal cytoprotective protein, Hsp70 is used to monitor the effects of environmental temperature changes, increasing the survival rate of cells exposed to stress, as well as the role of protein in cardiovascular disease, body decay and cell aging. Our research aims to to examine whether changes in serum protein 70 (Hsp70) values due to exposure to water temperature (41 °C and 44 °C) may indicate a mechanism of cardiomyocyte damage due to hyperthermia.
Methods
A total of 40 adult albino Wistar rats, of known gender and body weight from 250 g to 300 g were used as material in this research. Rats were housed in polypropylene cages with optimum environmental conditions. Depending on the temperature of the water (WT) to which they were exposed, rats were randomized into the following groups.
WT 37 °C (n = 8); WT 41 °C, antemortem (n = 8), WT 41 °C, postmortem (n = 8); WT 44 °C, antemortem (n = 8), WT 44 °C, postmortem (n = 8). Blood samples for determination of Hsp70 were taken before and after the expiration of the given duration of the experiment of 20 min or death. The Hsp70 level was determined by Enzyme-Linked Immunosorbent Assay (ELISA)
Results
Hsp70 basal values in serum were significantly different, p = 0.004 pg/ml, and in the groups depending on the duration of exposure to Hsp70, p = 0.002. Serum Hsp70 values after being exposed to water temperature were significantly different according to the group, p = 0.009. Significant lower Hsp70 levels were found in the control group according to G41 p = 0.006 and G44, p = 0.002. Insignificant differences in Hsp70 level were found among theseG41 and G44, p > 0.005. The concentration of Hsp70 in rat serum and ≥ 31.36 ng/ml indicates exposure to temperatures higher than 37 °C (sensitivity 85.71%, specificity 83.33%, PPV 96%, and NPV 55%, p = 0.002).
Conclusions
Altered concentration of serum Hsp70 may show exposition to the elevated water temperature.
Keywords
Overheating
Temperature
Cell damage
Hsp70
Wistar rats
1 Introduction
Heat shock proteins (HSPs) are essential for maintaining cellular homeostasis and for adapting cell functions. The first function associated with these proteins was thermotolerance. protection from subsequent exposure to heat shock. The protective role of HSPs also applies to protection against other forms of stress by controlling the structuring, sorting, degradation, translocation, and aggregation of proteins. They play an important role in signal transduction, cell skeletal organization, apoptosis, proliferation, and adhesion (Beere, 2004; Zininga et al.,2018).
Heat shock proteins are important in the adaptation of cells to many other stressors, for example infection, free radicals or mechanical stress, providing protection from harmful environmental influences and provoking an autoimmune response by cross-reacting between hsp microorganisms and their cellular components. According to molecular weight, they are classified into several families: small, hsp40-60, hsp70, hsp90 and hsp110. The expression of hsp70 is regulated at the transcriptional level by heat shock factor (HSF). The phenomenon of cross-protection in response to heat shock is the basis for the use of hsps in medicine, for example in cardiovascular diseases and in the protection of organ transplants (Farhan et al.,2021).
As a universal protective protein, Hsp70 is used to monitor the survival rate of cells exposed to stress, as well as the role of protein in cardiovascular disease, body decay and cell aging, to monitor the effects of the environment temperature changes on core temperature, his role as protein in cardiovascular disease, gastrointestinal disease, and cell aging (Yang et al., 2017, Njemini et al.,2007, Zhong et al., 2010, Wang et al., 2005).
The multifunctionality of Hsp70 protein can be divided into three related roles: prevention of aggregation, promotion of folding into a three-dimensional structure, and solubilization of aggregated proteins (Mayer and Bukau, 2005). Due to similarities in enzymatic functions, Hsp70 is believed to act by similar mechanisms in different species (Scheufler et al.,2000).
In a state of elevated body temperature in the human and animal body, it is the most sensitive protein (Hassan et al. 2019).
Heat stroke protein 70 prevents unwanted protein aggregation that occurs with aging and advanced cardiac stress (Bernardo et al., 2015). In the available literature on the role of Hsp70, the results are controversial. Chen et al. (Chen et al., 2015) investigated the role of Hsp70 in cardiac tissue due to hyperthermia exposure. Results showed its ratio of increased synthesis from the initiation of heat stress to a sharp decrease after 80 min of rat heat exposure. Decreased protein levels indicate a reduced protective role on myocytes, and increased expression of Hsp70 suggests a mechanism for improving the body's response to heat-induced stress. In their research, rats were exposed to dry heat (Chen et al., 2015). There are more and more reported cases of sudden deaths as a result of heat stroke during bathing (Bernardo et al., 2015, Chen et al., 2015). In our country, according to the available literature, such cases are less common.
Hsp levels were found to be significantly lower in subjects with cardiovascular disease and inversely proportional to the degree of atherosclerosis (Zhu et al.,2003). In a study by Eapen et al. (Eapen et al.,2014) high levels of Hsp70 are connected with a high chance of cardiac arrest. Excessive induction of Hsp70 in cultured cardiomyocytes has been shown to protect cells from thermal or ischemic stress (Mathew et al.,2000, Yoshida et al. 2000, Sugahara et al., 2003, Li et al., 2013).
Chen et al. (Chen et al., 2015) investigated the change in Hsp70 expression in cardiomyocytes, and found that temperature-induced damage was associated with a change in Hsp70 expression. Morphological changes in cells and Hsp70 levels were associated with reduced protective activity on cardiomyocytes indifferent environmental conditions. By examining the methods by which stress causes cell damage and alters cellular metabolism in vivo, Hsp70 has become a major challenge in experimental research. Hsp70 expression levels in cardiomyocytes of rats subjected to high temperature showed a slight but not significant increase, 20 min after induced heat stroke (Chen et al., 2015). The expression of Hsp70 in these cells was maximal 40 min after heat stroke. Postmortem levels of Hsp70 in rat heart tissue were significantly lower than in the control group (Chen et al., 2015). Induction of Hsp70 in the cell was also associated with myocardial protection. Elevated serum Hsp70 values after death speak in favour of exposure to high temperatures immediately before death. Oxidative stress and elevated ambient temperature have been confirmed to be responsible for the shock and sudden death of pigs and broilers as a result of sudden cardiac disease preceding cardiovascular damage (Maslov et al., 2011).
In view of the above, the aim of our study was to examine whether changes in serum Hsp70 values due to Wistar rat exposition to water temperature (41 °C and 44 °C) that cause hyperthermia, may indicate a mechanism of cardiomyocyte damage due to hyperthermia.
2 Material and methods
2.1 Experimental design
The research was carried out as a randomized, prospective, laboratory investigation done on animals who were brought to a state of hyperthermia.
After obtaining Ethical committee approval (02–3-4–1253/20) the research was conducted at the Faculty of Veterinary Medicine and Faculty of Medicine, University of Sarajevo, in agreement with the Principles of Care and Preservation of Laboratory animals (Balls M, 2022).
2.2 Experimental rats
A total number of 40 adults, albino Wistar rats, weight from 250 g to 300 g have been involved in the study. Animals were housed in polypropylene cages with optimum conditions (temperature 24 ± 2 °C, a light: dark cycle of 12:12 h), acclimatised for a week prior to the study and wached constantly for symptoms of diseases.
General anesthesia was provided by a combination injection of ketamine hydrochloride (USP Rotexmedica-Germany) and xylazine (5 mg/kg tw; 2% Xylazine, Cp Pharma, Bergdorf, Germany) once at a dose of 1.2 mL / kg tw +/- 10% before prior to a certain temperature during a certain time of exposure (Režić-Mužinić et al., 2018).
The providing general anesthesia, forty rats were exposed to priorly heated water in water bath. The rats were randomly assigned to the one of the following groups: control group (n = 8) − 37 °C WT (KG), G41-AM (antemortem) group – 20 min exposure to 41 °C WT (n = 8), G41-PM (postmortem) group − 41 °C WT exposure until death (n = 8), G44-AM (antemortem) group − 20 min exposure to 44 °C WT (n = 8), and G44-PM (postmortem) group − 44 °C WT exposure until death (n = 8).
2.3 Experimental protocol
An experimental protocol was performed for each anesthetized rat by immersion in preheated water bath water of the target temperature with the head above the surface. A probe was used to measure the core temperature of rats (RET-4 probe for mice and rats), and the core temperature was read on a thermometer (Physitemp Instruments Clifton, Physitemp Thermalert Model TH-8, USA).
For our research hyperthermia was defined as nternal temperature increase by 0.5 °C compared to normothermia, and heat stroke as core body temperature increase>40.5° C (Kidane and Peters, 2020, Bouchama and Knochel, 2002, Dervišević et al., 2022).
Serum from a blood was taken from the tail vein a week prior to the experiment and from the abdominal aorta during the experiment. After standing at room temperature (20 min), blood was centrifuged at 4000 rpm. for ten minutes, we obtained the serum and froze at −80 °C until the time we used to determine the Hsp70 value. A immunochemical enzyme labeled with an immunoabsorption method (Enzyme Linked Immuno Sorbent Assay ELISA reader, type 2100, Statfax, USA) was used for determination of levels of Hsp70 Heat shock protein 70 (Hsp70) was analysed in serum by sandwich ELISA. Optical density is measured spectrophotometrically at a wavelength of 450 ± 2 nm. The value of the optical taste is proportional to the concentration of Hsp70 rats. Sample: serum; Sensitivity 0.19 ng / ml; Detection range 0.31–20 ng / ml.
3 Results
Exposure to higher temperatures resulted in a shorter survival time, ie. that rats lived longer at lower temperatures (Fig. 1).
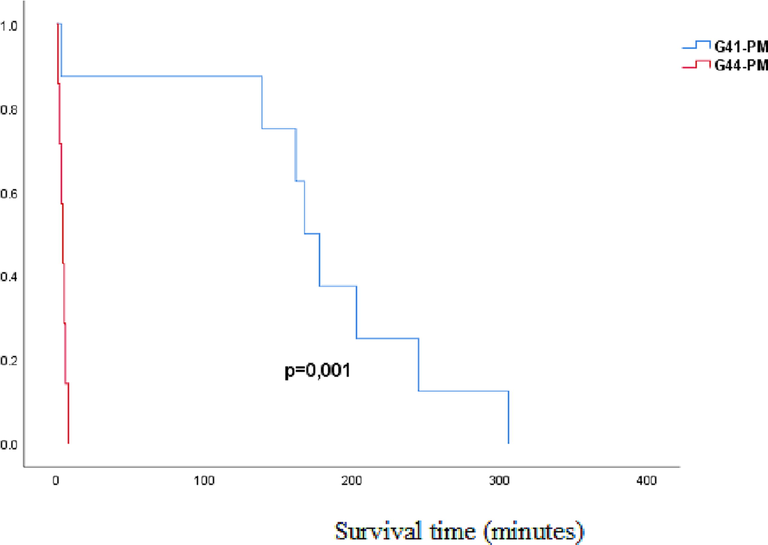
- Kaplan Mayer survival analysis of rats exposed to 41 °C and 44 °C temperatures: G41-PM-postmortem group exposed to water temperatures of 41˚C (exposure length to death); G44-PM- postmortem group exposed to water temperatures of 44˚C (exposure length to fatal outcome); p-probability.
The mean serum Hsp70 values prior to being exposed to the suitable WT in the experimental groups were different significantly p = 0.004, with the mean rank values for the groups being 9.00 ng / ml for KG37, 14.86 ng / ml for G41 and 23.79 ng / ml for G44 (Table 1, Fig. 2).
| Variable | Group | X25 | Xmed | X75 |
|---|---|---|---|---|
| Hsp-b (ng/ml) |
KG37 | 29.49 | 30.05 | 30.98 |
| G41 | 31.07 | 31.75 | 32.14 | |
| G44 | 32.02 | 33.42 | 34.25 |
Hsp-b-heat shock protein (basal values); Basal Hsp-b values (ng / ml) are represented by the median along the interquartile range (25–75 percentile); KG37-control group of rats exposed to water temperatures of 37˚C; G41- group exposed to water temperature 41˚C; G44-group of rats exposed to water temperatures of 44˚C;
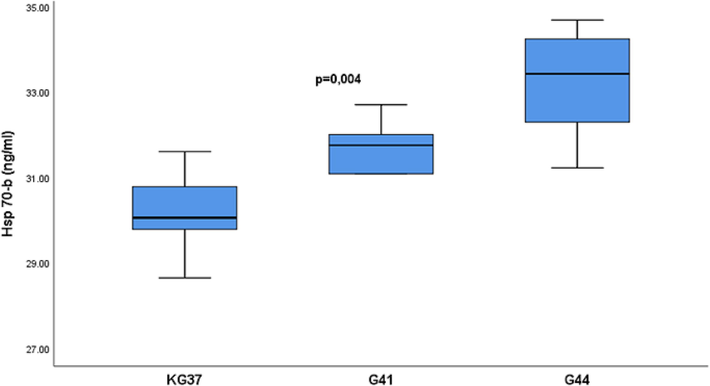
- Influence of temperature on the concentration of heat shock protein 70 in the serum of rats of experimental groups: Basal values of heat shock protein 70 are presented in median form with interquartile range (25-75percentile). Vertical lines represent the range of minimum and maximum values. Hsp70-heat shock protein 70 (basal values); p-probability; KG37-control group of rats exposed to water temperatures of 37˚C; G41- group of rats exposed to water temperature 41˚C; G44-group of rats exposed to water temperatures of 44˚C;
The difference between the mean values of the heat shock protein of isoform 70 in the serum prior to being exposed to the suitable WT depending on the experimental group is presented (Table 2).
| Variable | Group | X25 | Xmed | X75 |
|---|---|---|---|---|
| Hsp 70 (ng/ml) |
KG37 | 29.36 | 30.73 | 31.34 |
| G41 | 31.68 | 33.41 | 35.07 | |
| G44 | 32.43 | 34.15 | 35.44 |
Hsp-heat shock protein (medium values); Hsp70 values (ng / ml) are represented by the median along the interquartile range (25–75 percentile); KG37-control group of rats exposed to water temperatures of 37˚C; G41 group exposed to water temperature 41˚C; G44-group of rats exposed to water temperatures of 44˚C;
The Hsp70 mean concentration values after exposure to WT of 37˚C, 41˚C and 44˚C differed significantly (p = 0.009), with mean rank values for the groups being 6.25 ng/ml for KG37, 19.07 ng/ml for G41 and 20.75 ng/ml for G44. The highest values were detected in G44 with 34.15 ng/ml, and the lowest in the control group 30.73 ng/ml (Fig. 3).
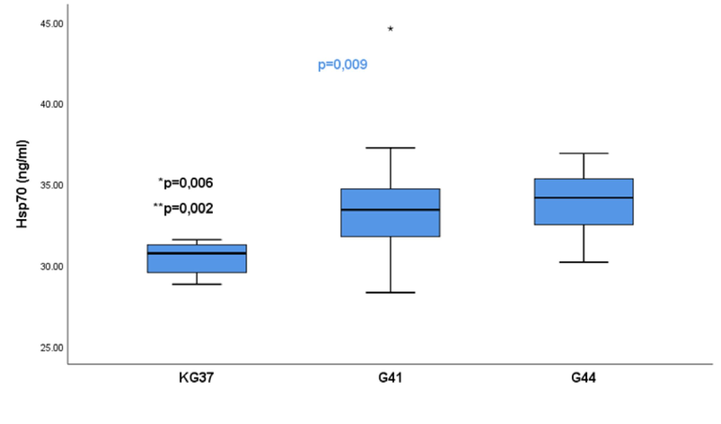
- Significance of the difference of serum values of heat shock protein 70 in the control group of rats in relation to groups G41 and G44: The values of the heat shock protein isoform 70 are presented in the form of a median with an interquartile range (25–75 percentile). Vertical lines represent the range of minimum and maximum values; p-significance of differences between groups 37,41,44; * p-KG37vs G41; ** p-KG37vsG4; KG37-control group of rats exposed to water temperatures of 37˚C; G41 group exposed to water temperature 41˚C; G44-group of rats exposed to water temperatures of 44˚C; star-extreme value in the group;
The Mann Whitney test showed that the KG group had significantly lower Hsp70 concentarion in comparison to G41, (p = 0.006) and G44, (p = 0.002). No statistical significance was found in Hsp70 value between G41 and G44, p > 0.05 (p = 0.667).
Fig. 4 shows that the values of heat shock protein 70 had different basal mean values in the serum of the experimental groups p = 0.002. The values showed individual variation and the lowest median was 30.05 ng/ml found in the KG37 group and the highest in G44-PM with 34.24 ng/ml (Table 3).
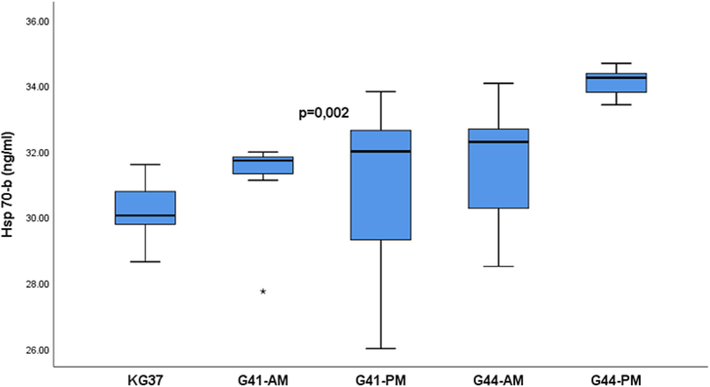
- Mean value of heat shock protein concentration 70 in rat serum in experimental groups: The values of heat shock protein 70 are presented in the form of a median with an interquartile range (25–75 percentile); Hsp 70-b-basal measurement of heat shock protein 70;. Vertical lines represent the range of minimum and maximum values; p-probability; KG37-control group of rats exposed to water temperatures of 37˚G41-AM- antemortem group exposed to water temperatures of 41˚C (exposure length 20 min); G41-PM- postmortem group exposed to water temperature 41˚C (length of exposure to death); G44-AM-antemortem group of rats exposed to water temperatures of 44˚C (exposure length 20 min); G44-PM-postmortem group of rats exposed to water temperatures of 44˚C (length of exposure to death); star-extreme value in the group;
| Variable | Group | X25 | Xmed | X75 |
|---|---|---|---|---|
|
Hsp 70-b (ng/ml) |
KG37 | 29.49 | 30.05 | 30.98 |
| G41-AM | 31.12 | 31.72 | 31.88 | |
| G41-PM | 27.54 | 32.00 | 32.70 | |
| G44-AM | 29.32 | 32.29 | 32.92 | |
| G44-PM | 33.42 | 34.24 | 34.44 |
The values of heat shock protein 70 are presented in the form of a median with an interquartile range (25–75 percentile); Hsp 70-b-basal measurement of heat shock protein 70; KG37-control group of rats exposed to water temperatures of 37˚G41-AM- antemortem group exposed to water temperatures of 41˚C (exposure length 20 min); G41-PM- postmortem group exposed to water temperature 41˚C (length of exposure to death); G44-AM-antemortem group of rats exposed to water temperatures of 44˚C (exposure length 20 min); G44-PM-postmortem group of rats exposed to water temperatures of 44˚C (length of exposure to death);
After exposure to the appropriate water temperature, a significant difference in serum Hsp70 values was found when testing the effect of temperature and length of exposure to water temperature according to the experimental groups p = 0.023 (Fig. 5).
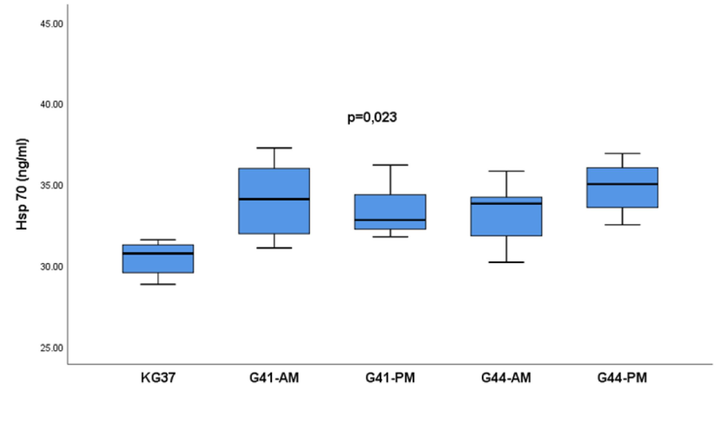
- Influence of the length of water temperature exposure of rats of experimental groups on the concentration of heat shock protein 70 in serum: The values of heat shock protein 70 are presented in the form of a median with an interquartile range (25-75percentile).; Hsp 70- measurement of heat shock protein 70;. Vertical lines represent the range of minimum and maximum values; p-probability; KG37-control group of rats exposed to water temperatures of 37˚G41-AM- antemortem group exposed to water temperatures of 41˚C (exposure length 20 min); G41-PM- postmortem group exposed to water temperature 41˚C (length of exposure to death); G44-AM-antemortem group of rats exposed to water temperatures of 44˚C (exposure length 20 min); G44-PM-postmortem group of rats exposed to water temperatures of 44˚C (length of exposure to death);
The mean serum Hsp70 was 32.81 ng/ml with an interquartile range (31.25–32.81). It was observed that the highest values of Hsp70 were reached in the group G44-PM and amounted to 35.00 ng/ml with an interquartile range (32.82–36.76), and the lowest in the control group KG37 with values of 30.73 (29.36–31.34) ng/ml (Fig. 5).
Roc analysis showed that Hsp 70 with its serum concentration ≥ 31.36 pg / ml can detect rats with a temperature higher than physiological p = 0.002 (Fig. 6).
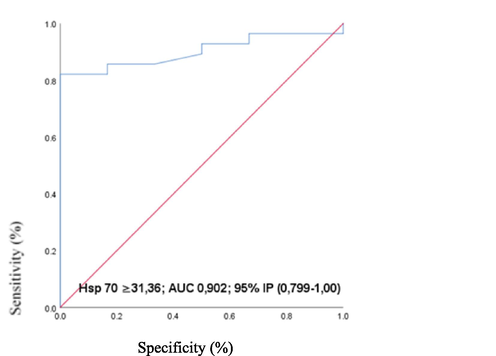
- ROC curve of serum heat shock protein levels 70 as a biomarker of rat exposure to ambient temperature higher than 37◦C: ROC-engl. Reciever Operating Characteristics; AUC-Engl. Area under curve, IP- (CI-confidence interval, confidence interval) and; p-probability; Hsp 70- isoform 70 heat shock protein;
Analysis of the role of Hsp70 as a biological marker of heat stroke and damage of the myocardium did not reveal statistically significant values, p > 0.005 (Table 4).
|
Variable |
AUC | Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
95% IP |
p |
|---|---|---|---|---|---|---|---|
| Hsp70 (ng/ml) | 0.902 | 85.71 | 83.33 | 96.0 | 55.5%0 | 0.799–1.00 | 0.002 |
AUC- area under the curve; PPV-positive predictive value, NPV-negative predictive value, 95% IP-confidence interval; p-probability; Hsp 70- isoform 70 heat shock protein;
4 Discussion
Increasingly significant changes in the temperature of the living environment are the cause of an increased number of deathsespecially in the summer months. Two significant causes of sudden death in athletes are death caused by arrhythmia and heat stroke. Death caused by arrhythmia is more the focus of medical attention while heat stroke is less considered (Yankelson et al., 2014, Lin et al., 2019). in the daily clinical practice of forensic medicine, there are great difficulties in recognizing hyperthermia as a sign of deathbecause of non-specific and even negative findings during microscopic and macroscopic examination. A lot of biochemical analyses are used for improving understanding mechanism of deadly hyperthermia (Fleshner and Johnson, 2005).
The objective of our research was to make an animal model of rat hyperthermia and to examine the importance of determining the concentration of biochemical parameter Hsp 70 in serum, which would indicate the mechanism of cardiomyocyte damage caused during exposure of rats to WT of 41 and 44°. In addition to its role in the detection of antemortem cardiomyocyte damage, the forensic significance of this biochemical marker was examined by determining the concentrations in the postmortem blood sample obtained.
Hsp 70 protein has a protective effect on the body, but it is one of the proteins that is inducible and whose expression and increase in concentration in the cell indicate hyperthermia (Heled et al.,2013). Studies have shown that the Hsp70 content in skeletal muscle increases after exposure to high temperature in the period 1 to 4 and 24 to 48 h depending on the type of fiber, while in the heart muscle a mean value is taken from the same values in skeletal muscle. This indicates that Hsp70 expression in the cell is a function of length and stress intensity. Previous researches indicate that deteriorated cardiovascular system is very often coomplication in patients with heat stress, which ultimately manifests as cardiomyocyte apoptosis and heart failure (Leon and Helwig, 2010). Due to heat stress, oxidative stress and accumulation of inflammatory mediators play a major role, which ultimately leads to cell apoptosis (Leon and Helwig, 2010). Hyperthermia in the rat model induces Hsp70 synthesis after exposure for a period of 3 to 72 h where the preconditioning effects are 48 to 72 h. In addition to acting as a molecular chaperone in myocardial tissue, Hsp70 is involved in the orchestration of the inflammatory response. Given that hyperthermia of the organism is observed in the context of the inflammatory response (Kruger et al., 2019), extracellular Hsp70 could be a link between cell damage and the pathogenesis of the inflammatory condition. In vitro data suggest that Hsp70 could be a biochemical stress sensor (Bathaie et al., 2010). The half-life of Hsp70 in the cell is two hours, and the values are extended to seven hours with continuous exposure to heat stress. The decrease in the value in the cell is related to the decrease in the acquired thermotolerance. A results from Bathaie et al. (Bathaie et al., 2010) indicates that long-term exposure to warm water has affected Hsp 70 serum level. Hsp70 has been reported to activate the autophagic process in response to heat stress and protects against heat stress induced organ damage (Tsai et al., 2016, Shen et al., 2019).
Our study did not aim to examine the protective effects of Hsp70 but to examine changes in serum concentrations that could be used to demonstrate exposure to high temperature as a cause of death. The basal values of Hsp70 in serum were significantly different between three groups (37,41,44), p = 0,004 and also between five subgroups groups (KG37, G41-AM, G41-PM, G44-AM and G44-PM), p = 0.002, which indicates interindividual variations. A study by Xiao et al. (Xiao et al., 2003) indicates the existence of interindividual variations in basal and inducible levels of Hsp70 which implies greater sensitivity or possible different response. A study by Shenn et al. (Shen et al., 2019) reported that Hsp70 protein expression in the heat stroke-group was significantly higher than that in the control group in the hearts. In the experimental model, taking into account these data, we can say that the measured serum Hsp70 concentration after exposure to water temperature is a reflection of changes in the cell membrane and leakage of contents into the circulation. By examining the influence of rat organism exposure to water temperatures of 37˚C, 41˚C and 44˚C, significant differences in Hsp70 concentration were found (p = 0.009). Hsp70 concentrations were significantly higher in G41 (p = 0.006) and G44 (p = 0.002) compared to KG37. Examination of the influence of the length of exposure to water temperature showed that Hsp70 values differed significantly, p = 0.023, and that differences in Hsp70 concentration by groups were different only according to the control group, and between other experimental groups the difference was not significant (p > 0.05). This indicates that despite the difference in the length of exposure to 41˚C and 44˚C, the concentration of Hsp70 was not significantly different in terms of terminal damage to the body by exposure to high temperature.
Based on the literature on the inducibility of Hsp70 with prolonged exposure to high temperatures, we can not exclude the possibility of additional synthesis of Hsp70 in the cell, its consumption in the cell for the purpose of repairing damage, but also its excretion into the circulation. Heat shock protein 70 with its serum concentrations taken antemortem indicated exposure to ambient temperatures higher than physiological, which we confirmed by measuring the core temperature. The performance of Hsp70 is optimal at a body temperature of about 37° C. At temperature levels above 41° C, proper functioning of the proteins is hampered, and a further increase destruction called denaturation (Zininga et al., 2018). The absence of a significant difference in the serum concentration of Hsp70 in rats taken antemortem and postmortem indicates that this protein, as measured by the serum concentration, does not indicate terminal myocardial damage caused by high water temperature in the model we studied.
In a study by Chen et al. (Chen et al.,2015) the association between the kinetics of Hsp70 expression and cardiomyocyte damage exposed to 42˚C in vitro and in vivo was investigated. The results showed that the activity of enzymes in serum such as aspartate aminotransferase (AST), alanine aminotransferase (ALT) and creatine kinase was increased as a reflection of acute histopathological lesions suggesting that heat stress alters cardiomyocyte membrane integrity in vitro and in vivo. The authors state that the critical period is 60 min of exposure because in that period the consumption of Hsp70 exceeds the synthesis, and that only after that period the cells produce enough Hsp70 to protect against hyperthermic damage.
Considering the results of our study that Hsp70 with its serum concentration in rats significantly differentiates rats exposed to water temperature of 37˚C from rats exposed to water temperatures of 41˚C and 44˚C, we examined serum concentrations indicating exposure of the body to water temperature>37˚C. Values of ≥ 31.36 ng / ml in rat serum may indicate exposure to temperatures>37 °C with a sensitivity of 85.71% and a specificity of 83.33%. The positive predictive value was 96% and the negative predictive value 55% on the animal model used.
It is developed an experimental model to prove that Hsp70 has a prooxidant role and that it has an advantage in conditions of hyperthermia over the activity of antioxidant enzymes. Induction of Hsp70 at temperatures above 40 °C helps monocytes survive oxidative stress during infection, as Hsp70 is known to protect cells from superoxide radicals.
The results of the study by Oehler et al. (Oehler et al., 2001) indicate that changes in body temperature and induction of Hsp70 in leukocytes caused by hyperthermia help leukocytes justify their key function in the immune response during heat stroke. Immunopositivity in cases of hyperthermia has been described in a study by Ishikawa et al. (Ishikawa et al., 2008), and refers to multiple organ dysfunction. In our experimental study, we did not examine the effect of hyperthermia on rats other than the heart.
The body's response to heat stroke is an adaptive, molecular, cytoprotective mechanism that protects cells and tissues from overexposure to heat and other harmful stimuli such as hypoxia, infection and ischemic reperfusion injury, and includes heat shock protein production, of which Hsp70 is considered among the most sensitive. In our study, we were guided by changes in the cardiomyocyte membrane that under controlled conditions of hyperthermia lead to the release of Hsp70 into the circulation where the concentration can be determined to assess the effects of hyperthermia.
5 Conclusion
Altered concentration of serum Hsp70 may show exposition to the elevated water temperature. In Hsp70, the importance of serum concentrations in the detection of exposure to high ambient temperature was determined, but not the role in the detection of terminal heart muscle damage.
Also, there was a limitation, further research should consider more complex models that include several integrators and other afferent signals taking into account the stages of the heat flow mechanism, but also the interindividual variations of the response that cannot be excluded.
Institutional Review Board Statement
The animal study protocol was approved by the Ethical Committee of Medical Faculty University of Sarajevo under registration number 02–3-4–1253/20, Bosnia and Herzegovina, for studies involving animals.
Informed Consent Statement
Not Applicable.
Funding
This research received no external funding.
CRediT authorship contribution statement
Emina Dervišević: Writing – original draft, Conceptualization, Methodology. Sabaheta Hasić: Supervision, Conceptualization, Methodology. Emina Kiseljaković: Data curation, Resources, Software, Validation, Formal analysis. Radivoj Jadrić: Resources. Lejla Dervišević: Writing – review & editing, Data curation, Software, Validation, Formal analysis. Zurifa Ajanović: Writing – review & editing, Software, Validation, Formal analysis. Adis Salihbegović: Writing – original draft, Software, Validation, Formal analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alternatives to Laboratory Animals: Trends in Replacement and the Three Rs. Altern Lab Anim.. 2022;50(1):10-26. Epub 2022 Mar 20 PMID: 35311373
- [CrossRef] [Google Scholar]
- The effect of hot-tub therapy on serum Hsp70 level and its benefit on diabetic rats: a preliminary report. Int J Hyperthermia. 2010;26(6):577-585.
- [Google Scholar]
- The stress of dying: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci.. 2004;117:2641-2651.
- [Google Scholar]
- Long-Term Overexpression of Hsp70 Does Not Protect against Cardiac Dysfunction and Adverse Remodeling in a MURC Transgenic Mouse Model with Chronic Heart Failure and Atrial Fibrillation. PLoS ONE. 2015;10(12):145-173.
- [Google Scholar]
- Localization and expression of heat shock protein 70 with rat myocardial cell damage induced by heat stress in vitro and in vivo. Mol Med Rep. 2015;11(3):2276-2284.
- [Google Scholar]
- Forensic significance of cTnI serum for the detection of terminal myocardial damage in rats (Rattus norvegicus) caused by hyperthermia. JKSUS.. 2022;34(2):1018-3647.
- [Google Scholar]
- The Evaluation of Novel Biomarkers and the Multiple Biomarker Approach in the Prediction of Cardiovascular Disease. Curr Cardiovasc Risk Rep. 2014;8:408.
- [Google Scholar]
- The Role of Heat Shock Proteins in Cellular Homeostasis and Cell Survival. Cureus.. 2021;13(9):e18316.
- [Google Scholar]
- Endogenous extra-cellular heat shock protein 72: Releasing signal(s) and function. Int J Hyperthermia. 2005;21:457-471.
- [Google Scholar]
- Prospects of HSP70 as a genetic marker for thermo-tolerance and immuno-modulation in animals under climate change scenario. Anim Nutr.. 2019;5(4):340-350.
- [CrossRef] [Google Scholar]
- Cytokines and their role in hyperthermia and heat stroke. J Basic Clin Physiol Pharmacol. 2013;24(2):85-96.
- [Google Scholar]
- Postmortem biochemistry and immunohistochemistry of adrenocorticotropic hormone with special regard to fatal hypothermia. Forensic Sci Int. 2008;179:147-151.
- [Google Scholar]
- Role of heat shock proteins 70/90 in exercise physiology and exercise immunology and their diagnostic potential in sports. J Appl Physiol. 2019;126(4):916-927.
- [Google Scholar]
- Leon, L.R., Helwig, B.G. 2010. Heat stroke: role of the systemic inflammatory response. J Appl Physiol (1985). 109(6), 1980-8.
- Heat shock protein 70 acts as a potential biomarker for early diagnosis of heart failure. PLoS One. 2013;8(7):e67964.
- [Google Scholar]
- Biochemical detection of fatal hypothermia and hyperthermia in affected rat hypothalamus tissues by Fourier transform infrared spectroscopy. Biosci Rep. 2019;39(3):BSR2018163.
- [Google Scholar]
- Role of heat shock proteins in the mechanism of cardioprotective effect of transient hyperthermia and delayed preconditioning. Patol Fiziol Eksp Ter. 2011;4:64-73.
- [Google Scholar]
- Analysis of the mammalian heat-shock response. Inducible gene expression and heat-shock factor activity. Methods Mol Biol. 2000;99:217-255.
- [Google Scholar]
- Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci.. 2005;62(6):670-684.
- [CrossRef] [Google Scholar]
- Heat shock proteins and chemokine/cytokine secretion profile in ageing and inflammation. Mech Ageing Dev.. 2007;128(7–8):450-454.
- [Google Scholar]
- Cell type-specific variations in the induction of hsp70 in human leukocytes by feverlike whole body hyperthermia. Cell Stress Chaperones. 2001;6(4):306-315.
- [Google Scholar]
- Expression of adhesion molecules on granulocytes and monocytes following myocardial infarction in rats drinking white wine. PLoS One. 2018;13(5):e0196842.
- [Google Scholar]
- Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70–Hsp90 multichaperone machine. Cell. 2000;101(2):199-210.
- [Google Scholar]
- Alpha-Lipoic Acid Protects Cardiomyocytes against Heat Stroke-Induced Apoptosis and Inflammatory Responses Associated with the Induction of Hsp70 and Activation of Autophagy. Mediators Inflamm.. 2019;2019:8187529.
- [Google Scholar]
- Heat shock transcription factor HSF1 is required for survival of sensory hair cells against acoustic overexposure. Hear Res. 2003;182(1–2):88-96.
- [Google Scholar]
- Heat shock protein 70 and AMP-activated protein kinase contribute to 17-DMAG-dependent protection against heat stroke. J Cell Mol Med.. 2016;20(10):1889-1897.
- [Google Scholar]
- Effects of intrauterine growth retardation on development of the gastrointestinal tract in neonatal pigs. Biol Neonate. 2005;88(1):66-72.
- [Google Scholar]
- Basal and inducible levels of Hsp70 in patients with acute heat illness induced during training. CSC. 2003;8(1):86-92.
- [Google Scholar]
- Integrating a human thermoregulatory model with a clothing model to predict core and skin temperatures. Appl Ergon. 2017;61:168-177.
- [Google Scholar]
- Life-threatening events during endurance sports: is heat stroke more prevalent than arrhythmic death? JACC. 2014;64(5):463-469.
- [Google Scholar]
- Sound conditioning reduces noise-induced permanent threshold shift in mice. Hear Res. 2000;148(1–2):213-219.
- [Google Scholar]
- Heat shock protein 70 is upregulated in the intestine of intrauterine growth retardation piglets. Cell Stress Chaperones.. 2010;15(3):335-342.
- [CrossRef] [Google Scholar]
- Increased serum levels of heat shock protein 70 are associated with low risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2003;23:1055-1059.
- [Google Scholar]
- Heat shock proteins as immunomodulants. Molecules.. 2018;23:2846. PMID: 30388847; PMCID: PMC6278532
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102654.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







