Cardioprotective effects of Callicarpa tomentosa leaf extract in Wistar albino rats against isoproterenol-induced myocardial necrosis: Phytochemical analysis and in vitro antioxidant study
⁎Corresponding author at: Department of Pharmacy Practice, College of Pharmacy, AlMaarefa University, Dariyah 13713, Riyadh, Saudi Arabia (S.M.B. Asdaq); Department of Pharmacology, Yenepoya Medical College, 575018, Mangalore, Karnataka, India (R.P. Nayak). roopapnayak@yenepoya.edu.in (Roopa P. Nayak), sasdag@um.edu.sa (Syed Mohammed Basheeruddin Asdaq), sasdaq@gmail.com (Syed Mohammed Basheeruddin Asdaq),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Globally, cardiovascular disease (CVD) remains the foremost cause of mortality. Conventional medications are associated with several complications, necessitating an alternative, safe and efficacious medicine. Therefore, the study was planned to assess the cardioprotective efficacy of Callicarpa tomentosa (CT) leaf extract against isoproterenol (ISO)-induced myocardial failure in experimental rats.
Materials and methods
CT leaf extract was subjected to phytochemical analysis, total phenolic and flavonoid content measurement, and in vitro antioxidant evaluation. Additionally, the protection from myocardial injury was evaluated based on the estimation of biomarkers including CK-MB, CKNAC, and LDH in both the low-dose (200 mg/kg) and high-dose (400 mg/kg) groups of rats. We measured endogenous antioxidants in heart tissue to estimate the antioxidant potential. Histopathological analysis confirmed the biochemical results.
Results
The findings of this study indicated that the CT leaf extract contained several phytoconstituents such as alkaloids, flavonoids, steroids and triterpenoids, cardiac glycosides, carbohydrates, phenols, saponins, and tannins. The CT's total phenol content was 33.3 ± 0.14 mg GAE (Gallic Acid Equivalent) per gram of extract, while its total flavonoid concentration was 76.5 ± 1.2 mg QUE (Quercetin Equivalent). In the DPPH assay, CT had a mean inhibitory effect of 60.06 ± 1.358 g/ml, whereas its IC50 in the H2O2 radical scavenging activity assay was 102.85 ± 0.194 g/ml. The electrocardiograms of the treated animals demonstrated considerable recovery from ISO-induced electrophysiological abnormalities as well and the amount of endogenous biomarkers was significantly reduced in animals that received CT treatment. Additionally, the use of CT reversed the effects of ISO administration on SOD activity, increased GSH levels, and lowered LPO levels. The damage to the heart tissues was significantly reduced, with the tissue architecture appearing closer to normal in groups that received high doses of CT.
Conclusion
These results suggest that C. tomentosa leaf extract has potent antioxidant activity and provides protection against isoproterenol-induced cardiac necrosis in Wistar albino rats. The cardioprotective benefits are attributed to the presence of active components in the extract, which aid in the restoration of antioxidant equilibrium and the reduction of damage caused by oxidative stress.
Keywords
Antioxidant
Cardioprotective activity
Callicarpa tomentosa
Isoproterenol
Myocardial infarction
- CVD
-
Cardiovascular disease
- MI
-
Myocardial infarction
- C. tomentosa(CT)
-
Callicarpa tomentosa
- ISO
-
Isoproterenol
- H2O2
-
Hydrogen peroxide
- NO
-
Nitric oxide
- DPPH
-
2,2-diphenyl-1-picrylhydrazyl
- AST
-
Aspartate Aminotransferase
- ALT
-
Alanine Aminotransferase
- ALP
-
Alkaline Phosphatase
- CK-MB
-
Creatine Kinase-MB
- CKNAC
-
Creatine Kinase-N-Acetyl Cysteine
- LDH
-
Lactate Dehydrogenase
- GSH
-
Glutathione
- LPO
-
Lipid Peroxidation
- SOD
-
Superoxide Dismutase
Abbreviations
1 Introduction
Cardiovascular diseases (CVD) persist as a predominant global health concern, maintaining their status as the leading cause of mortality. Despite advancements in medical interventions, ischemic heart conditions, particularly acute myocardial infarction (MI), remain a formidable threat. MI is marked by a high mortality rate (7%) and significant morbidity (22%), contributing to adverse cardiac hypertrophy and decreased left ventricular capacity. Current therapeutic approaches, including beta-blockers, calcium antagonists, and angiotensin-converting enzyme inhibitors, while effective, often fall short in addressing the complex pathogenesis of MI, marked by an imbalance in leukocytes, triacylglycerol, and cholesterol levels (Ong et al., 2018; Fajobi et al., 2020; Wahid et al., 2022). Recognizing the limitations of conventional treatments, there is a growing interest in exploring alternative, natural sources for potential cardioprotective agents.
Amidst the pursuit of alternative treatments, studies have highlighted the potential of antioxidant-rich fruits, vegetables, and plants in mitigating the risk of cardiovascular diseases. The consumption of such natural products has demonstrated notable cardio-protective properties attributed to their antioxidant, anti-inflammatory, and thrombolytic characteristics (Morakinyo et al., 2018). This has fueled the investigation of plant-derived compounds as potential therapeutic options. In this context, Callicarpa tomentosa, a species of the beauty berry plant, has garnered attention for its diverse phytoconstituents, including lupeol, oleanolic acid, sterol B, β-sitosterol-glucoside, and ursolic acid. The leaves of Callicarpa tomentosa are reputed for their myriad medicinal properties, presenting an opportunity for exploring its potential as a natural cardioprotective agent (Ray et al., 2022).
Callicarpa tomentosa's candidacy as a therapeutic agent aligns with the broader interest in natural products for cardiovascular health. Phytochemical analysis of medicinal plants has consistently revealed bioactive substances such as flavonoids, phenolic compounds, alkaloids, and terpenoids, showcasing antioxidant and cardioprotective potential (Zulfkar et al., 2014). Unraveling the phytochemical composition of Callicarpa tomentosa leaves becomes imperative to understand the mechanisms underpinning its potential cardioprotective effects.
The induction of myocardial injury, a pivotal aspect of cardiovascular research, often involves the use of isoproterenol, a synthetic sympathomimetic amine. Isoproterenol administration triggers oxidative stress by promoting the excess production of reactive oxygen species (ROS), leading to cardiac tissue damage (Asdaq et al., 2021). In this study, we aim to assess the antioxidant activity of Callicarpa tomentosa leaf extract in scavenging ROS and potentially protecting against oxidative stress-induced myocardial necrosis. Wistar albino rats, chosen for their genetic uniformity and physiological similarity to humans (Das et al., 2015), will serve as valuable experimental models to evaluate the cardioprotective activity of Callicarpa tomentosa leaf extract against isoproterenol-induced myocardial injury. Through this research, we aspire to uncover the phytochemical composition, antioxidant potential, and cardioprotective effects of the ethanolic extract of Callicarpa tomentosa leaves, laying the groundwork for potential therapeutic interventions in acute myocardial infarction and other cardiovascular disorders.
2 Materials and methods
2.1 Collection of C.tomentosa leaves
Leaves of Callicarpa tomentosa were gathered from the Udupi Manjunath Melkaje Medicinal Garden. The leaves were authenticated by Dr. H.S Shenoy, Principal Scientist and Head of the Division at Pilikula Nisargadama, Mangalore. A voucher specimen (No:106/Callicarpa tomentosa (L). L).
2.2 Preparation of the Plant Extract
The collected leaves were dried in the shade and then processed through a dry grinder to produce a coarse powder. The powder of the leaf was sieved through a 20-mesh sieve. The 50 g of powdered sample was subjected to extraction in a soxhlet apparatus method by using 500 mL of 99% ethanol as a solvent and maintaining a temperature of 55 °C. After extraction, the solvents were dried using a water bath the temperature was maintained at 80°C, and the extract was subjected to phytochemical tests. Phytochemical analysis was performed following standard methods (El Faqer et al., 2022; (Mani et al., 2015); Rai et al., 2019; Sarma and Kaur, 2016).
2.3 Estimation of total Phenolics
The number of phenolic compounds present in, specifically gallic acid (GA) and leaf extract was determined using a (Shahinuzzaman et al., (2021) method. Initially, the leaf extract was first combined with methanol (10 µg/ml) in a mixture of 20 µl. The resulting solution was then mixed with 100 µl of the Folin-Ciocalteaus reagent and 1500 µl of water. For eight minutes, this mixture was incubated at room temperature in a darkened space. After that, 300 µl of a 20% sodium bicarbonate solution was taken and added to the mixture, and it was then incubated for an additional 2 h in the dark at 30 °C. Finally, a wavelength of 765 nm was used to measure the solution’s absorbance.
2.4 Estimation of total flavonoids
Quercetin was dissolved in methanol to prepare a solution with a concentration of 0.1% (10 mg per milliliter). This was achieved by dissolving 0.1 g of quercetin in 10 mL of methanol. Quercetin was used as a standard, and its flavonoid content was measured in terms of quercetin equivalent. Dilutions of quercetin ranging from 0.025 to 5 mg per milliliter were prepared using the standard solution in methanol. Then, 100 µl of each quercetin dilution was mixed with 500 µl of distilled water. To this mixture, 100 µl of a 5% sodium nitrate solution was added, followed by a 6-minute incubation period. Afterward, 150 µl of a 10% aluminum chloride solution was added, and the mixture for 5 min. Finally, 200 µl of a 1 M sodium hydroxide solution was added (Martínez-Inda et al., 2023)., and the resulting reaction solution was then subjected to measurement of its absorbance at 510 nm using a UV spectrophotometer.
2.5 DPPH Radical Scavenging Assay
Accordance with the method of Rumpf et al., (2023), a solution of DPPH (0.75 mL) was combined with plant extract (0.25 mL) at various concentrations ranging from 20 to 100 µg/mL. The mixture was subsequently placed in a dark environment area at a temperature of 30◦C for 30 min. The solution’s absorbance was measured at a wavelength of 517 nm, using ascorbic acid as a reference. The calculation for determining the DPPH scavenging effect was as follows:
The outcomes were reported as the average percentage inhibition ± standard deviation, based on a sample size of three (n = 3).
2.6 Hydrogen Peroxide (H2O2) Radical Scavenging Assay
In their 2023 study, (Hlophe and Bassey, 2023)conducted experiments to evaluate the scavenging efficacy of an extract against hydrogen peroxide (H2O2). A solution of H2O2 was prepared with a concentration of 43 mM in a 0.1 M phosphate buffer solution at pH 7.4. Then, they mixed 1 mL of the samples with the H2O2 solution and allowed the mixture to incubate for 10 min. After the incubation period, they measured the absorbance of the reaction mixture at 230 nm. To serve as a baseline, they prepared a blank solution containing only the phosphate buffer without H2O2. Additionally, they used ascorbic acid as a reference compound to compare the results obtained from the extract.
2.7 Nitric Oxide (NO Radical Scavenging Assay
The Sarma et al. protocol (2016) was followed to measure the scavenging activity of nitric oxide radicals. Firstly, a solution containing sodium nitroprusside (SNP) at a concentration of 10 mM was prepared. This SNP solution was mixed with the extract in a phosphate buffer at a pH of 7.4 and a concentration of 0.2 M. The solution was then incubated at a temperature of 25◦C for 150 min. After the incubation period, the Griess reagent, consisting of 1% naphthalene diamine dichloride and 2% phosphoric acid, was added to the mixture Anusmitha et al., (2022). The resulting solution was analyzed by measuring its absorbance at a wavelength of 546 nm.
2.8 Animal Study
2.8.1 Drugs, reagents, and chemicals
Isoproterenol hemisulfate and drug metoprolol were procured from Sigma Pvt.Ltd (Man- galore, India. The enzyme kits of AST, ALT, CK-MB, and LDH were purchased from agappe Pvt. Ltd. (Mangalore, India).
2.8.2 Experimental animals
The study utilized healthy adult male Wistar albino rats with weights ranging from 170 to 220 g. The rats used in the study were obtained from a breeder who was authorized by the Committee for Control and Supervision of Experiments on Animals (CPCSEA). They were housed in an animal facility that also received approval from the same organization. The research protocol was reviewed and approved by the Institutional Animal Ethical Committee of Yenepoya Medical College (Approval No. YU/IAEC/8/2020), located in Deralakatte, Mangalore. The rats were divided into five groups, each containing six animals, and the study was conducted following the guidelines and approval of the institutional animal ethics committee.
2.8.3 Study design: (n = 6)
Group 1: Normal group treated with a control substance. Group 2: Disease group treated with ISO (85 mg/kg). Group 3: Standard drug group treated with metoprolol (10 mg/kg) in addition to ISO (85 mg/kg). Group 4: Low dose group treated with Callicarpa tomentosa ethanolic extract (CTLD) (200 mg/kg) in addition to ISO (85 mg/kg). Group 5: High dose group treated with Callicarpa tomentosa ethanolic extract (CTHD) (400 mg/kg) in addition to ISO (85 mg/kg).
2.8.4 ISO-induced myocardial necrosis
In the experiment, mice were treated with CTLD and CTHD for 28 days. After the last treatment, Isoproterenol (ISO) was injected subcutaneously at a dose of 85 mg/kg for two successive days to induce myocardial necrosis. Electrocardiographic parameters were recorded 48 hours after the first ISO dose while the animals were under anesthesia. Blood samples were collected to measure serum biomarkers including AST, ALT, ALP, CK- MB, CKNAC, and LDH. The heart tissue was removed and homogenized to measure tissue antioxidants (Rong et al., 2023).
2.8.5 In vivo antioxidant assay
2.8.5.1 Lipid Peroxidation Assay
The method reported by Thiruchelvam et al., (2003) was applied to assess the level of lipid peroxidation using thiobarbituric acid (TBA). To experiment, the following components were combined: 1.5 mL of acetic acid (20% concentration, pH 3.5), 0.5 mL of fly homogenate, 1.5 mL of a TBA solution (0.8% concentration), and 0.2 mL of sodium lauryl sulfate (8% concentration). The mixture was then subjected to heat in a boiling water bath for 45 min. Afterward, the resulting compounds were extracted into 3 mL of 1-butanol. The extract’s absorbance was measured at 532 nm and converted into malondialdehyde equivalents, which are used as an indicator of lipid peroxidation.
2.8.5.2 Estimation of Reduced Glutathione
The method described by Fun et al., (2011) was used to measure the levels of reduced glutathione at room temperature using a technique called Ellman’s method (1959). In this method, a supernatant solution of 0.75 mL was combined with 0.75 mL of a 4% sulpho salicylic acid solution. The resulting mixture was then subjected to centrifugation at 1200 rpm for 5 min at a temperature of 40◦C. After centrifugation, the absorbance of the solution was measured at a wavelength of 412 nm using a UV–visible spectrophotometer. For the measurement, 0.5 mL of the mixture was mixed with 4.5 mL of a 0.01 M solution of Ellman’s reagent (also known as DTNB or 5,5′-dithiobis-(2-nitrobenzoic acid).
2.8.5.3 Catalase activity
According to the procedure described by Khalil et al., (2005), estimation of catalase activity was performed at room temperature. The process involved mixing a 100 µL sample of liquid remaining after centrifugation with 10 µL of pure ethanol and cooling it in an ice bath for 30 min. After cooling, the tubes were returned to room temperature, and 10 µL of Triton X-100 was added. Then, 50 µL of the resulting mixture was combined with 0.066 M H2O2 (250 mL) in phosphate buffer within a cuvette containing 200 mL of phosphate buffer.The spectrophotometer measured the reduction in light absorption at 240 nm over 60 s. To calculate catalase activity, a molar extinction coefficient of 43.6 Mcm-1 was used, which represents the number of moles of H2O2 broken down per milligram of protein per minute.
2.8.5.4 Superoxide dismutase assay (SOD)
The method described by Hassan et al., (2012) was used to measure the activity of Superoxide Dismutase (SOD), a mixture was prepared by combining 100 µL of the supernatant with 880 µL of a carbonate buffer containing 0.05 M concentration and 0.1 mM of EDTA at pH 10.2. Then, 20 µL of a solution containing epinephrine at a concentration of 30 mM in 0.05% CH3COOH was added to the mixture. The optical density (OD) values were recorded at 480 nm using a UV–UV-visible spectrophotometer for 4 min. The activity of SOD was determined by measuring the quantity of the enzyme required to inhibit the oxidation of epinephrine by 50%, which is defined as 1 unit of SOD activity.
2.9 Histological analysis
Histological sections of the heart were prepared and treated with hematoxylin and eosin stain. The stained section slides were examined to identify any alterations in the tissue structure. The extent of myocardial damage was evaluated using a scoring system that takes into account the severity of the observed changes (Ahmed et al., 2023).
2.10 Statistical analysis
The data was reported as the mean standard error of the mean (SEM) and statistical analysis was performed using a one-way analysis of variance (ANOVA) followed by the Tukey-Kramer post hoc test. If the p-value was<0.05, then the results were considered statistically significant.
3 Results
3.1 Plant phytochemicals
The ethanolic extraction of Callicarpa tomentosa leaves resulted in a yield of 8%. Qualitative phytochemical tests were conducted to analyze the compounds present in the extract. CT leaf extract contained alkaloids, flavonoids, steroids and triterpenoids, cardiac glycosides, carbohydrates, phenols, saponins, and tannins.
The phenolic content of a C. tomentosa extract was assessed by analyzing its absorbance using a calibration curve. The curve was obtained by measuring the absorbance of different concentrations of Gallic acid in methanol, ranging from 0.025 to 5 mg/ml. The calibration curve exhibited a linear relationship with a slope of 0.212 and a regression coefficient of 0.985. Utilizing this curve, the total phenolic content of the C. tomentosa extract was determined to be 33.3 ± 0.14 mg GAE (Gallic Acid Equivalent) per gram of extract (supplementary Table 1).
The concentration of flavonoids in the C. tomentosa leaf extract was measured by determining the amount of flavonoids using a calibration curve constructed with Quercetin dissolved in methanol. The calibration curve consisted of various concentrations of Quercetin (ranging from 0.025 to 5 mg/ml), and the corresponding absorbance values were recorded. The calibration curve exhibited a linear with a gradient of 0.163 and a regression coefficient of 0.990. Employing this calibration curve, the overall flavonoid content in the C. tomentosa extract was determined to be 76.5 ± 1.2 mg QUE (Quercetin Equivalent) per gram of extract (supplementary Table 2).
3.2 Antioxidant activity
In the DPPH assay, the leaf extract of C.tomentosa demonstrated good antioxidant activity. Ascorbic acid exhibited a dose-dependent increase in mean inhibition, reaching a high inhibition percentage of 90.24% at 100 µg/ml. C.tomentosa also showed an increase that varies based on the dosage in mean inhibition, with a maximum inhibition of 86.58% at 100 µg/ml. The mean ± SD values for ascorbic acid and C. tomentosa were 56.71 ± 0.356 µg/ml and 60.06 ± 1.358 µg/ml, respectively.
In the hydrogen peroxide (H2O2) assay, ascorbic acid at 100 µg/ml showed a mean inhibition of 49.12%. In contrast, C.tomentosa exhibited relatively lower antioxidant activity in this assay, with a mean inhibition ranging from 0.25% to 29.18% across the tested concentrations. The mean SD values for the IC50 (half-maximal inhibitory concentration) of ascorbic acid and C.tomentosa were 96.25 ± 0.767 µg/ml and 102.85 ± 0.194 µg/ml, respectively.
In the NO assay, C.tomentosa demonstrated antioxidant effects. Ascorbic acid displayed a 49.99% at 100 µg/ml. C.tomentosa also showed an increase that varies based on the dosage in mean inhibition, with a maximum inhibition of 42.59% at 100 µg/ml. The IC50 values for ascorbic acid and C. tomentosa were 128.27 ± 0.372 µg/ml and 96.25 ± 1.716 µg/ml, respectively.
3.3 Animal model experiment:
Our study also investigated the effects of C.tomentosa leaf extract on electrocardiographic parameters in rats. We compared the results to a normal control group and an ISO control group (rats administered with isoproterenol). Furthermore, we evaluated the effects of Metoprolol, a known medication for cardiovascular conditions.
The examination of the electrocardiogram revealed significant observations. The experimental group treated with ISO displayed an elevated heart rate in comparison to the control group without any treatment. Additionally, the ISO control exhibited a decrease in the QRS complex and QT interval when compared to the normal control group. However, when Metoprolol or C. tomentosa was administered at doses of 200/400 mg/kg, there was a notable increase in the QRS complex and QT interval compared to the ISO control group (Table 1).
| Parameters | R-R (ms) | P-R (ms) | P-Q (ms) | Q T (ms) | QRS (ms) | ST (mV) |
|---|---|---|---|---|---|---|
| NC | 204.42 ± 0.12 | 60.40 ± 0.11*** | 35.42 ± 0.13*** | 113.41 ± 0.41 | 66.38 ± 0.13 | 0.160 ± 0.001 |
| ISO 85 mg/ml | 141.42 ± 0.10abc | 35.40 ± 0.11abc | 16.33 ± 0.10abc | 75.44 ± 0.15abc | 43.31 ± 0.10abc | 0.711 ± 0.002abc |
| Metoprolol 10 mg/ml | 200.36 ± 1.10*** | 59.46 ± 0.14*** | 34.48 ± 0.11*** | 103.57 ± 0.41*** | 63.40 ± 0.13*** | 0.207 ± 0.001*** |
| C.tomentosa 400 mg/ml | 184.29 ± 0.09*** | 50.34 ± 0.14*** | 32.38 ± 0.14*** | 97.23 ± 0.08*** | 60.48 ± 0.14*** | 0.250 ± 0.001*** |
| C.tomentosa 200 mg/ml | 172.43 ± 0.10*** | 40.39 ± 0.12*** | 22.41 ± 0.14*** | 75.44 ± 0.13 | 47.24 ± 0.08*** | 0.458 ± 0.001*** |
Values were expressed as mean ± SEM. Values were considered statistically significant when aP < 0.05, abP < 0.01, abcP < 0.001 as compared to the normal group and *P < 0.05, **P < 0.01, ***P < 0.001 compared to the ISO treated group (n = 6).
In addition, the group of subjects treated with ISO (isoproterenol) showed shorter PR, PQ, and RR intervals compared to the control group. However, when treated with either Metoprolol or C.tomentosa at doses of 200/400 mg/kg, the ISO group exhibited improvements in their PR and RR intervals, returning them closer to normal levels.
The study also observed ST-segment elevation (red arrow in Fig. 1 b) in the ISO control group, which indicates myocardial infarction, as opposed to the normal control group. However; the observed effects of C.tomentosa on electrocardiographic parameters in this study suggest a potential protective role against ISO-induced alterations in the heart. The increase in QRS complex and QT interval, as well as the recovery from abnormal PR and RR intervals, indicate a positive influence on cardiac function.
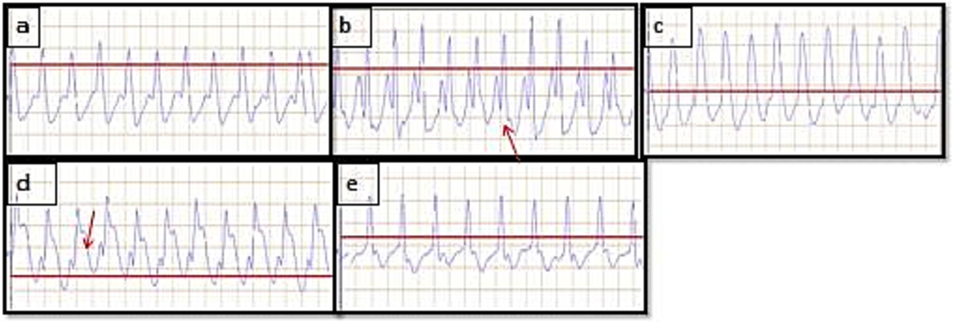
-
Effect of C.tomentosa on ISO 85 mg/kg induced changes of ECG parameters in rats. Images of (a) Normal control (b) ISO control, (c), Metoprolol (10 mg/kg), (d) C.tomentosa (200 mg/kg) treated (e) C.Tometosa (400 mg/kg) treated treated rats. The red arrow represents ST elevation in rats treated with ISO. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Moreover, our result illustrates a significant rise in the levels of certain cardiac marker enzymes, including AST, ALT, CK-MB, CK-NAC, ALP, and LDH, in the ISO control rats when compared to the normal control rats. Nevertheless, administering Metoprolol and C. tomentosa extract before the treatment led to a reduction in the levels of these enzymes, as observed in the ISO control group.
The ISO control group exhibited noticeably higher levels of ALT, AST, CK-MB, CK-NAC, ALP, and LDH compared to the normal control rats. Nonetheless, prior administration of Metoprolol and various doses of C. tomentosa extract effectively decreased the enzyme levels in comparison to the ISO control group (Table 2).
| Parameter | ALT (IU/L) |
AST (IU/L) |
CK-MB (IU/L) |
CK-NAC (IU/L) |
ALP (IU/L) |
LDH (IU/L) |
|---|---|---|---|---|---|---|
| NC | 38.88 ± 2.74 | 63.14 ± 2.36 |
144.18 ± 2.92 |
236.41 ± 3.83 | 278.32 ± 4.64 | 284.84 ± 3.34 |
|
ISO 85 mg/ml |
113.1 ± 1.12abc |
216.17 ± 1.23abc | 545.87 ± 4.25abc | 728.81 ± 2.36abc | 522.47 ± 4.52abc | 1152.09 ± 4.09abc |
| Metaprolol 10 mg/ml | 50.76 ± 0.91*** | 68.43 ± 1.11*** | 335.88 ± 2.32*** | 249.41 ± 4.51*** | 345.82 ± 0.83*** | 425.15 ± 2.87*** |
| C.tomentosa 400 mg/ml | 68.65 ± 4.68*** | 99.49 ± 4.82*** | 349.53 ± 4.53*** | 260.58 ± 4.20*** | 376.10 ± 4.43*** |
475.43 ± 3.64*** |
| C. tomentosa 200 mg/ml | 75.88 ± 0.55*** | 124.51 ± 1.69*** | 373.14 ± 4.38*** | 447.25 ± 4.79*** | 480.73 ± 4.33*** | 677.09 ± 4.11*** |
The results were reported as mean SEM and SD analyzed using one-way ANOVA followed by Tukey-Karmer multiple comparison tests. Values were considered statistically significant when aP < 0.05, abP < 0.01,abcP < 0.001 as compared to the normal group and ∗P < 0.05, ∗∗P < 0.01,∗∗∗P < 0.001 compared to the ISO 85 mg/ml group (n = 6). ALT (Alanine transaminase), AST(Aspartate transaminase), CK-MB(creatine kinase-MB), CK-NAC (creatine kinase-NAC), ALP- Alkaline phosphatase, LDH- Lactate dehydrogenase.
These findings suggest that the subcutaneous injection of ISO caused damage to the heart muscle, which was confirmed by a notable rise in levels of cardiac marker enzymes in the bloodstream (Thiruchelvam et al., 2003). However, pretreatment with Metoprolol and C. tomentosa extract mitigated these alterations, indicating their potential cardioprotective effects.
In terms of the in vivo antioxidant enzymes, Table 3 demonstrates that ISO administration resulted in a reduction in cardiac GSH (glutathione) levels and a rise in LPO (lipid peroxidation) levels, indicating oxidative stress and damage. ISO also decreased the activity of the antioxidant enzyme SOD (superoxide dismutase). However, treatment with Metoprolol and C. tomentosa extract restored GSH levels, decreased LPO levels, and counteracted the decrease in SOD activity induced by ISO administration.
| Parameter |
Glutathione (µg/mg of protein) |
LPO (µg/mg of protein) |
Catalase (µg/mg of protein) |
SOD (µg/mg of protein) |
|---|---|---|---|---|
| Normal control |
54.16 ± 0.54 |
2.07 ± 0.12 |
93.47 ± 1.239 |
19.98 ± 0.714 |
| ISO 85 mg/kg |
6.36 ± 0.35 abc | 13.01 ± 0.34 abc | 41.13 ± 0.124 abc | 2.29 ± 0.036 abc |
| Metoprolol 10 mg/kg |
31.11 ± 0.27*** | 4.95 ± 0.039*** | 87.80 ± 0.702*** | 8.66 ± 0.542*** |
|
C. tomentosa 400 mg/kg |
22.61 ± 0.31*** |
3.06 ± 0.21*** |
78.85 ± 0.219*** |
13.73 ± 0.033*** |
|
C. tomentosa 200 mg/kg |
19.05 ± 0.56*** |
10.27 ± 0.10*** |
44.038 ± 2.191** |
8.76 ± 0.303*** |
The results were reported as mean ± SEM and analyzed using one-way ANOVA followed by Tukey-Karmer multiple comparison tests. Values were considered statistically significant when aP < 0.05, abP < 0.01, abcP < 0.001 as compared to the normal group and *P < 0.05, **P < 0.01, ***P < 0.001 compare ISO 85 mg/ml group (n = 6). LPO-Malondialdehyde, (Lipid peroxidation), SOD- Superoxide dismutase.
These results suggest that ISO administration led to oxidative stress and impaired antioxidant defense mechanisms. However, pretreatment with Metoprolol and C. tomentosa extract exhibited antioxidant properties that protect against ISO-induced oxidative damage. However, the observed cardioprotective effects of Metoprolol and C. tomentosa extract reduced serum cardiac marker enzymes and restored antioxidant enzyme levels.
In a histopathological examination of heart tissues stained with hematoxylin and eosin (H&E), the microscopic analysis revealed that the heart tissues of normal control rats displayed a normal architecture. However, significant damage was observed in the rats treated with ISO (isoproterenol) at a dosage of 85 mg/kg. The observed damage included features such as myocardial necrotic cells, nuclear pyknosis (shrinkage and increased staining intensity of cell nuclei), and inflammatory cell infiltration in response to ischemia. These changes were classified as grade 3, indicating severe damage (Fig. 2).
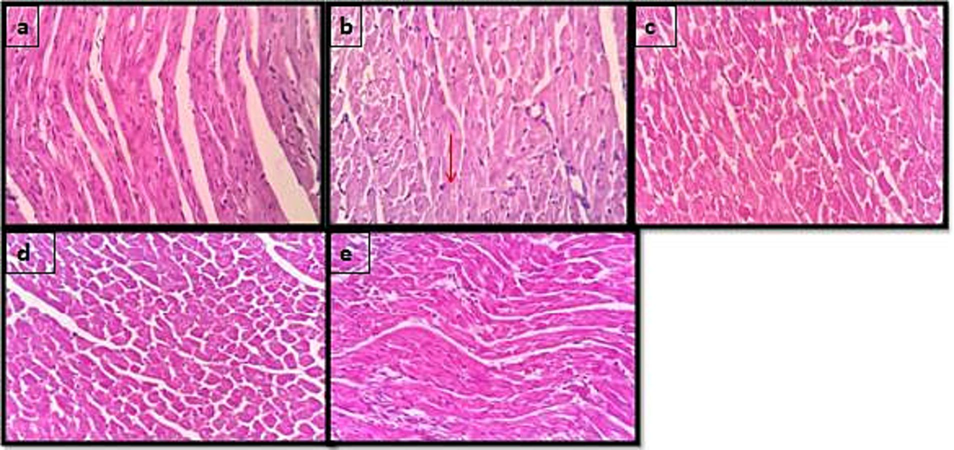
-
Effect of C.tomentosa on the morphology of heart in ISO-induced myocardial damage in Wistar albino rats (magnification 40×). 2(a) Normal control, 2(b) ISO 85 mg/kg, 2(c) Metaprolol 10 mg/kg, 2(d) C.tomentosa 200 mg/ and 2(e) C.tomentosa 400 mg/kg.
On the other hand, rats treated with a lower dose of C.tomentosa (a plant extract) at 200 mg/kg showed grade 2 changes in the heart tissues, which refer to moderate damage. When animals were treated with a higher dose of C.tomentosa at 400 mg/kg, grade 1 changes were observed, which refer to mild damage. In this case, the damage to the heart tissues was notably improved, with the architecture of the tissue appearing closer to normal. Furthermore, the heart tissues of animals treated with metoprolol (a commonly used beta-blocker medication) resembled the normal architecture of the control group.
4 Discussion
In this study, we selected the leaf of Callicarpa tometosa which is recommended for a wide range of ailments including fever, hepatic obstruction, hepatic eruption, and skin diseases, and used as a wash for aphthae in the mouth. The Phytochemical analysis revealed the presence of various bioactive compounds in the leaf extract of Callicarpa tomentosa. These compounds include flavonoids, phenolic compounds, terpenoids, and other phytoconstituents. These findings are consistent with a previous study by Zulfkar (2014) that reported the presence of similar phytochemicals. In various medicinal plants, the study by Yilmaz et al. (2023), Phong et al. (2022), and Kısa et al. (2022) shows the presence of these phytoconstituents known for their cardioprotective properties. Flavonoids and phenolic compounds, in particular, have been associated with potent antioxidants which could potentially contribute to the observed protective effects on the heart tissue.
The antioxidant assessment using DPPH, H2O2, and nitric oxide assays demonstrated the scavenging ability of Callicarpa tomentosa leaf extract against free radicals and reactive oxygen species (ROS). Previous studies conducted on C. tomentosa also indicated that it possesses scavenging potential against DPPH radicals at the concentration of 17.12 µg/ml. (Ray et al., 2022). The DPPH assay measures the ability of the extract to scavenge stable radicals, while the H2O2 and nitric oxide assays assess its ability to quench hydrogen peroxide and nitric oxide radicals, respectively. The observed antioxidant activity indicates that the leaf extract may effectively protect cardiac tissues from oxidative damage, which is a significant factor in myocardial necrosis, these results are in line with previous studies (Thanh et al., 2023; Derbali et al., 2015) on myocardial infarction can be defined from diverse perspectives involving clinical, biochemical, and pathological characteristics. However, one of the primary criteria for definitively diagnosing myocardial infarction often relies on electrocardiogram (ECG) abnormalities. A significant indicator has been shown by the conducted study which observed an ST-segment elevation in rats with isoproterenol-induced myocardial infarction. This change in the amplitude of the ST segment serves as a key indicator of myocardial ischemia.
The recent findings from ECG assessments indicated notable improvements in various electrocardiographic parameters among rats treated with extract of Callicarpa tomentosa leaves, in comparison to the group of rats with isoproterenol-induced necrosis. This suggests that the plant extract could potentially have positive effects on cardiac electrophysiology and conduction, thereby reinforcing its potential for cardioprotection. The influence of natural compounds on cardiac function underscores the possibility that the regulation of cardiac electrophysiology might constitute a common mechanism of action for cardioprotective agents (Beyazcicek and Beyazcicek, 2023).
The analysis of biochemical markers revealed a decrease in cardiac biomarkers like creatine kinase-MB (CK-MB), CK-NAC, and lactate dehydrogenase (LDH) in rats treated with extract from Callicarpa tomentosa leaves. These biomarkers are typically elevated in cases of myocardial injury, and the observed reduction implies a safeguarding effect on heart tissue. Similar outcomes have been documented in studies conducted by Shen et al. (2019) and Kushwah et al. (2022) where they investigated the impact of injected ISO on myocardial cells. The research indicated that ISO could cause damage to the myocardial cell membrane, leading to the release of cardiac enzymes into the bloodstream. Elevated enzyme levels in the blood are abnormal and suggest compromised plasma membrane integrity or permeability, serving as a diagnostic indicator for assessing the severity of myocardial infarction (MI).
In the current study, the in vivo antioxidant activity was investigated, revealing a noteworthy reduction in oxidative stress markers—such as malondialdehyde (MDA) and reactive oxygen species (ROS)—among the treated rats, when compared to the group with isoproterenol-induced necrosis. This compelling outcome suggests that the administered plant extract effectively bolstered the endogenous antioxidant defense system, thereby contributing to the substantial alleviation of oxidative stress within the myocardium. Notably, Callicarpa tomentosa leaf extract emerged as a potent agent in mitigating oxidative damage to the cardiac tissue. Additionally, a related study conducted by Sajid et al. (2022) involving rats exposed to ISO exhibited a discernible safeguarding effect on cardiac tissue when treated with a specific substance. This protective action is likely attributable to the substance's adeptness in scavenging free radicals. Encouragingly, the study's findings indicated a reduction in lipid peroxidation (LPO) and a decrease in the levels of cellular antioxidant defense enzymes, along with smaller antioxidants like reduced glutathione.
The body's inherent enzymatic defense against oxidative stress plays a pivotal role in countering tissue damage caused by oxygen-free radicals. Key enzymes, notably superoxide dismutase (SOD), catalase, and glutathione peroxidase (GSH-Px), act as the primary line of defense by efficiently scavenging free radicals and offering initial cellular protection against oxidative damage. Functioning by neutralizing oxygen (O2) and hydrogen peroxide (H2O2), intercept potential reactive molecules before they can instigate the formation of highly reactive hydroxyl radicals, thus upholding the integrity of cellular structures (Sajid et al., 2022).
In the present research, a histopathological examination demonstrated a notable decrease in myocardial damage, inflammation, and necrosis among the rats subjected to treatment with Callicarpa tomentosa leaf extract these findings strongly indicate that the plant extract offers a safeguarding effect on cardiac tissue, effectively counteracting the detrimental consequences of isoproterenol-induced necrosis. The studies conducted earlier (Dianita et al., 2015), and Hosseini et al (2022) revealed significant histopathological evidence, illustrating severe myofibrillar degeneration along with extensive neutrophil infiltration and interstitial edema within the tissues of the isoproterenol-induced control group.
Flavonoids are the largest group of naturally occurring phenolic compounds occurring in plants. They are present in free states as well as glycosides (Zulfikar et al., 2014). According to the literature, the presence of phenolic compounds exhibits cardioprotective properties against several diseases (Ullah et al., 2020). Some of the important actions documented for cardioprotection are anti-inflammation, anti-hypertension, hypolipidemic, and mitigation of endothelial dysfunction (Wen et al., 2021). In addition, alteration of oxidative stress, sirtuin activity, survival signaling and calcium homeostasis were found to be linked to the phenolic compound-induced cardiac protection (Bailly, 2021). Studies have suggested that inhibition of nuclear factor kappa B, tumor necrosis factor-α, interleukin-6, macrophage inflammatory protein-1, and p-selectin activities are some of the mechanisms responsible for the beneficial effects in cardiac diseases after the administration of phenolic compounds (Ali et al., 2021).
In comparison with other similar studies, the findings of the current study align well with the existing literature on the cardioprotective effects of natural compounds. Many medicinal plants, including those rich in flavonoids, phenolic compounds, and other phytoconstituents, have been reported to possess antioxidant properties and exert protective effects on the heart tissue in various experimental models of myocardial necrosis. Callicarpa tomontosa might have exerted a similar mechanism due to the antioxidant potential in attenuating the cardiac damages induced by isoproterenol in rats. Chemical profiling to identify the precise bioactive constituent as well as studies involving the parameters of safety and efficacy might determine the true potentiality of the plant in different pharmacological activities including cardiac protection.
5 Conclusion
The protective effects were assessed in rats with myocardial necrosis, showing that Callicarpa tomentosa leaf extract treatment notably enhanced cardiac biomarkers, reduced myocardial damage, and improved histopathological changes in heart tissue. The activity could be linked to the presence of phytochemicals such as phenolic compounds in the extract. These bioactive compounds could have mitigated the oxidative stress induced by isoproterenol leading to cardiac damages. These results suggest the extract's cardioprotective properties, highlighting its potential in preventing or managing myocardial necrosis. Being derived from nature, the compound could be a promising agent in reducing the complications of cardiovascular diseases. However, more research is needed to establish the safety and efficacy of the extract and also to identify the precise active phytoconstituents responsible for the biological effects.
Funding
The authors would like to express gratitude to King Saud University, Riyadh, Saudi Arabia, for extending financial support to do this research project through the Researchers Supporting Project number (RSPD2023R853). The authors are also thankful to AlMaarefa University for supporting this research.
Acknowledgments
The authors would like to acknowledge the financial support offered by the Researchers Supporting Project number (RSPD2023R853) at King Saud University, Riyadh, Saudi Arabia. This work was financially supported by the government of Karnataka fellowship (43/2019-20/154). The researchers express their gratitude to the administration of Yenepoya (Deemed to be University), Mangalore, for granting them the necessary facilities to carry out this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cardioprotective Effect of Flibanserin against Isoproterenol-Induced Myocardial Infarction in Female Rats: Role of Cardiac 5-HT2A Receptor Gene/5-HT/Ca2+ Pathway. Pharmaceuticals. 2023;16(4):502.
- [CrossRef] [Google Scholar]
- Current Updates on Potential Role of Flavonoids in Hypoxia/Reoxygenation Cardiac Injury Model. Cardiovasc. Toxicol.. 2021;21(8):605-618.
- [Google Scholar]
- Phytochemical analysis, antioxidant, anti-inflammatory, anti-genotoxic, and anticancer activities of different Ocimum plant extracts prepared by ultrasound-assisted method. Physiol. Mol. Plant Pathol.. 2022;117:101746
- [CrossRef] [Google Scholar]
- The potential benefits of using garlic oil and its active constituent, dially disulphide, in combination with carvedilol in ameliorating isoprenaline-induced cardiac damage in rats. Front. Pharmacol.. 2021 Sep;27(12):739758
- [CrossRef] [Google Scholar]
- The subgroup of 2'-hydroxy-flavonoids: Molecular diversity, mechanism of action, and anticancer properties. Bioorg. Med. Chem.. 2021;15(32):116001
- [Google Scholar]
- Protective effects of Lacticaseibacillus rhamnosus on isoprenaline-induced myocardial infarction in rats. J. Appl. Microbiol.. 2023;134(1):lxac008.
- [CrossRef] [Google Scholar]
- Natural products in the treatment of cardiovascular diseases: Lessons learned from traditional medicine. In: Bagchi D., Preuss H.G., Swaroop A., Bagchi M., eds. Nutraceuticals and Functional Foods in Human Health and Disease Prevention. Boca Raton, FL: CRC Press; 2015. p. :643-665.
- [Google Scholar]
- Cardioprotective effect of linseed oil against isoproterenol-induced myocardial infarction in Wistar rats: a biochemical and electrocardiographic study. J. Physiol. Biochem.. 2015;71:281-288.
- [CrossRef] [Google Scholar]
- Protective effects of Labisia pumila var. alata on biochemical and histopathological alterations of cardiac muscle cells in isoproterenol-induced myocardial infarction rats. Molecules. 2015;20(3):4746-4763.
- [CrossRef] [Google Scholar]
- Phytochemical characterization and immunomodulatory effects of aqueous, ethanolic extracts, and essential oil of Syzygium aromaticum L. on human neutrophils. Scientific African. 2022;18:e01395.
- [Google Scholar]
- Cardioprotective activities of Pterocarpus mildbraedii leaves on isoproterenol-induced myocardial infarction in rats. Eur. J. Med. Plants. 2020;31(10):20-37.
- [Google Scholar]
- 4-[2-(4-Chlorophenyl)hydrazinylidene]-3-methyl-1H-pyrazol-5(4H)-one. Acta Crystallographica Section E. 2011;67:o2670
- [CrossRef] [Google Scholar]
- Synthesis and antimicrobial evaluation of novel Pyrazolones and Pyrazolo nucleosides. Nucleosides Nucleotides Nucleic Acids. 2012;31:783-800.
- [CrossRef] [Google Scholar]
- Phytochemical Profiling, and Antioxidant Potentials of South African and Nigerian Loranthus micranthus Linn. The African Mistletoe Exposé. Plants. 2023;12(10):2016.
- [CrossRef] [Google Scholar]
- Attenuation of isoprenaline-induced myocardial infarction by Rheum turkestanicum. Biomed. Pharmacother.. 2022;148:112775
- [CrossRef] [Google Scholar]
- Metal salt-catalyzed diazo coupling of 3-substituted-1H-pyrazol-2-in-5-ones in an aqueous medium. Dyes Pigm.. 2005;66:241-245.
- [CrossRef] [Google Scholar]
- Assessment of antimicrobial and enzymes inhibition effects of Allium kastambulense with in silico studies: Analysis of its phenolic compounds and flavonoid contents. Arab. J. Chem.. 2022;15(6):103810
- [CrossRef] [Google Scholar]
- Cardioprotective Activity of Cassia fistula L. Bark Extract in Isoproterenol-Induced Myocardial Infarction Rat Model. Evidence-Based Complem. Alternative Med. 2022
- [CrossRef] [Google Scholar]
- Phytochemicals as potential cardioprotective agents: Role in oxidative stress and inflammation. In: Bagchi D., Preuss H.G., Swaroop A., Bagchi M., eds. Nutraceuticals and Functional Foods in Human Health and Disease Prevention. Boca Raton, FL: CRC Press; 2015. p. :353-375.
- [Google Scholar]
- Valorization of agri-food waste through the extraction of bioactive molecules. Prediction of their sunscreen action. J. Environ. Manage.. 2023;325:116460
- [CrossRef] [Google Scholar]
- Evaluation of antioxidant, anti-inflammatory and phytochemical constituents of Aframomum melegueta aqueous leaf extract. Int. J. Med. Plants Photonics. 2018;112:888-903.
- [Google Scholar]
- Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther.. 2018;186:73-87.
- [CrossRef] [Google Scholar]
- Phytochemical screening, total phenolic, flavonoid contents, and antioxidant activities of four spices commonly used in Vietnamese traditional medicine. Mater. Today:. Proc.. 2022;56:A1-A5.
- [Google Scholar]
- Cardioprotective potential of phytochemicals. In: Gupta R.C., ed. Nutraceuticals in Veterinary Medicine. Cham: Springer; 2019. p. :395-420.
- [Google Scholar]
- Chemical composition and antioxidant property of essential oil of Callicarpa tomentosa. Chem of Natural Compounds.. 2022;58(4):756-759.
- [Google Scholar]
- Cardioprotective role of scopoletin on isoproterenol-induced myocardial infarction in rats. Appl. Biochem. Biotechnol.. 2023;195(2):919-932.
- [CrossRef] [Google Scholar]
- Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol.. 2023;233:123470
- [CrossRef] [Google Scholar]
- Cardioprotective potential of aqueous extract of Fumaria indica on isoproterenol-induced myocardial infarction in SD rats. Oxid. Med. Cell. Longev.. 2022;2022
- [CrossRef] [Google Scholar]
- Phytochemicals as potential cardioprotective agents: Unraveling the mechanism(s) of action. Oxid. Med. Cell. Longev.. 2016;2016:9576342.
- [CrossRef] [Google Scholar]
- New insights into phenolic compounds from optimized fruit extract of Ficus auriculata. Sci. Rep.. 2021;11(1):12503.
- [CrossRef] [Google Scholar]
- Antioxidative and cardioprotective effects of Schisandra chinensis bee pollen extract on isoprenaline-induced myocardial infarction in rats. Molecules. 2019;24(6):1090.
- [CrossRef] [Google Scholar]
- Antioxidant, anti-inflammatory, and anti-proliferative activities of green and yellow zucchini (Courgette) Appl. Nanosci.. 2023;13(3):2251-2260.
- [CrossRef] [Google Scholar]
- Age-related irreversible, progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson's disease phenotype. Eur. J. Neurosci.. 2003;18:589-600.
- [Google Scholar]
- Important flavonoids and their role as a therapeutic agent. Molecules. 2020;11(25(22)):5243.
- [Google Scholar]
- Metabolomics analysis delineates the therapeutic effects of hydroethanolic extract of Cucumis sativus L. seeds on hypertension and isoproterenol-induced myocardial infarction. Biomed. Pharmacother.. 2022;148:112704
- [CrossRef] [Google Scholar]
- Recent Research on Flavonoids and their Biomedical Applications. Curr. Med. Chem.. 2021;28(5):1042-1066.
- [Google Scholar]
- Phytochemical analysis, antioxidant, and enzyme inhibition activity of five Salvia taxa from Turkey. S. Afr. J. Bot.. 2023;152:212-221.
- [Google Scholar]
- Antioxidant activity of ethanolic extract of Callicarpa linata leaf. Pharmacologyonline. 2014;3(5):121-125.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103100.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







