Translate this page into:
Butin prevent liver damage triggered by D-galactosamine via regulating lipid peroxidation and proinflammatory cytokines in rodents
⁎Corresponding authors. h.althurwi@psau.edu.sa (Hassan N. Althurwi), ikazmi@kau.edu.sa (Imran Kazmi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Liver damage is becoming a more severe problem around the world. Unfortunately, there is still a great need for clinically effective pharmacological therapy for liver injury. The investigation aimed to assess the favorable outcome of butin hepatoprotective efficacy against D-galactosamine (D-GalN)-produced liver damage in experimental rats.
Methods
A single dose of D-GalN 400 mg/kg b. wt. was injected intraperitoneally, 24 hr prior to scarification of rats to cause liver damage. The liver injury was assessed biochemically, investigating parameters like aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT), γ-glutamyl transferase (GGT), total-bilirubin, -albumin and -protein. Butin (25 and 50 mg/kg b. wt.) was managed for 21st days and at the end of the experimental period, normal control, D-GalN control, and butin treatment group rats for further biochemical as well as histopathological consideration.
Results
Butin pretreatment significantly attenuated D-GalN-induced hepatocytes damage by reducing hepatic markers and its ability in attenuating oxidative damage (MDA, GSH, SOD, CAT) and pro-inflammatory cytokine (TNF-α, IL-6, IL-1β) and MPO. Histopathological assessment of the liver and biochemical estimation confirmed the hepatoprotective effects of butin. Conclusions: These results demonstrated that butin protects experimental rats against D-GalN-induced liver injury by modulating lipid peroxidation and pro-inflammatory cytokine. Current research will give preliminary experimental evidence for butin, and possible therapeutic effects in liver disorders.
Keywords
Butin
D-galactosamine
Experimental design
Hepatoprotective activity
1 Introduction
Liver injury is a threatening disorder that poses significant clinical problems with a 30% mortality rate (Stravitz and Lee 2019). Hepatic impairment occurs rapidly, leading to progressive multiorgan failure and hepatic encephalopathy. Viruses, drug-induced liver damage, alcoholic hepatitis, cryptogenic liver failure, and pregnancy-related acute liver disease are the most prevalent reasons for liver damage (Marrone et al., 2021). Hepatotoxic substances react with liver cell components, resulting in a variety of liver ailments. Chemical poisons including galactosamine, acetaminophen, thioacetamide, and carbon tetrachloride have been successfully utilized in earlier to establish in vitro and in vivo models of experimental hepatocyte injury (Tsai et al., 2021). D-Galactosamine (D-GalN), which is generated from D-galactose, is a renowned hepatotoxic drug that causes a diffuse kind of liver injury with necrosis, inflammation, and regeneration that is morphologically and functionally comparable to human viral hepatitis (Chen et al., 2021). In animal models of liver injury, D-GalN is often employed due to its great repeatability and ease of dosage management. D-GalN inhibits hepatocyte RNA synthesis and lowers the quantity of cellular uridine-5′-triphosphate (UTP) to impede transcription in the liver (Park et al., 2020).
The flavonoid butin can be obtained from a variety of medicinal plants, including Rhus verniciflua Stokes, Parkinsonia aculeata L, Vernonia anthelmintica Willd, Dalbergia odorifera and Adenanthera pavanina (Su et al., 2007, Chen et al., 2016, Abdelaziz et al., 2020). Butin has been shown to be an influential antioxidant in the treatment of stress-induced illnesses such as liver disease, diabetes and cancer (Brusselmans et al., 2005, Kuzu et al., 2008, Shu et al., 2009). Butin reduces apoptosis and the inflammatory response in intracerebral hemorrhage (ICH) rats, which alleviates the altered behaviour and neuronal state (Li and Jiwu 2018). Butin has previously been revealed to defend cells and activate antioxidant enzymes by apoptosis triggered by hydrogen peroxide by scavenging reactive oxygen species (ROS) (Zhang et al., 2008). Butin has anti-myocardial I/R properties. The cardioprotection in the setting of diabetes by butin is likely mediated through the activation of AMPK/Akt and GSK-3/Nrf2 signalling pathways (Duan et al., 2017). Butin has been shown to provide a successful response in a hydroquinone-induced experimental vitiligo model, and its putative tools include increased tyrosinase-related protein-1 (TRP-1) and tyrosinase (TYR) expression and decreased cholinesterase activity as well as malondialdehyde (MDA) (Huo et al., 2017). Despite the fact that butin reported anti-inflammatory, antioxidant, anti-apoptic, antiplatelet, skin whitening, and anti-implantation effects (Chen et al., 2016). The hepatoprotective impact of butin in the context of liver damage caused by D-GalN has never been investigated. As a result, the goal of this work was to investigate butin produce hepatoprotective properties and underlying mechanisms against D-GalN-produced liver toxicity via lipid peroxidation and pro-inflammatory cytokine modulation.
2 Methods
2.1 Experimentation
Male Wistar rats weighing 150 to 200 gm and aged 10 to 12 weeks were used. Animals were randomly divided into groups, each with six animals. Before the experiments, the experimental rats were given free access to a normal diet as well as unlimited access to tap water adlibitum. Animal Ethics Committee of the Institution agreed the protocol, and adheres to the CPCSEA, Government of India (IAEC/918/CPCSEA/01).
2.2 Chemicals
D-GaIN was obtained from Sigma Aldrich (USA). Butin (>97% purity, gift sample from MSW Pharma, Maharashtra, India) was used in the study. The rest of the reagents and chemicals used were high-quality.
2.3 Experimental research model and D-GalN-induced hepatoprotective treatment protocol
The following treatments were administered to 24 rats equally divided into four groups. Group I: preserved as normal for a 21-day study period, with distilled water at a rate of 1 ml/kg. Group II: D-GalN control treated with a D-GalN 400 mg/kg intraperitoneal single dose on the 21st day to produced hepatotoxicity (Raish et al., 2016). Groups III and IV: Butin 25 and Butin 50 mg/kg group was administered for a period of 21st days and challenged with a single D-GalN dose at 400 mg/kg/i.p. on the day of 21st. Biochemical and histopathological assessment was performed on the 22nd day.
2.4 Evaluation parameters
2.4.1 Biomarkers estimation
Biomarkers like aspartate aminotransferase (AST) (Frankel and Meyer 2000), alanine aminotransferase (ALT), alkaline phosphatase (ALP) (Goodla et al., 2017), triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL) (Adewale et al., 2019), total protein (TP) (Gornall et al., 1949), albumin (GRANT 1987) and total bilirubin (TB) (Gupta et al., 2005) and were assessed. The liver specimens were extracted and homogenates (10% w/v). The resulting supernatant was utilized to determine catalase (CAT)(Aebi 1984), glutathione peroxidase (GSH) (Sedlak and Lindsay 1968), superoxide dismutase (SOD)(Nandi and Chatterjee 1988), γ-glutamyl transferase (GGT) (Persijn and van der Slik 1976) myeloperoxidase (MPO) (Van Dielen et al., 2004) and lipid peroxidation (MDA)(Ohkawa et al., 1979).
2.4.2 Measurement of tumor necrosis factor-α (TNF-α), interleukin beta (IL-1β) and IL-6 in serum
The IL-6, IL-1β, and TNF-α serum concentrations were quantified by immunoassay kit (Chen et al., 2016).
2.4.3 Histopathological estimation
Experiments ended with the animals being sacrificed. In order to fix the liver tissue, a buffered neutral formalin solution of 10% was immediately used. The tissues were carefully embedded in molten paraffin with the help of metallic blocks, covered with flexible plastic moulds and kept under freezing plates to allow the paraffin to solidify. Cross sections (5 mm thick) of the fixed tissues were cut. The microarchitecture of the tissues was observed under a light microscope using hematoxyline and eosin-stained sections. An investigator who was not aware of the biochemical results and treatment allocation performed the histological assessment (Shang et al., 2020).
2.5 Statistical analysis
Statistical correlation was accomplished by Prism software. The data are accessible as the as mean ± S.E.M. One-way ANOVA followed by Tukey’s post-hoc test, p < 0.05 being considered as a significant difference.
3 Results
3.1 Hepatic injury markers
D-GalN-induced liver damage (control group) caused a substantial escalation in serum biomarkers ALT, ALP, AST, GGT, and TB levels (p < 0.05) as correlated to the normal. Conversely, treatment with butin at different doses shows a considerable decline in the raised liver marker enzymes ALT, AST, and ALP, as depicted in Fig. 1A-C. In addition, butin with higher as well as lower doses substantially (p < 0.05) abridged GGT levels and TB content in the treatment group (Fig. 1D and 2A). TP levels were noticeably (p < 0.05) reduced in D-GalN-induced rats correlated with normal, whereas in both doses of butin treated group were appreciably improved (p < 0.001) (Fig. 2B). D-GalN-induced rats albumin levels were substantially higher than those of normal (Fig. 2C). Compared to D-GalN group rats, butin significantly restored the elevated albumin levels (p < 0.05, p < 0.001).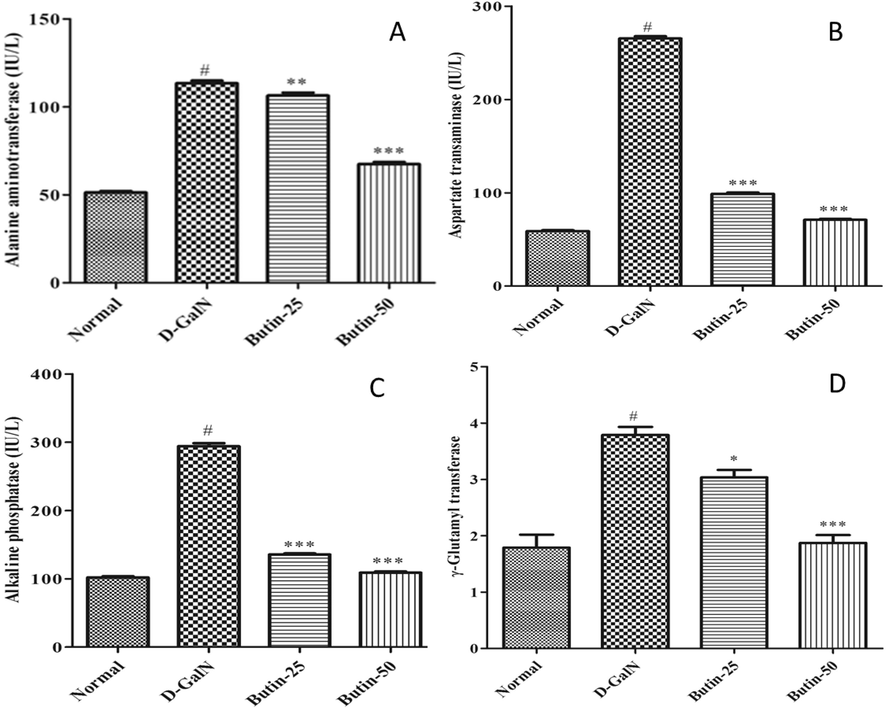
(A). Effect of butin on ALT against D-GalN-induced hepatotoxicity (B). Effect of butin on AST against D-GalN-induced hepatotoxicity. (C). Effect of butin on ALP against D-GalN-induced hepatotoxicity. (D). Effect of butin on the GGT level against D-GalN-induced hepatotoxicity. Values are expressed as Mean ± SEM, # p < 0.05 Normal VS D-GalN; **p < 0.01, ***p < 0.001 D-GalN VS Butin-25, Butin-50.
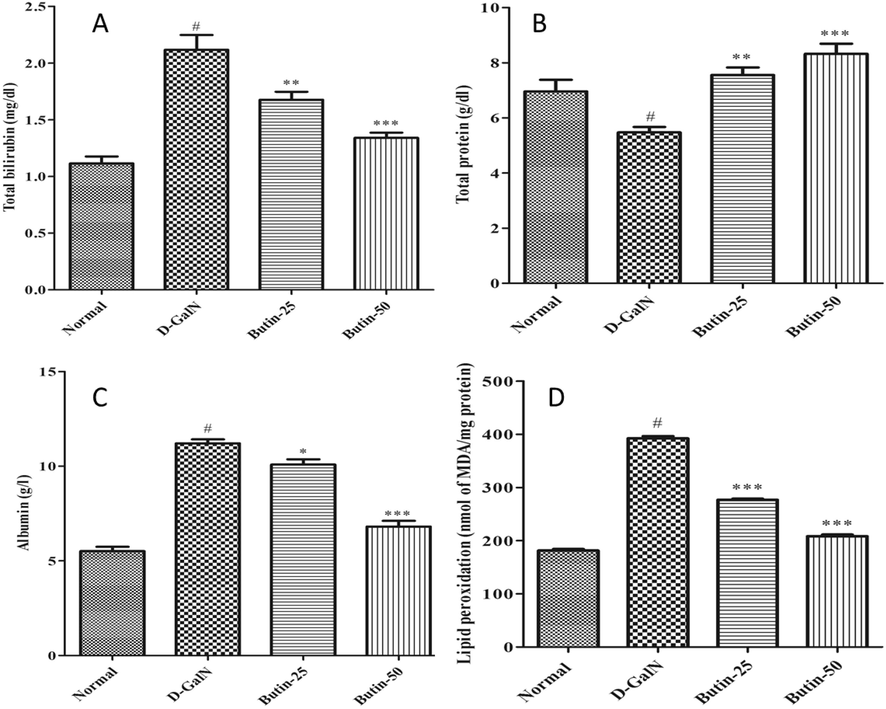
(A). Effect of butin on the total bilirubin level against D-GalN-induced hepatotoxicity. (B). Effect of butin on total protein levels against D-GalN-induced hepatotoxicity. (C). Effect of butin on Albumin levels against D-GalN-induced hepatotoxicity. (D). Effect of butin on the liver LPO level against D-GalN-induced hepatotoxicity. Values are expressed as Mean ± SEM, # p < 0.05 Normal VS D-GalN; **p < 0.01 ***p < 0.001 D-GalN VS Butin-25, Butin-50.
3.2 Oxidative markers
Fig. 2D demonstrates the substantial (p < 0.05) gain in the MDA (lipid peroxidation) in the D-GalN-induced rats. Butin treatment, on the other hand, considerably (p < 0.001) reduced lipid peroxidation than the D-GalN group.
3.3 Endogenous antioxidant enzyme markers
The hepatic GSH, SOD, and CAT levels were remarkably reduced in the D-GalN-control (p < 0.05) than the normal (Fig. 3A-C). Treatment with butin (25, 50 mg/kg) restored endogenous antioxidant enzyme-GSH, SOD, and CAT activities in the treatment group (p < 0.001).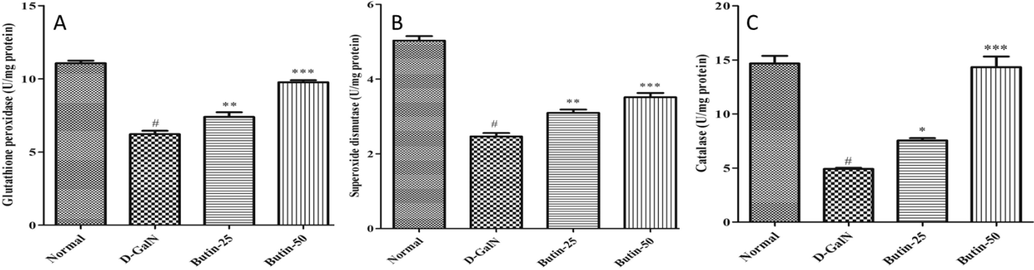
(A). Effect of butin on the liver GSH level against D-GalN-induced hepatotoxicity. (B). Effect of butin on SOD content against D GalN-induced hepatotoxicity. (C). Effect of butin on CAT content against D-GalN-induced hepatotoxicity. Values are expressed as Mean ± SEM, # p < 0.05 Normal VS D-GalN; **p < 0.01, ***p < 0.001 D-GalN VS Butin-25, Butin-50.
3.4 Pro-inflammatory cytokines and MPO markers
The TNF-α, IL-1β, and IL-6-pro-inflammatory cytokines were all dramatically upregulated the activity of pro-inflammatory factors in response to the D-GalN challenge. Butin (25, 50 mg/kg) with lower-higher doses effectively prevented the increase TNF-α, IL-1β, and IL-6 levels (Fig. 4A-D). MPO activity was significantly higher in D-GalN- control. However, treatment with butin significantly (p < 0.001) improved the MPO level produced by a D-GalN in the treatment group.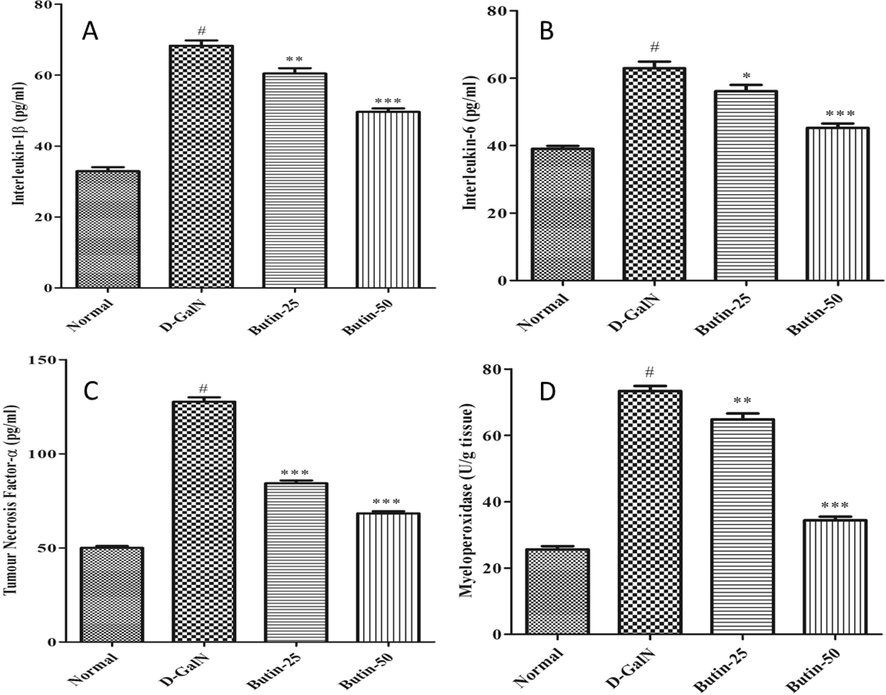
(A). Effect of butin on Interlukin-1 β content against D GalN-induced hepatotoxicity. (B) Effect of butin on Interlukin-6 content against D-GalN-induced hepatotoxicity. (C). Effect of butin on TNF-α content against D GalN-induced hepatotoxicity. (D). Effect of butin on the liver MPO level against D-GalN-induced hepatotoxicity. Values are expressed as Mean ± SEM, # p < 0.05 Normal VS D-GalN; **p < 0.01, *** p < 0.001 D-GalN VS Butin-25, Butin-50.
3.5 Lipid profile
The TC and TG were significantly increased, whereas the HDL was substantially (p < 0.05) reduced by D-GalN as correlated with normal. In treatment groups, (Butin 25 and 50 mg/kg) TC and TG were suggestively reduced (p < 0.001) as correlated to D-GalN animals. Interestingly, pre-treatment of rats with butin (p < 0.001) improvement in these abnormalities, as shown in Fig. 5A-C. Butin-treated rats favorably modulated lipid parameters.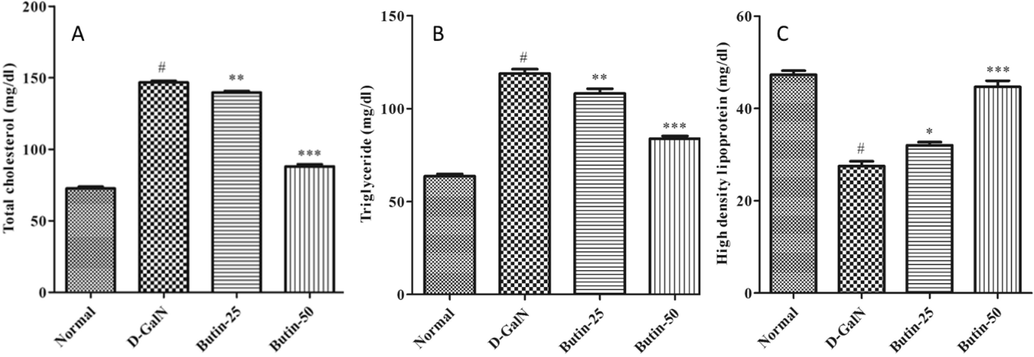
(A). Effect of butin on the TC level against D-GalN-induced hepatotoxicity. (B). Effect of butin on the TG level against D-GalN-induced hepatotoxicity. (C). Effect of butin on the HDL level against D-GalN-induced hepatotoxicity. Values are expressed as Mean ± SEM, # p < 0.05 Normal VS D-GalN; **p < 0.01, ***p < 0.001 D-GalN Butin-25, Butin-50.
3.6 Changes of hepatic tissue
Rats with normal hepatocyte architecture were observed in a photomicrograph of the liver in normal. (Fig. 6 A). In difference, the liver cells of D-GalN control group rats exhibited massive coagulative necrosis, hemorrhage, inflammation and central vein congestion as associated to normal group (Fig. 6B). Butin (25 mg/kg) lower dose pretreated induced D-GalN, there was mild inflammation and moderate tissue necrosis histoarchitecture of liver sections of butin (50 mg/kg) demonstrated a progress in the pathological characteristics, including mild inflammation. All these results suggest that pretreatment with butin prior to D-GalN injection provides defensive measures in rats (Fig. 6C and D).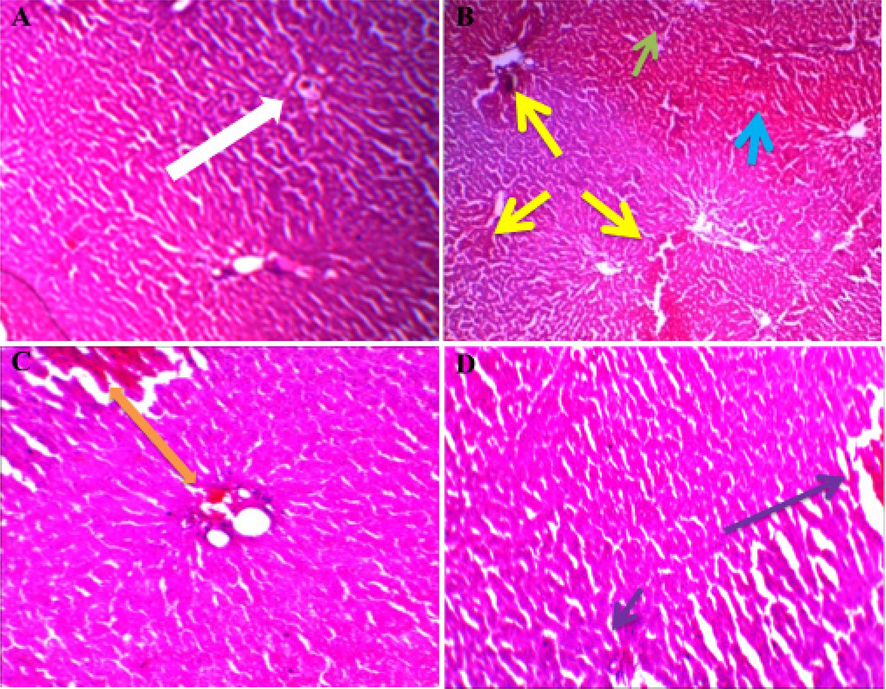
Representative photographs demonstrating histopathological finding of liver tissue sections stained with H and E among various experimental groups. Histological examination of liver sections from different groups (A) Group I: White arrow indicated a normal arrangement of hepatocytes. (B) Group II (section of liver tissue of the D-GalN-treated group): yellow arrow indicating massive coagulative necrosis, a green arrow indicating central vein congestion, and blue arrow indicating inflammation. (C) Group III (section of liver tissue pretreated with 25 mg/kg Butin followed by D-GalN): an orange arrow indicating moderate tissue necrosis and mild inflammation. (D) Group IV (section of liver tissue pretreated with 50 mg/kg butin followed by D-GalN): a purple arrow indicating normal histology with mild inflammation.
4 Discussion
The liver is a metabolically active organ that is in charge of biotransformation and clearance of xenobiotics from the body. It is a popular target for medications and pathogens, which can harm liver cells and decrease general liver function, leading to hepatitis, cirrhosis, and hepatocellular carcinoma. Unfortunately, clinically effective pharmacological treatments for hepatic illness are still desperately desirable (Dong et al., 2020). D-GalN-induced hepatic damage is a well-known method of xenobiotic-induced hepatotoxicity that is commonly used to identify anti-hepatotoxic or hepatoprotective agents. A rising body of support recommended that stress and inflammation are both linked to the enlargement of hepatic damage and have a part in the disease pathogenesis (Hu et al., 2020). As a result, medicines that can improve liver functions, act as antioxidants and anti-inflammation properties could be evaluated as viable therapeutic approaches for liver injury.
The purpose of the experimental was to gain an enhanced perceptive of the action underlying D-GalN-produced liver damage and investigate the influence of butin on D-GalN-produced hepatic damage. The study confirmed the hepatoprotective effects of butin in rats challenged with D-GalN. The ALT, GGT, AST, and ALP are directly implicated key biomarkers in hepatic injury and indicate the rigor of D-GalN impairment. This study found that giving D-GalN to rats caused a considerable rise in ALT, ALP, AST and GGT as well as increased inflammatory infiltration, massive coagulative necrosis, hemorrhage, and central vein congestion as well as a deterioration of hepatic integrity, which is in accordance with prior findings (Rouf et al., 2021). The hepatic cell membrane permeability is disrupted by D-GalN injection, leading to hepatic enzyme loss from the cell and an increase in serum enzyme. In addition, excess reactive free radicals formed by D-GalN change the antioxidant prominence of some organs, rendering them further liable to stress (Prakash et al., 2018). As a result, the current in vivo study may be appropriate for assessing hepatoprotective drugs. It is possible that pre-treatment of butin reduces hepatic injuries by preventing ionic pump dysfunction, cell leakage, and membrane damage. Serum TB, on the contrary, is a well-known functional hepatic marker linked to hepatic and biliary disorders, as well as acute disturbance of hepatocellular structural design and function, greater serum TB levels are commonly detected in D-GalN-induced liver damage. However, butin treatment appreciably attenuated the deleterious effects of TB. To assess the noxious effects of toxicants, TP, and albumin levels can be used. Proteins are synthesized most efficiently in hepatocytes. The equilibrium of synthesis, use and breakdown is reflected in plasma protein levels. Severe hepatic damage has been linked to a decrease in the production of various proteins (Rouf et al., 2021). Butin showed protective effects on total protein and albumin. These findings are in agreement with those of the Prakash et al. (2018) (Prakash et al., 2018). Butin may exhibit hepatoprotective effects based on histopathological and biochemical studies. Butin could considerably diminish the biomarkers expression of stress while mounting the activity of anti-oxidant enzymes. Meanwhile, cytokines, which are important controllers of inflammation in hepatic impairment, may be suppressed by butin treatment. Butin scavenged activating endogenous antioxidant enzymes and ROS to diminish stress-induced mitochondrial dysfunction, declined stress-induced 8-hydroxy-2′-deoxyguanosine levels by activating oxoguanine glycosylase 1, protected cells against apoptosis triggered by hydrogen peroxide (Zhang et al., 2008). The intracellular organelles that produce the most ROS in cells are crucial in the advance of oxidative stress in normal and pathological sittings and mitochondrial malfunction is mainly probable triggered oxidative stress-induced apoptosis. Lipid peroxidation is a well-known stress and cell injury mechanistic pathway. The stress caused by D-GalN- accumulates hepatic lipid peroxides and depletes antioxidant enzymes such as CAT, GSH, and SOD. Butin reduced the toxic effects of D-GalN by suggestively dropping lipid peroxidation. Butin inhibitory impact on lipid peroxidation suggested that it could protect the liver from free radical-induced injury and consequent pathological alterations.
Antioxidant enzymes, such as SOD, are crucial antioxidant defense systems that protect the body from ROS. SOD has the capacity to alter SOD radicals to hydrogen peroxide, which can then be catalysed by GSH-px to produce water and oxygen (Han et al., 2020). The treatments with butin also showed restored GSH, SOD, and CAT levels. Hence, natural flavonoids are proving to be effective treatments for diseases caused by free radicals.
MPO was not only thought to be a neutrophil marker, but it could also make hypochlorite from hydrogen peroxide and chloride ions, resulting in free radicals that could cause oxidative stress and tissue damage(Chen et al., 2020). Butin significantly decreased levels of MPO in a D-GalN-treated model according to our findings. When D-GalN causes liver damage, it increases cytokines expression, such as TNF-α, IL-1β, IL-6 and generates ROS, which triggers a downstream signaling cascade (Lv et al., 2017). Furthermore, butin may reduce the cytokines expression, which are important controllers of inflammation in liver injury. D-GalN significantly improved the TNF-α level in this study. TNF-α is the most important pleiotropic cytokine, capable of initiating an inflammatory cascade that mains to the production of other cytokines, such as IL-1β and IL-6. Butin reduced the acute liver impairment induced by D-GalN by regulating inflammatory cytokines. Previous work demonstrated the pro-inflammatory modulatory effect of butin on ICH condition by changed NF-κB in the tissues brain (Trajkovska and Topuzovska 2017). Molecularly, cellular functions and homeostasis are regulated and maintained by lipids. Cholesterol, bile acid production, and phospholipid metabolism are the primary functions of the liver. Increased serum TG and TC levels, as well as lower HDL levels, are classic signs of liver injury, which matches our findings. When D-GalN-injected animals were compared to normal rats, we found a rise in serum TC and TG levels as well as a decrease in HDL levels, which agreed with the former outcomes (Mondal et al., 2020). As result of hepatocellular injury, a large amount of TG accumulates in the parenchymal cells, which then discharge a substantial amount of TG into the bloodstream. Butin appears to have a protective effect produced D-GalN toxic effect, as seen by raised HDL and reduction in TC and TG.
To summaries, the current study established the butin hepatoprotective efficacy in rats exposed to D-GalN-produced heptic injury, including down-regulation of key factors such as MDA, GSH, SOD, CAT, MPO, IL-6, IL-1β and TNF-α which may be adequate to battle cellular damage. Butin may protect hepatocytes and enhance liver tissue regeneration, according to histological recovery toward normalcy. However, more research is needed to corroborate these findings and to establish butin therapeutic benefits as a conventional hepatoprotective medication.
5 Conclusion
This study examines butin ability to protect the D-GalN-induced liver damage. The D-GalN prompted hepatic toxicity (elevated in hepatic markers, amplified oxidative stress, decreased antioxidants and increased pro-inflammatory markers) was ameliorated by pretreatment of butin. These findings demonstrated butin protective effects against rats challenged with D-GalN-induced liver impairment by modulating pro-inflammatory cytokine and lipid peroxidation as well as buildup, as demonstrated by the clear morphology of liver cells.
Funding
This research was supported by the Ministry of Education in Saudi Arabia project number (IFPIP: 778-130-1443).
Acknowledgments
This research work was funded by Institutional Fund Projects under grant no. (IFPIP: 778-130-1443). The authors gratefully acknowledge the technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phytochemical profile, antioxidant and cytotoxic potential of Parkinsonia aculeata L. growing in Saudi Arabia. Saudi Pharmaceutical Journal.. 2020;28:1129-1137.
- [Google Scholar]
- Curcumin protects sodium nitrite-induced hepatotoxicity in Wistar rats. Toxicol. Rep.. 2019;6:1006-1011.
- [Google Scholar]
- Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem.. 2005;280:5636-5645.
- [Google Scholar]
- Targeting Myeloperoxidase (MPO) Mediated Oxidative Stress and Inflammation for Reducing Brain Ischemia Injury: Potential Application of Natural Compounds. Front. Physiol.. 2020;11:433.
- [CrossRef] [Google Scholar]
- Gut microbiota and chemical-induced acute liver injury. Front. Physiol.. 2021;12:688780
- [Google Scholar]
- LY294002 prevents lipopolysaccharide-induced hepatitis in a murine model by suppressing IκB phosphorylation. Mol. Med. Rep.. 2016;13:811-816.
- [Google Scholar]
- Isolation of Sulfuretin and Butin from Rhus verniciflua Stokes Using Medium-pressure Liquid Chromatography and their Tyrosinase Inhibitory Effects. BioResources. 2016;11:759-771.
- [Google Scholar]
- Protective effect of butin against ischemia/reperfusion-induced myocardial injury in diabetic mice: involvement of the AMPK/GSK-3β/Nrf2 signaling pathway. Sci. Rep.. 2017;7:1-14.
- [Google Scholar]
- The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric.. 2000;80:1925-1941.
- [Google Scholar]
- Preventive and curative effects of Cocculus hirsutus (Linn.) Diels leaves extract on CCl4 provoked hepatic injury in rats. Egyptian journal of basic and applied sciences.. 2017;4:264-269.
- [Google Scholar]
- Determination of serum proteins by means of the biuret reaction. J. Biol. Chem.. 1949;177:751-766.
- [Google Scholar]
- GRANT, G. H., 1987. Amino acids and proteins. Fundamentals of clinical chemistry.
- Nutritional and hypoglycemic effect of fruit pulp of Annona squamosa in normal healthy and alloxan-induced diabetic rabbits. Ann. Nutr. Metab.. 2005;49:407-413.
- [Google Scholar]
- Danger signals in liver injury and restoration of homeostasis. J. Hepatol.. 2020;73:933-951.
- [Google Scholar]
- Lactobacillus plantarum LP33 attenuates Pb-induced hepatic injury in rats by reducing oxidative stress and inflammation and promoting Pb excretion. Food Chem. Toxicol.. 2020;143:111533
- [Google Scholar]
- The effect of butin on the vitiligo mouse model induced by hydroquinone. Phytother. Res.. 2017;31:740-746.
- [Google Scholar]
- Epigallocatechin gallate attenuates experimental non-alcoholic steatohepatitis induced by high fat diet. J. Gastroenterol. Hepatol.. 2008;23:e465-e470.
- [Google Scholar]
- Butin attenuates brain edema in a rat model of intracerebral hemorrhage by anti inflammatory pathway. Transl. Neurosci.. 2018;9:7-12.
- [Google Scholar]
- Asiatic Acid Exhibits Anti-inflammatory and Antioxidant Activities against Lipopolysaccharide and d-Galactosamine-Induced Fulminant Hepatic Failure. Front. Immunol.. 2017;8:785.
- [CrossRef] [Google Scholar]
- Acute liver failure in Still’s disease relapse during pregnancy: case report and discussion of a possible trigger role of DILI. BMC Gastroenterol.. 2021;21:1-6.
- [Google Scholar]
- Hepatoprotective and antioxidant capacity of Mallotus repandus ethyl acetate stem extract against D-galactosamine-induced hepatotoxicity in rats. ACS Omega. 2020;5:6523-6531.
- [Google Scholar]
- Assay of superoxide dismutase activity in animal tissues. J. Biosci.. 1988;13:305-315.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- A mixture of mulberry and silk amino acids protected against D-galactosamine induced acute liver damage by attenuating oxidative stress and inflammation in HepG2 cells and rats. Exp. Ther. Med.. 2020;19:3611-3619.
- [Google Scholar]
- Persijn, J. and á. van der Slik, 1976. A new method for the determination of γ-glutamyltransferase in serum.
- Sodium fluoride-induced oxidative stress and histological changes in liver of Swiss albino mice and amelioration by Ocimum sanctum Linn. Asian J. Pharm. Clin. Res.. 2018;11:195-199.
- [Google Scholar]
- Hepatoprotective activity of Lepidium sativum seeds against D-galactosamine/lipopolysaccharide induced hepatotoxicity in animal model. BMC Complement. Altern. Med.. 2016;16:501.
- [CrossRef] [Google Scholar]
- Evaluation of hepatoprotective effects of arogyavardhini against D-galactosamine-induced hepatotoxicity in rats. Journal of Pharmacognosy and Phytochemistry.. 2021;10:12-19.
- [Google Scholar]
- Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem.. 1968;25:192-205.
- [Google Scholar]
- Kidney ischemia-reperfusion elicits acute liver injury and inflammatory response. Front. Med.. 2020;7:201.
- [Google Scholar]
- Antihyperglycemic effects of total flavonoids from Polygonatum odoratum in STZ and alloxan-induced diabetic rats. J. Ethnopharmacol.. 2009;124:539-543.
- [Google Scholar]
- Studies on chemical constituents from stems and leaves of Adenanthera pavanina. Zhongguo Zhong yao za zhi= Zhongguo Zhongyao Zazhi= China Journal of Chinese Materia. Medica. 2007;32:2135-2138.
- [Google Scholar]
- High-density lipoprotein metabolism and reverse cholesterol transport: strategies for raising HDL cholesterol. Anatol. J. Cardiol.. 2017;18:149.
- [Google Scholar]
- The ameliorative effects of fucoidan in thioacetaide-induced liver injury in mice. Molecules. 2021;26:1937.
- [Google Scholar]
- Macrophage inhibitory factor, plasminogen activator inhibitor-1, other acute phase proteins, and inflammatory mediators normalize as a result of weight loss in morbidly obese subjects treated with gastric restrictive surgery. J. Clin. Endocrinol. Metab.. 2004;89:4062-4068.
- [Google Scholar]
- Protective effect of butin against hydrogen peroxide-induced apoptosis by scavenging reactive oxygen species and activating antioxidant enzymes. Mol. Cell. Biochem.. 2008;318:33-42.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102934.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







