Translate this page into:
Brain response after treatment of Trypanosoma evansi-infected mice with Indigofera oblongifolia
⁎Corresponding author at: Zoology Department, College of Science, King Saud University, P.O. Box: 2455, Riyadh 11451, Saudi Arabia. mdkhil@ksu.edu.sa (Mohamed A. Dkhil)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Trypanosomiasis is one of the most important diseases affecting economic development for humans and livestock. The current research aimed to assess the in vitro antitrypanosomal effect of methanol extracts of Indigofera oblongifolia leaf (IME) and to examine the brain response after IME treatment of infected mice. In the in vitro method, IME (1.25 mg/ml) was able to kill all parasites after 30 min of exposure. The percentage of the parasite inhibition reached 100% after 180 min of the IME application. Swiss albino mice were infected with 103 Trypanosoma evansi then were daily treated with 100 mg/Kg IME for four days. IME was able to significantly decrease the number of trypanosomes in mice blood. In addition, IME improved the histopathological changes in mice brain induced by the infection. Furthermore, following treatment with IME, the level of epinephrine and nitric oxide in the mice brain homogenate decreased significantly. Taken together, I. Oblongifolia has antitrypanosomal activity and might enhance the brain response to T. evansi.
Keywords
Trypanosomiasis
In vitro
In vivo antioxidant
Brain
Mice
Epinephrine
Nitric oxide
1 Introduction
Human trypanosomiasis is a parasitic disease due to trypanosomes of genus Trypanosoma that still affects an estimated population of 65 million people in 36 sub-Saharan African countries (WHO, 2017).
Trypanosoma evansi is a protozoan hemoflagellate capable of infecting various animal species causing ‘sura’. The pattern of disease caused by T. evansi is similar to that caused by T. brucei gambiense from human sleeping sickness. The transmission occurs through tsetse flies (Desquesnes et al., 2013). In absence of treatment, fever, anaemia, weakness, and nervous symptoms developed and cause animal production loss (Otto et al., 2009). The trypanosome invades the host in the blood to replicate and then in the central nervous system. This requires energy that the host provides for the trypanosome's survival (Habila et al., 2012).
T. evansi's pathogenicity varies considerably between its strains and across species of animals (De Menezes et al., 2004). Like T. brucei, T. evansi affects the central nervous system but the symptoms are different in various animals (Luckins, 1988). Some reports inducated human infection with T. evansi (Joshi et al., 2005; Powar et al., 2006).
During trypanosomiasis, there is an imbalance in the brain neurotransmitters. Kalu and Haruna (1985) reported that, the epinephrine significantly increased parasitaemia during trypanosomiasis induced by T. congolense in ruminant blood within 45 min of administration. In addition, Sanchez (1973) reported the increased epinephrine concentration after T. lewisi infection in rats. For our knowledge, there are no reports for the status of epinephrine during T. evansi infection in the brain. In this study, we measured the oxidative stress induced in the brain through measuring the level of nitric oxide and the level of epinephrine.
Due to the induced parasite resistance to the commonly used anti-trypanosomal drugs, scientists seek to find efficient agents from natural resources to overcome the induce infection. Medicinal plants were found to be suitable protective antiparasitic agents. Indigofera oblongifolia is considered one of the traditionally used plants against blood parasites (Lubbad et al., 2015; Dkhil et al., 2019). I. oblongifolia belongs to family fabaceae and the leaf extracts were found to be with antimicrobial and hepatoprotective activity (Dahot, 1999; Lubbad et al., 2015). Recently, our group reported the spleen protective role of Indigofera oblongifolia methanolic leaf extracts (IME) against the induced injury by T. evansi (Dkhil et al., 2019). In the current study, we showed the brain response after treatment of T. evansi-infected mice with IME.
2 Materials and methods
2.1 Preparation of of I. oblongifolia leaf extracts
After collection of I. oblongifolia leaves from Jazan, Saudi Arabia (during summer), they were dried and methanolic extraction was carried out according to Lubbad et al. (2015).
2.2 Animals and infection
Thirty Swiss albino female mice (12 ± 2 weeks old) were obtained from the animal facility of the Zoology Department, College of Science, King Saud University. Infection of mice was carried out according to Dkhil et al. (2019). Calculation of parasitemia was done according to Herbert and Lumsden (1976).
Animals were allocated into three groups (G1, G2 and G3) (10 mice per group). G1 was considered the non-infected control group. This group was only gavaged with distilled water. G2 was Intraperitoneally infected with 103 T. evansi. G3 was infected as G2 then after 1 h, animals were treated with 100 mg/Kg of IME (Dkhil et al., 2019) for 4 days (one dose per day). After 4 days p.i., Brains were collected after killing of mice by CO2 asphyxiation.
2.3 In vitro test
For blood separation, blood-containing trypomastigotes was collected from T. evansi infected mice by cardiac puncture in heparinized tubes. Each 200 µL of the blood washed in 2 ml Roswell Park Memorial Institute (RPMI) 1640 culture medium (Sigma-Aldrich, Darmstadt, Germany), stored in microcentrifuge tubes and centrifuged at 1000 ×g for 10 min. The supernatant was removed and resuspended in RPMI 1640 and the number of parasites was counted according to Herbert and Lumsden (1976). Twelve wells plates were used, each well received 200 µL of media containing 2.8 × 105 T. evansi. IME was at doses of 1.25 mg/ml, 0.3125 mg/ml, 0.078125 mg/ml and 0.0195 mg/ml. The reference drug, Cymelarsan (Merial, Lyon France) was used at a dose of 0.625 µg/ml (according to IC50 test). The tests were performed in duplicates and the parasites were counted at 30, 60, 90, 120 and 180 min. Incubation was carried out in a 5% CO2 incubator at 37 °C.
2.4 Brain histopathology
Mice brains were separated from the skull then fixed in formalin (10%) for 24 hs, processed in ethanol and xylene, then embedded in paraffin wax and sectioned to obtain 4 μm thickness paraffin sections. According to Drury and Wallington (1980), sections were stained with hematoxylin and eosin.
2.5 Brain nitric oxide and epinephrine
According to Tsakiris et al. (2004), the brain homogenate was prepared using Tris-HCl and sucrose (10%, w / v). The level of nitric oxide (NO) was determined according to Green et al. (1982). Commercially available ELISA kit (Abnova GmbH, Heidlberg, Germany) was used to determine epinephrine concentration.
2.6 Statistical analysis
The results were presented as mean ± standard error of the mean. Data for multiple variable comparisons were analyzed by one-way ANOVA. Duncan's test was used as a post-hoc test to compare the significance between classes. The acceptable level of significance was set at P ≤ 0.05.
3 Results
The results of in vitro analysis using IME is shown in Fig. 1. The highest concentration of IME (1.25 mg/ml) was able to kill all parasites after 30 min of exposure. The percentage of the parasite inhibition reached 100% after 180 min of the IME application. The reference drug, cymelarsan induced the parasite inhibition of 100% after 90 min. For the concentration of 0.3125 mg/ml, it was possible to identify a reduction of about 30% % after 120 min and 100% after 180 min. Lower concentrations of 0.07812 and 0.0195 mg/ml induced the parasite inhibition after 180 min (Fig. 1). In our previous work, we reported that IME was able to decrease the number of trypanosomes in mice blood by about two fold (Dkhil et al., 2019). This antitrypanosomal effect was due to the presence of some active compounds found in our results of the IME phytochemical analysis (Dkhil et al., 2019).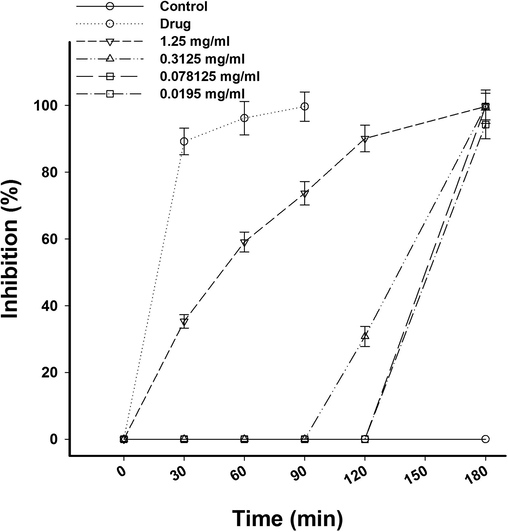
Effect of I. oblongifolia on T. evansi in concentration 1.25, 0.3125, 0.07812 and 0.0195, mg/ml at time 0, 30, 60, 90, and 180 min.
In this study, T. evansi infection caused considerable neurohistopathological changes in the form of inflammation, hemorrhage, and structural changes in Purkinje cells. Moreover, the infection induced decrease in number of cells in Purkinje’s layer (Fig. 2).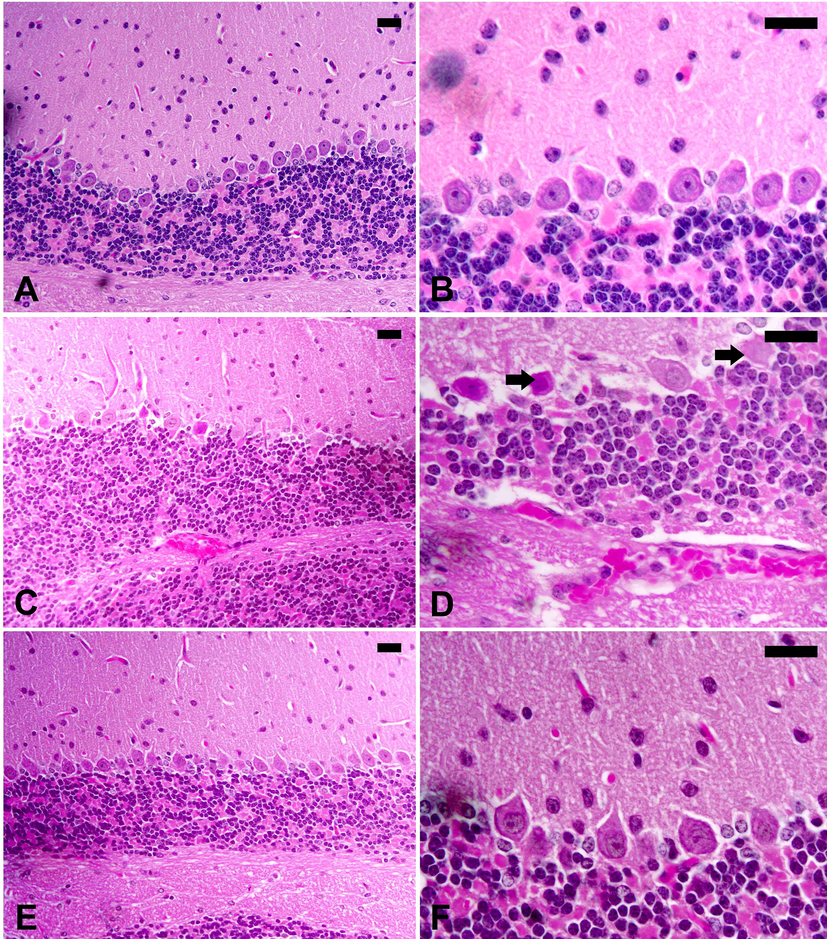
I. oblongifolia improves the brain histopathology after infection with T. evansi. (A, B), Control cerebellum of mice with normal structure. (C, D), Infected cerebellum of mice with injured Purkinje cells (arrow) and hemorrhage in the dilated sinusoids between the molecular layers. (E, F), I. oblongifolia -treated infected cerebellum with improved structure. Scale bar = 25 mm.
In Fig. 3, the level of epinephrine in the brain homogenate of mice infected with T. evansi was significantly (p < 0.01) increased to77 ± 2.1 ng/g. Treatment of the infected mice with IME significantly decreased the elevated epinephrine level (Fig. 3).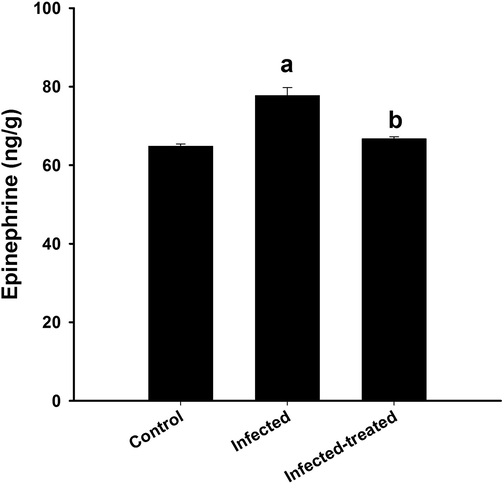
Effect of I. oblongifolia on brain epinephrine of mice infected with T. evansi. a, significance against control group at p < 0.01. b, significance against infected group at p < 0.01.
The IME was able to ameliorate the oxidative stress induced by T. evansi through decreasing the level of NO from 22 ± 2 (µmol/g) in the infected brain to12.5 ± 1.7 (µmol/g) in the infected-treated mice (Fig. 4).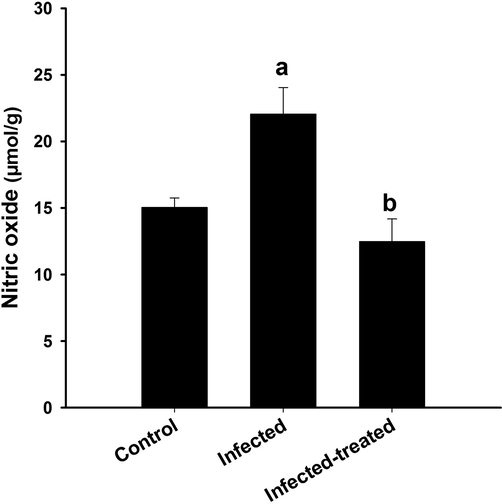
Effect of I. oblongifolia on brain nitric oxide of mice infected with T. evansi. a, significance against control group at p < 0.01. b, significance against infected group at p < 0.01.
4 Discussion
Trypanosomiasis induced by T. evansi involves a complex interaction between the defenses of the host and the effects of the parasite, particularly the oxidative stress produced. The increased oxidative damage, however, may also contribute to pathophysiological changes that increase in severity along with disease progression. Increasing the antioxidant potential of the host's defenses may therefore serve as an intervention strategy to slow down trypanosomiasis progression.
Our previous work reported the enrichment of I. oblongifolia with active compounds like alkaloids and flavonoids (Lubbad et al., 2015; Dkhil et al., 2019). These compounds were able to increase the suppression rate of parasitemia due to the presence of T. evansi in blood (Dkhil et al., 2019).
T. evansi causes severe brain damage and could faster penetrating the brain tissue than African Trypanosomes (Biswas et al., 2010). It has been reported that pathological changes in the brain are due to constant irritation caused by the presence of parasites or by the toxins. Moreover, in the second stage of the disease, severe and potentially fatal clinical symptoms were reported to cause infiltration and dissemination of T. evansi in the central nervous system (Berlin et al., 2009). Besides, pathogenic brain lesions are reported in both sleeping sickness patients (Schmidt and Bafort, 1987) and in animals infected with T. brucei (Keita et al., 1997), but there is less study of cerebral trypanosomiasis in surra in naturally infected and experimental animals.
Biswas et al. (2010) related the death of animals infected with T. evansi to the failure or dysfunction of spleen, lung, heart, kidney, liver or brain or as a result of synergistic effect. In this study, IME, could improve the histopathological alteration induced by the parasite and may be this is due to the enrichment of IME with active compounds. Al-Quraishy et al. (2016) reported beneficial effects of IME against lead acetate-induced neurotoxicity through its antioxidant activity.
In this study, the epinephrine concentration increased by the infection with T. evansi. Sanchez (1973) indicates that physiological and antigenic transformations can be induced in T. lewisi by increasing the epinephrine levels of the host. Dkhil et al. (2016) reported the increased level of epinephrine after infection of mice with the blood parasite Plasmodium berghei and they related that to the induced oxidative damage in the brain.
Trypanosome infection results in the development of large quantities of reactive oxygen species that act as cytotoxic agents that destroy vital components of the cell and lead to the pathogenesis of trypanosomiasis (Gupta et al., 2009). Nitric oxide (NO) produced by activated macrophages is cytotoxic for a variety of pathogens, including T. cruzi (Gazzinelli et al., 1992). Increased NO synthesis has been observed in the brain of mice infected with T. brucei, indicating that the cytotoxicity of NO and its derivatives may cause neurological signs (Keita et al., 2004). The produced NO is considered as a marker for evaluating immune response (Bogdan, 2001) and oxidative stress status (Beckman and Koppenol, 1996). Interestingly, it was reported that NO is involved in many physiological processes including neurotransmission (Dusse et al., 2000; Filho and Zilberstein, 2000) where ONOO– is produced when NO rises in the central nervous system and could be implicated not only in the pathogenesis of brain lesions but also in the development of neurological disorders in T. brucei infection (Keita et al., 2004). Also, Bombeiro et al. (2010) reported that over production of NO during infection with T. cruzi is contributed to the neurodegenerative process. In our study, the IME could ameliorate the induced oxidative damage in the brain where it is considered antioxidant agent (Lubbad et al., 2015; Dkhil et al., 2019).
5 Conclusion
We concluded that IME has trypanocidal activities against T. evansi in vitro. Also, it could enhance the brain response against infection with the blood parasite T. evansi but intensive studies should be carried out to identify the mechanism of IME action.
Acknowledgment
This study was supported by Research Supporting Project (RSP-2019/23), Riyadh, King Saud University.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Neuroprotective potential of Indigofera oblongifolia leaf methanolic extract against lead acetate-induced neurotoxicity. Neural. Regen. Res.. 2016;11:1797-1803.
- [Google Scholar]
- Nitric oxide, superoxide and peroxynitrite the good the bad and the ugly. Am. J. Physiol.. 1996;271:C1424-C1437.
- [Google Scholar]
- Disseminated central nervous system disease caused by Trypanosoma evansi in a horse. Vet. Parasitol.. 2009;161(3–4):316-319.
- [Google Scholar]
- Histopathology of Trypanosoma (Trypanozoon) evansi infection in bandicoot rat. II Brain and choroid plexus. Proc. Zool. Soc.. 2010;63:27-37.
- [Google Scholar]
- Neurodegeneration and increased production of nitrotyrosine, nitric oxide synthase, IFN-gamma and S100beta protein in the spinal cord of IL-12p40-deficient mice infected with Trypanosoma cruzi. Neuroimmunomodulation. 2010;17:67-78.
- [Google Scholar]
- Antimicrobial and antifungal activity of small protein of Indigofera oblongifolia leaves. J. Ethnopharmcol.. 1999;64:277-282.
- [Google Scholar]
- Trypanosoma evansi in inbred and Swiss-Webster mice: distinct aspects of pathogenesis. Parasitol. Res.. 2004;94(3):193-200.
- [Google Scholar]
- Trypanosoma evansi and Surra: a review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. Biomed. Res. Int.. 2013;2013:194176
- [Google Scholar]
- Indigofera oblongifolia protects against trypanosomiasis-inducedspleen injury. J. Infect. Public. Health.. 2019;12:660-665.
- [Google Scholar]
- Impact of sex differences in brain response to infection with Plasmodium berghei. Parasitol. Res.. 2016;115(1):415-422.
- [Google Scholar]
- Drury, R.A.B., Wallington, E.A., 1980. Carleton’s Histological Technique. 5th ed. Oxford, UK: Oxford University Press. 188–189, 237–240, 290–291.
- Revisão sobre óxido nítrico. Jornal Brasileiro de Patologia e Medicina Laboratorial. 2000;39:343-349.
- [Google Scholar]
- Óxido nítrico: o simples mensageiro percorrendo a complexidade. Metabolismo, síntese e funções. Revista da Associação de Medicina Brasileira. 2000;46:265-271.
- [Google Scholar]
- The microbicidal activity of interferon-y-treated macrophages against Trypanosoma cruzi involves an L-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur. J. Immunol.. 1992;22:2501-2506.
- [Google Scholar]
- Analysis of nitrate, nitrite, and [15n] nitrate in biological fluids. Anal. Biochem.. 1982;126:131-138.
- [Google Scholar]
- Interdiscip. Perspect. Infect. Dis.. 2009;2009:1-8.
- [CrossRef]
- Pathogenic mechanisms of Trypanosoma evansi infections. Res. Vet. Sci.. 2012;93(1):13-17.
- [Google Scholar]
- Trypanosoma brucei: a rapid “matching” method for estimating the host’s parasitemia. Exp. Parasitol.. 1976;40:427-431.
- [Google Scholar]
- Human trypanosomiasis caused by Trypanosoma evansi in India: the first case report. Am. J Trop. Med. Hyg.. 2005;73:491-495.
- [Google Scholar]
- Effects of vasopressor drugs on number of Trypanosoma congolense in ruminant blood. Vet. Parasitol.. 1985;17(4):287-294.
- [Google Scholar]
- Trypanosoma brucei brucei: a long-term model of human African trypanosomiasis in mice, meningoencephalities, astrocytosis, and neurological disorders. Exp. Parasitol.. 1997;85:183-192.
- [Google Scholar]
- Inducible nitric oxide synthase and nitrotyrosine in the central nervous system of mice chronically infected with Trypanosoma brucei brucei. Exp. Parasitol.. 2004;95(1):19-27.
- [Google Scholar]
- Antimalarial and antioxidant activities of Indigofera oblongifolia on Plasmodium chabaudi-induced spleen tissue injury in mice. Parasitol. Res.. 2015;114:3431-3438.
- [Google Scholar]
- Susceptibility of Trypanosoma evansi to human blood and plasma in infected mice. Vet. Parasitol.. 2009;168:1-4.
- [Google Scholar]
- A rare case of human trypanosomiasis caused by Trypanosoma evansi. Indian J. Med. Microbiol.. 2006;24:72-74.
- [Google Scholar]
- Physiological and antigenic changes in Trypanosoma lewisi mediated by epinephrine. Comp. Gen. Pharmacol.. 1973;41:327-332.
- [Google Scholar]
- African trypanosomiasis: haematogenic brain parasitism early in experimental infection through bypassing the blood-brain barrier, with consideration on brain trypanosomiasis in man. Parasitol. Res.. 1987;73:15-21.
- [Google Scholar]
- Protective effect of lcysteine and glutathione on the modulated suckling rat brain Plasmodium berghei ANKA after chronic exposure. Parasitol. Res.. 2004;108:807-814.
- [Google Scholar]
- World Health Organization (WHO), 2017. Trypanosomiasis, human African (sleeping sickness) fact sheet. www.who.int/meidacentre/factsheets/fsn259/en/accessed on 6/4/2017.







