Translate this page into:
Brain oxidative status and behavioral response of mice infected with Trypanosoma evansi
⁎Corresponding author. fathagfan@pnu.edu.sa (Felwa A. Thagfan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
There is a growing awareness of the importance of incorporating behavioral changes into infectious disease treatments. In this study, we evaluated whether behavioral changes in mouse brains were altered following infection with the Trypanosoma evansi parasite. Infection significantly reduced locomotor activity as well as vertical and horizontal movements. In the grip strength test, infected mice showed a lower peak than the control group, and in the rotator test, parasite-infected mice spent less time on the rod compared with control mice. The parasite decreased the concentration of both glutathione and catalase in the brain. In addition, the infection caused a significant increase in the levels of malondialdehyde and nitric oxide. The infection induced marked histopathological changes in the brain parts and considerably increased the concentrations of both dopamine and serotonin in the brains of mice. Collectively, T. evansi infection resulted in an oxidative imbalance in the brain. This resulted in neurodegenerative alterations in the brain, as well as a shift in the behavior of mice.
Keywords
Trypanosomiasis
Behavior
Oxidative damage
Neurotransmitter
Mice
1 Introduction
Evaluation of behavioral changes during infection is necessary in biomedical research, including when screening for the pharmacological and toxic effects of novel anti-parasitic compounds. The effects of these agents on general health and on the neurological and locomotor function are important parameters to be considered (Karl et al., 2003).
Trypanosomiasis is an infectious disease caused by trypanosome infection. Both humans and animals can be infected with several Trypanosoma species (Kumar et al., 2021). Surra, also known as animal trypanosomiasis, is caused by Trypanosoma evansi and affects both wild and domestic animals. T. evansi infection causes weakness, fever, anemia, and neurotic symptoms in mice (Desquesnes et al., 2013).
Baldissera et al. (2015) reported that infection with T. evansi induced cognitive deficit in rats, which may have resulted from brain inflammation (Berlin et al. 2009). In addition, Joshi et al. (2005) found that T. evansi-infected humans developed sensory deficits, confusion, anxiety, and irritability. Moreover, neurological and behavioral impairments have been reported in parasitic infections with Plasmodium berghei (Desruisseaux et al., 2008).
Several studies have shown that oxidative stress plays a role in a variety of disorders, including those caused by T. evansi (Avery 2011; Ienco et al., 2011; Baldissera et al., 2014). T. evansi was also reported to induce oxidative stress in several brain regions (Da Silva et al., 2011). Because the brain is ultimately responsible for organizing and controlling behavior, this study was conducted to determine how behavioral changes in mice brains evolved when they were infected with the T. evansi parasite.
2 Materials and methods
2.1 Animals and infection
Twenty male C57BL/6 mice (13 ± 2 weeks old) were used in this study. The animals were allocated into two groups (10 mice per group). The non-infected control received only water while the infected group was intraperitoneally infected with 1000 T. evansi. According to Dkhil et al. (2019), the mice were infected with parasites. On day 4 post-infection, the mice were sacrificed by CO2 asphyxiation, and their brains were extracted.
2.2 The behavior experiments
Locomotor activity was measured using the activity cage (Basile, Milan, Italy; Catalog No 7400) as previously described by Pontieri et al. (2001).
The grip strength of the mouse forelimbs (i.e., peak force and time resistance) was measured using a Grip-Strength Meter (COMERIO, Varese, Italy). Each mouse was tested three times, and the peak force was recorded.
The mice balance, coordination, and motor activity were measured using the rota-rod instrument (COMERIO). The mouse was horizontally positioned and mechanically rotated at a rate of 15 revolutions per minute. The amount of time an animal spent on the rotating rod was calculated.
2.3 Brain histopathology
The brains of mice were extracted from the skull and fixed in 10% formalin for 24 h before being processed in ethanol and xylene, embedded in paraffin wax, and sectioned into 4-µm sections. Hematoxylin and eosin staining was performed as described by Drury and Wallington (1980).
2.4 Oxidative status in the brain
Mouse brain tissue was homogenized in ice-cold medium containing 300 mM sucrose and 50 mM Tris-HCl. The homogenates were centrifuged for 10 min at 500 × g and 4 °C. The supernatant (10%) was used to determine the level of malondialdehyde (Ohkawa et al. 1979) and nitric oxide (Green et al., 1982). The enzymatic antioxidant, glutathione peroxidase, and catalase activities were determined according to the protocols of Paglia and Valentine (1967) and Aebi (1984), respectively.
2.5 Brain neurotransmitters
The brain was weighed and kept at −80 °C until it was used. The levels of dopamine and serotonin were determined as described by Ciarlone (1978).
2.6 Statistical analysis
Significance was evaluated by unpaired Student’s t-test. Data are expressed as the mean and standard error of the mean. p ≤ 0.05 was considered as significant for all statistical analyses.
3 Results
On day 4 post-infection, the weight of the mice was significantly decreased (Fig. 1) compared to that of control animals. Comparison of the locomotor activity of infected mice and non-infected mice showed that infection significantly decreased locomotor activity. Both vertical and horizontal activities significantly decreased following infection (Fig. 2).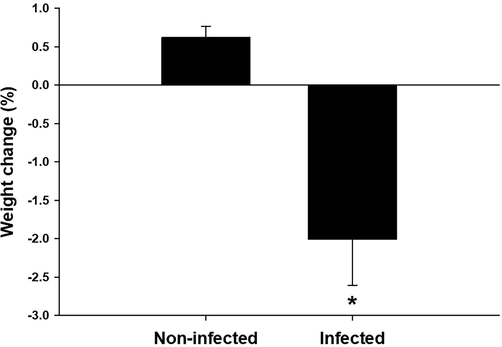
Change in weight of mice. *, significance against non-infected mice at P ≤ 0.5.
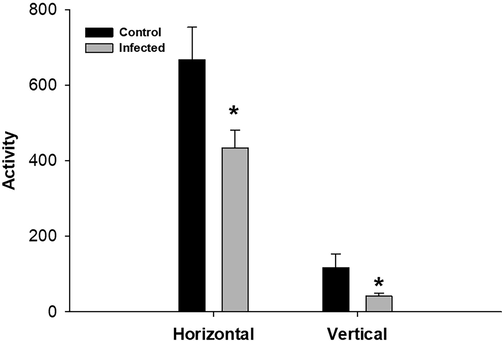
T. evansi infection causes changes in vertical and horizontal locomotor activities in mice.*, significance against non-infected mice at P ≤ 0.5.
In the grip strength test, the forelimb muscles of infected mice had a lower break force compared to the control group (Fig. 3). In the rotator test, parasite-infected mice spent less time on the rod compared to control mice (Fig. 4).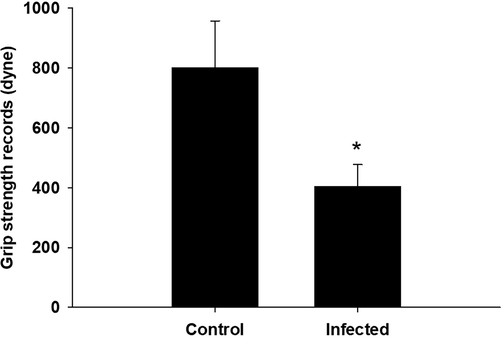
T. evansi infection alters mouse fore limb grip strength records. *, significance against non-infected mice at P ≤ 0.5.
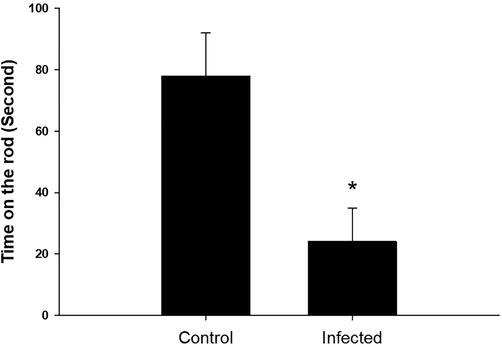
T. evansi infection alters mouse records in the rota rod. *, significance against non-infected mice at P ≤ 0.5.
The cerebrum, cerebellum, and medulla oblongata appeared as normal in the control non-infected mice; however, after infection, the pyramidal cells of the cerebrum were pyknotic. Purkinje cells were degenerated and spindle-shaped in the cerebellum of infected brains. Examination of the medulla oblongata showed that the medullary neurons had become pyknotic (Fig. 5).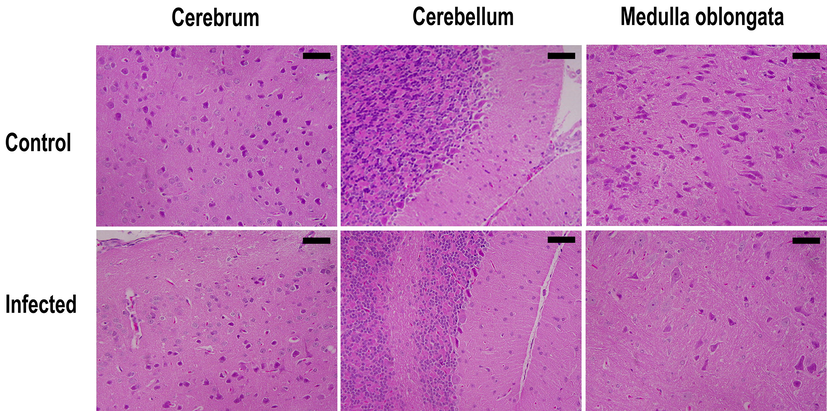
Sagittal sections of the cerebrum, cerebellum, and medulla oblongata of T. evansi-infected and non-infected mice brains. Bar = 50 µm.
Infection by the parasite led to decreased glutathione levels to 0.39 ± 0.02 mmol/g in the mouse brains compared to in non-infected animals (Table 1). Similar results were observed for catalase activity, with the parasite significantly reducing catalase levels. In addition, the infection caused a significant increase in the levels of both malondialdehyde and nitric oxide (Table 1). Fig. 6 shows that the concentrations of dopamine and serotonin in the brain were considerably increased by T. evansi infection. Values are means ± SD. *Significant change at P < 0.01 between control and infected animals.
Group
Glutathione peroxidase (mmol/g)
Malondialdehyde (nmol/g)
Nitric oxide (µmol/g)
Catalase (U/g)
Control
0.54 ± 0.07
540 ± 87
28.4 ± 20
3.4 ± 0.8
Infected
0.39 ± 0.02*
863 ± 116*
82.8 ± 15*
1.9 ± 0.1*
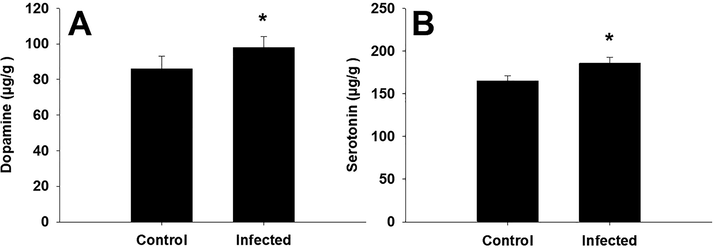
T. evansi infection change the concentration of both of dopamine and serotonin in the brain of mice. *, significance against non-infected mice at P ≤ 0.5.
4 Discussion
The behavioral and physiological responses of animals are altered during illness, which may occur through pathogen-dependent or host-dependent processes (Lopes et al., 2016).
As behavior is controlled by the brain, changes in the brain physiology due to infection can lead to changes in behavior. Kent et al. (1992) reported that pathogens stimulate the immune system to produce inflammatory cytokines that affect brain function and cause sickness-related behavior.
The decrease in mice weight following T. evansi infection occurred because of decreased animal motivation for food when the secretion of phagocytic cells was increased by pro-inflammatory cytokines (Johnson, 2002).
Forelimb and hind limb motor coordination and balance in the rotarod test requires intact cerebral function and motor coordination (Carter et al. 1999). In our study, the infection induced cellular oxidative damage and histopathological alterations the brain, which may be responsible for the decreased time on the rod.
Neuroinfectious diseases produce an inflammatory microenvironment that can have a detrimental impact on an infected person's quality of life, as well as their social and behavioral habits; this microenvironment can lead to brain dysfunction (Alves et al., 2020).
Because locomotor activity is required for several specific behavioral tasks, changes in locomotor activity should be evaluated before other behavioral characterizations are performed (Karl et al., 2003). Rotarod is a common test to evaluate neuromotor abilities such as motor control, balance, and ataxia (Carter et al., 1999). Here, T. evansei led to reductions in locomotor activity that were very similar to those induced by Toxoplasma (Stibbs, 1985).
In the body of mice, oxidative stress can cause tissue injury due to physiobiochemical reactions. These effects occur when the antioxidant and pro-oxidant status is disrupted, resulting in oxidative injury due to the reaction with free radicals. Various antioxidant mechanisms and antioxidant protection systems are induced in animals to restrict the prooxidant activity of reactive oxygen species to prevent or to lower the formation of these species (Pamplona and Costantini, 2011).
In this study, T. evansi decreased the level of glutathione, an endogenous antioxidant biomarker that functions by decreasing the enzyme binding of inactive disulfide to the active sulfhydryl group. By donating an electron to free radicals to neutralize them and avoid cell oxidation, the sulfhydryl group of glutathione is oxidized (Yadav et al., 2018). Thus, glutathione plays an important role in the defense against membrane peroxidation and decreases hydrogen peroxide levels (Dean et al., 2009). Malondialdehyde is a biological indicator of oxidative stress and was found to be increased in the liver during trypanosome infection (Dkhil et al., 2020a).
In this study, the levels of nitric oxide were increased following parasite infection, which agrees with the results of Dkhil et al. (2020b). The concentration of nitric oxide is used to assess the immunological response and oxidative stress state (Bogdan, 2001). Nitric oxide is involved in a variety of physiological activities, including neurotransmission (Dusse et al., 2000). Bombeiro et al. (2010) also found that excessive nitric oxide production during T. cruzi infection contributes to neurodegenerative processes.
Catalase is an important antioxidant enzyme that can help to reduce oxidative stress (Abd Ellah, 2010). Oxidative stress has been linked to a variety of diseases in several studies, such as T. evansi infection (Da Silva et al., 2011; Baldissera et al., 2014; Dkhil et al., 2020a). In our study, infection altered the oxidative status of the brain (Table 1).
The parasite's ability to affect the natural host's behavior is unknown. Several studies have suggested that parasitic brain infections alter neurotransmitter levels in the brain (Prandovszky et al., 2011; Skallová et al., 2006), among other effects. However, the precise mechanism underlying host behavior modification remains unknown.
Rats infected with T. evansi develop behavioral changes associated with alterations in the levels of neurotransmitters (Wolkmer et al., 2013). The brain and sympathetic nerves that innervate secondary lymphoid organs contain dopamine (Klein et al., 2019). Moreover, T and B cells have been shown to contain dopamine (Cosentino et al., 2007). Dopamine regulates biological effects such as movement, emotion, memory, cardiovascular, and endocrine functions by acting on G-protein receptors (Missale et al., 1998). Serotonin is also formed by the raphe nuclei in the brain and affects mood and cognition and also is produced by lymphocytes (Young et al., 1993). In this study, both serotonin and dopamine levels were increased in the mouse brain after infection with T. evansi, indicating disturbances in neurotransmitters.
T. evansi infection induced an imbalance in the brain oxidative status, leading to neurodegenerative changes in the brain and behavioral changes of the mice. We limited this study to know the brain oxidative status and both of the behavioral and histological changes induced by the parasite. This could help in diagnosis and treatment of trypanosomiasis in future.
Acknowledgment
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Involvement of free radicals in animal diseases. Comp. Clin. Pathol.. 2010;19(6):615-619.
- [Google Scholar]
- Purinergic signaling in infectious diseases of the central nervous system. Brain Behav. Immun.. 2020;89:480-490.
- [Google Scholar]
- Treatment with essential oil of Achyrocline satureioides in rats infected with Trypanosoma evansi: relationship between protective effect and tissue damage. Pathol. Res. Pract.. 2014;210(12):1068-1074.
- [Google Scholar]
- Relationship between behavioral alterations and activities of adenylate kinase and creatine kinase in brain of rats infected by Trypanosoma evansi. Exp. Parasitol.. 2015;151-152:96-102.
- [Google Scholar]
- Disseminated central nervous system disease caused by Trypanosoma evansi in a horse. Vet. Parasitol.. 2009;161(3-4):316-319.
- [Google Scholar]
- Neurodegeneration and increased production of nitrotyrosine, nitric oxide synthase, IFN-gamma and S100beta protein in the spinal cord of IL-12p40- deficient mice infected with Trypanosoma cruzi. Neuroimmunomodulation.. 2010;17(2):67-78.
- [Google Scholar]
- Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J. Neurosci.. 1999;19(8):3248-3257.
- [Google Scholar]
- Further modification of a fluorometric method for analyzing brain amines. Microchem. J.. 1978;23(1):9-12.
- [Google Scholar]
- Human CD4+CD25+regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood. 2007;109(2):632-642.
- [Google Scholar]
- Acetylcholinesterase activity and lipid peroxidation in the brain and spinal cord of rats infected with Trypanosoma evansi. Vet. Parasitol.. 2011;175(3-4):237-244.
- [Google Scholar]
- A role for glutathione in the pathophysiology of bipolar disorder and schizophrenia? Animal models and relevance to clinical practice. Curr. Med. Chem.. 2009;16(23):2965-2976.
- [Google Scholar]
- Trypanosoma evansi and surra: A review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. Biomed. Res. Int.. 2013;2013:1-22.
- [Google Scholar]
- Cognitive dysfunction in mice infected with Plasmodium berghei strain ANKA. J. Infect. Dis.. 2008;197(11):1621-1627.
- [Google Scholar]
- Indigofera oblongifolia as a fight against hepatic injury caused by murine trypanosomiasis. Saudi. J. Biol. Sci.. 2020;27(5):1390-1395.
- [Google Scholar]
- Brain response after treatment of Trypanosoma evansi-infected mice with Indigofera oblongifolia. JKSU-Sci.. 2020;32(4):2311-2315.
- [Google Scholar]
- Indigofera oblongifolia protects against trypanosomiasis-induced spleen injury. J. Infect. Public. Health.. 2019;12(5):660-665.
- [Google Scholar]
- Carleton’s Histological Technique. Oxford University Press, Oxford, UK. 1980;188–189(237–240):290-291.
- [Google Scholar]
- Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem.. 1982;126(1):131-138.
- [Google Scholar]
- Oxidative stress treatment for clinical trials in neurodegenerative diseases. J. Alzheimer. Dis.. 2011;24(s2):111-126.
- [Google Scholar]
- The concept of sickness behavior: a brief chronological account of four key discoveries. Vet. Immunol. Immunopathol.. 2002;87(3-4):443-450.
- [Google Scholar]
- Human trypanosomiasis caused by Trypanosoma evansi in India: the first case report. Am. J. Trop. Med. Hyg.. 2005;73(3):491-495.
- [Google Scholar]
- Behavioral phenotyping of mice in pharmacological and toxicological research. Exp. Toxicol. Pathol.. 2003;55(1):69-83.
- [Google Scholar]
- Sickness behavior as a new target for drug development. Trends Pharmacol. Sci.. 1992;13(1):24-28.
- [Google Scholar]
- Dopamine: Functions, signaling, and association with neurological diseases. Cell. Mol. Neurobiol.. 2019;39(1):31-59.
- [Google Scholar]
- Development of a loop-mediated isothermal amplification assay based on RoTat1.2 gene for detection of Trypanosoma evansi in domesticated animals. Parasitol. Res.. 2021;120(5):1873-1882.
- [Google Scholar]
- Infection-induced behavioural changes reduce connectivity and the potential for disease spread in wild mice contact networks. Sci. Rep.. 2016;6:31790.
- [Google Scholar]
- Protective effects of Spinacia oleracea seeds extract in an experimental model of schizophrenia: Possible behavior, biochemical, neurochemical and cellular alterations. Biomed. Pharmacother.. 2018;105:1015-1025.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95(2):351-358.
- [Google Scholar]
- Molecular and structural antioxidant defenses against oxidative stress in animals. Am. J. Physiol. Regul. Integr. Comp. Physiol.. 2011;301(4):R843-R863.
- [Google Scholar]
- Behavioral sensitization to heroin by cannabinoid pretreatment in the rat. Eur. J. Pharmacol.. 2001;421(3):R1-R3.
- [Google Scholar]
- The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS One.. 2011;6(9):e23866.
- [CrossRef] [Google Scholar]
- The role of dopamine in Toxoplasma-induced behavioural alterations in mice: an ethological and ethopharmacological study. Parasitology.. 2006;133(Pt 5):525-535.
- [Google Scholar]
- Changes in brain concentrations of catecholamines and indoleamines in Toxoplasma gondii–infected mice. Ann. Trop. Med. Parasitol.. 1985;79(2):153-157.
- [Google Scholar]
- Wolkmer, P., Paim, F.C., DA Silva, C.B., Gai, B.M., Carvalho, F.B., DA Souza, A.C., DA Rosa, M.M., DA Silva, A.S., Pereira, P.R., Lopes, S.T., 2013. Nogueira CW, Rubin MA, Monteiro SG, Mazzanti CM. Trypanosoma evansi infection impairs memory, increases anxiety behaviour and alters neurochemical parameters in rats. Parasitology. 140(11),1432-41.
- Stimulation of splenic T-lymphocyte function by endogenous serotonin and by low-dose exogenous serotonin. Immunology. 1993;80(3):395-400.
- [Google Scholar]







