Translate this page into:
Biosynthesis, characterization and monitoring in vitro antibacterial efficiencies of AgNPs with filamentous cyanobacteria Spirulina sp. And S. Subsalsa against pathogenic bacteria
⁎Corresponding author at: Central Research Laboratory, Institute of Medical Sciences, Siksha ‘O’ Anusandhan Deemed to be University, Bhubaneswar 751003, Odisha, India. rnpadhy54@gmail.com (Rabindra Nath Padhy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Study of the in-vitro antibacterial efficiencies of cyanobacteria-biosynthesized AgNPs. Analysis by UV–Vis, FESEM, FTIR, DLS, and XRD of biosynthesized AgNPs with Spirulina sp. and S. subsalsa.
Abstract
Silver nanoparticles are a fascinating nanomaterial group among metallic nanoparticles for several possible uses, mainly for controlling multidrug-resistant human pathogenic bacteria. The objectives here are to focus on the characterization and observation of antibacterial efficacies of AgNPs biosynthesized using the aqueous extracts of two filamentous non-nitrogen fixing cyanobacteria, Spirulina sp. and Spirulina subsalsa against bacteria. Characterizations of biosynthesized AgNPs with ultraviolet–visible spectroscopy, field emission scanning electron microscopy, Fourier transform infrared spectroscopy, dynamic light scattering, and X-ray diffraction analysis were done. The ultraviolet–visible spectrum demonstrated the reduction of S. subsalsa-AgNPs with a plasmon resonance peak of 458 nm and Spirulina sp.-AgNPs with a peak value of 432 nm, indicative of the yield of biosynthesized AgNPs. The antibacterial efficiencies were observed against MDR strains isolated from clinical samples of this hospital with Gram-negative bacteria, Escherichia coli (MTCC443), Acinetobacter baumannii (MTCC1425), and Gram-positive bacterium, Streptococcus pyogenes (MTCC1982) by the agar-well diffusion method. The minimum inhibitory concentration was 50–100 µg/ml, and the minimum bactericidal concentration value was 80–140 µg/ml in both Spirulina species. The synthesized AgNPs were more potent during in vitro control of MDR h-pathogenic bacteria with the zones of inhibitions at 14 and 15 mm for Spirulina sp.-AgNPs. and 15, 14, and 13 mm for S. subsalsa-AgNPs. Additionally, FESEM images were used to analyze spherical surface morphology with sizes ranging from 40 to 50 µm. The novelty of the study highlights the biosynthesis of AgNPs individually with S. subsalsa and Spirulina sp.; eventually, each cyanobacterial biomass holds some great promise for antimicrobial use against h-pathogenic MDR bacteria. Furthermore, research signifies the AgNP biosynthesis with cyanobacteria, evaluates the biocompatibility, and check for host-toxicities of the biosynthesized AgNPs for future possible therapeutic applications.
Keywords
Cyanobacteria
Biosynthesis
Silver nanoparticle
Characterization
Antibacterial efficiency
- AgNPs
-
Silver nanoparticles
- DLS
-
Dynamic light scattering
- DPPH
-
Diphenyl picrylhydrazyl
- FESEM
-
Field emission scanning electron microscopy
- FRAP
-
Ferric reducing/ antioxidant power
- FTIR
-
Fourier transform infrared spectroscopy
- GLA
-
Gamma-linoleic acid
- MBC
-
Minimum bactericidal concentrations
- MDR
-
Multidrug-resistant
- MHA
-
Mueller-Hinton Agar
- MHB
-
Mueller Hinton Broth
- MIC
-
Minimum inhibitory concentration
- NPs
-
Nanoparticles
- PBPs
-
Phycobiliproteins
- PDI
-
Polydispersity index
- ROS
-
Reductive oxygen species
- SPR
-
surface plasmon variation
- Ss-AgNPs
-
Spirulina subsalsa-AgNPs
- Ssp-AgNPs
-
Spirulina sp.-AgNPs
- UV–Vis
-
Ultraviolet–visible spectroscopy
- XRD
-
X-ray diffraction analysis
- ZOI
-
Zone of inhibition
Abbreviations
1 Introduction
Due to inherent distinct qualities, such as high surface area, reactivity and durability, silver nanoparticles (AgNPs) have become popular as antimicrobial agents. Antibacterial overuses, which concomitantly lead to bacterial resistance have made antibiotics obsolete and/ or less efficient over the past decades; nevertheless, several tactics such as chemical modifications of the obsolete drug and administration of an antibiotic member of a higher generation or combinatorial therapy could be employed to counteract critical clinical problems. Amino acids and DNA interact in the biosynthesis of AgNPs from broken cell proteins, aiding in possible druggable use as antibacterials (Shantkriti et al., 2023). Particularly, compared to conventional physicochemical methods, the synthesis of AgNPs using biological systems offers several advantages, such as the ability to control the size and form of the particles and less negative environmental impacts without any use of external energy. In contrast to expensive and hazardous physicochemical synthesis techniques, biologically generated AgNPs have solubility in water, and yields. Moreover, these techniques produce nanoparticles (NPs) that are bound to health-sensitive substances, which eventually compromise their application in medicine (Zahin et al., 2020). An easy one-step method can be utilized to create biosynthesis of AgNPs using plant extracts, with examples, the dried root of Zingiber officinale (Vijaya et al., (2017), aqueous leaf extracts of Passiflora edulis f. flavicarpa (Thomas et al., 2019), Basella alba leaf (Mani et al., 2021), and using Aspalathus linearis leaf extract by α-Ni(OH)2 (Diallo et al., 2018) for semiconductor metal oxide nanocrystals via photocatalytic application, which could generate health-sensitive chemicals (Jahangir et al., 2023). Thus, developing host-safe, NP-processing methods with organisms is coveted (Bhatt et al., 2022).
As it is, marine algae synthesis of NPs is done, and those have antibacterial characteristics (Al Nadhari et al., 2021). Currently, the focus is on locating and evaluating more contemporary natural sources as antioxidants in algae. Algal antioxidant qualities are due to phytochemicals that can be crucial and have high redox potentials (Ismail et al., 2021). For example, cyanobacterial phycobiliproteins (PBPs), namely, C-phycocyanin are abundant as the unique pigments employed by cyanobacteria for oxygenic photosynthesis, which explains oxygenation of the nascent earth; oxygenation could be the bold reaction in waiving out reductive oxygen species (ROS) from tissues suffering from ROS; at body metabolism how, NPs might lessen and stabilize the developing cell, as shown (Kannaujiya et al., 2020). Several tests proved the antioxidant activities of AgNPs, the ferric reducing/ antioxidant power (FRAP) assay, metal chelating activity, superoxide radical scavenging activity, and 2,2-diphenylpicrylhydrazyl (DPPH) radical scavenging activity, etc. (Prajapati et al., 2022). Herein, the green synthesis and monitoring of the antibacterial activities of the biosynthesized AgNPs for their plausible health advantages since both cyanobacterial species used have vitamins, minerals, and vital amino acids (Sharma and Phutela, 2023). Indeed, Spirulina sp. and S. subsalsa are photosynthetic and spiral, or coiled filamentous cyanobacteria in morphology belonging to the order Oscillatoriales and with pharmaceutics. Because of the high gamma-linoleic acid (GLA) content, Spirulina is a good dietary supplement (Matufi and Choopani, 2020). Various Spirulina strains have various lipid and fatty acid compositions, which may also depend on the conditions under which they are cultivated (Lupatini et al., 2017). This study evaluates the biosynthesis of AgNPs with the extract of Spirulina sp. and S. subsalsa were characterized and their antibacterial efficacies were examined in vitro.
2 Materials and methods
2.1 Unialgal biomass production and optimization
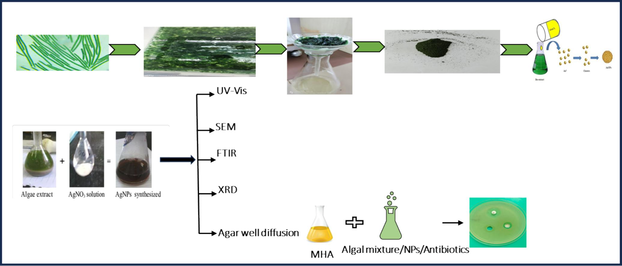
Spirulina sp. (strain no. BDU51381) (Ssp) and S. subsalsa (strain no. BDU101022) (Ss) were obtained from Bharathidasan University, Tiruchirappalli, TN, India; and those two taxa were mass-cultured in ASN III medium in a culture room. Individual biomasses were shade-dried for a month at room temperature. The obtained powders were stored in airtight containers. The extracts of Ssp and Ss were individually combined and incubated for one hour at 90 °C with 2 gm of shade-dried cyanobacterial biomass in 100 ml of deionized water for aqueous extracts to get aqueous extracts. Each extract was filtered to produce a clear solution, and each extract was combined individually with ethanol, methanol, butanol, acetone, and water separately to prepare each alga for antibacterial monitoring (Fig. 1).
Experimental setup and biomass production of Ssp and Ss extracts.
2.2 Biosynthesis of AgNPs of Spirulina sp. (Ssp-AgNPs) and S. Subsalsa (Ss-AgNPs)
Two lots of 10 mg of dried biomass of Ssp and Ss separately were roughly mixed at 40° C for 24 hrs (Sahoo et al., 2020). An aliquot of one milliliter of AgNO3 in a 1 mM solution was added to the aqueous medium. The reaction color was monitored every hour for 48 hrs. When the color of the incubated mixture changed from pale green to deep brown, the lot was repeatedly centrifuged at 4,000 rpm for 10 min (Sahoo et al., 2020). After centrifuging the mixture with AgNPs for 10 min., the individual final pellets were dried at 40° C for ultraviolet–visible spectroscopy (UV–vis), field emission scanning electron microscopy (FESEM), Fourier transform infrared spectroscopy (FT-IR), dynamic light scattering (DLS), and X-ray diffraction analysis (XRD), spectral characterizations. The visible colour change was verified by UV–visible spectrophotometry, which obtained spectral readings at 432 nm and 458 nm for Ssp-AgNPs and Ss-AgNPs, respectively.
2.3 Spectral characterization by standardization of suitable analytical tools
2.3.1 UV–Vis
The adsorption of each derived tan solution was evaluated using a spectrophotometer (JASCO V-670 UV–VIS-NIR Spectrophotometer; Tokyo, Japan). UV–vis spectra were used to identify the synthesis of silver nanoparticles. The sample was extracted in 0.2 ml aliquots of the colloidal suspension diluted to 2 ml with deionized water. The appearance of a plasmon resonance peak indicated the biogenesis of AgNPs (Zaheer et al., 2023).
2.3.2 FE-SEM
Every sample was photographed at different magnifications, and investigations were conducted into the distribution of particle sizes, presence, aggregation, and density. The morphologies of the nanoparticles, which range from spherical to more complex geometries, may be observed in detail using the great spatial resolution of FESEM, providing insight into their synthesis and stabilization processes. Furthermore, AgNP interactions with bacterial cells are clarified by FESEM, which also helps to understand the mechanisms behind the nanoparticles' antibacterial activity (Bhuyar et al., 2020).
2.3.3 FT-IR
For a Fourier transformer infrared (FTIR), JASCO FT/IR4600-ATR spectrophotometer, at the range of 400–4,000 cm−1 was used to identify the functional groups that were thought to be responsible for the reduction of silver ions in the extracts of Spirulina sp. and S. subsalsa. (Gnanakani et al., 2019).
2.3.4 DLS
The polydispersity index (PDI), hydrodynamic size (Z average), and particle size distribution of NPs were investigated using a DLS method by the MALVERN instrument (ZEN 1600). The size distribution and stability of AgNPs in solution are revealed by DLS, which measures the changes in light scattering caused by the Brownian motion of the nanoparticles. This approach is effective for monitoring size changes over time and assessing the homogeneity of suspensions of nanoparticles (Patil and Chougale, 2021).
2.3.5 XRD
X-ray diffraction was used to analyze the crystal structure of AgNPs, with information on the phase composition, crystallite size, and lattice parameters of the synthesized particles. Following drying, a glass substrate was coated with NP powder and then scanned in the 2θ area at 0.5°/min. Based on the line width of the maximum intensity reflection peak in the XRD pattern and Scherrer's equation, the mean particle diameter of AgNPs was determined (Jebril et al., 2020).
2.4 Applications of biosynthesized AgNPs
2.4.1 Antibacterial activities of Ssp-AgNPs & Ss-AgNPs
The Gram-positive bacterial strain, Streptococcus pyogenes (S. pyogenes), and the Gram-negative, Acinetobacter baumannii (A. baumannii), Escherichia coli (E coli) were cultivated on nutrient, Mueller-Hinton Agar (MHA) and in Mueller Hinton Broth (MHB). Before being maintained in a lab environment at 8 °C for the antibacterial experiment, the bacteria were grown in Mueller Hinton broth (MHB). After isolating one microbe into an aliquot, it was placed in a sterile 15 ml centrifuge tube with a laminar air-flow chamber and 5 ml of nutrient broth (Himedia, M002) medium. Once isolated from clinical samples, these pathogenic bacteria were regrown in laboratory conditions. The MDR strains of E. coli, A. baumannii, and S. pyogenes were considered for antibacterial activities of the study had previously been identified (Dubey and Padhy, 2013; Rath and Padhy, 2015, Swain et al., 2024).). The isolated bacteria were subjected to antibiotic-susceptibility tests, as described, as signposted: E. coli (MTCC443), A. baumannii (MTCC1425), and S. pyogenes (MTCC1982). MHA plates were used to store the pure bacterial cultures. The microbial strains were swabbed onto MHA plates. The media was stabbed with a sterile puncher to create the 8 mm diameter wells. The positive control was gentamycin. Additionally, a micropipette was used to add 50 µl of the extract to each well. MDR strains of the reported bacteria were used for monitoring biosynthesised nanoparticles' antibacterial efficacies by the agar-well diffusion test and the determination of MICs and MBCs using a 96-well plate and the conventional serial dilution method. Using a standard scale, the inhibitory zone surrounding the dropping was measured in mm. The OD600 of each bacterial strain was also used to determine its vitality, which was used to monitor the anti-microbial qualities of AgNPs.
3 Results
3.1 Biosynthesis of AgNPs
Additionally, a color reaction was observed, whereby the transparent AgNO3 solution took on a reddish-brown color, suggesting that silver nanoparticles may eventually surface, as illustrated (Fig. 2). The appearance of a brown color upon visual inspection indicated that Ssp and Ss were utilized in the synthesis of AgNPs.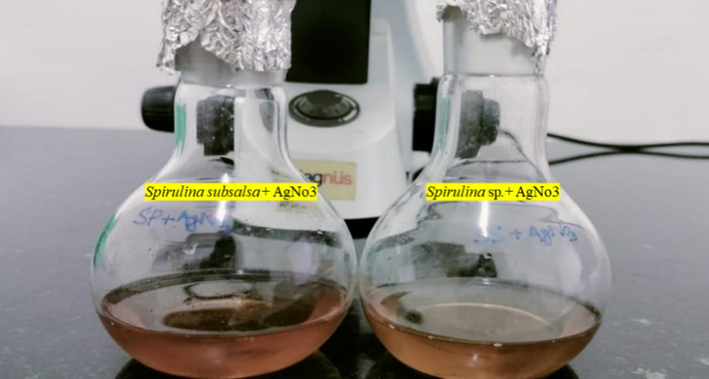
Formation of color changed after the biosynthesis of Ss-AgNPs and Ssp-AgNPs.
3.2 Characterizations
3.2.1 Uv–vis spectroscopy of biosynthesized Ssp-AgNPs and Ss-AgNPs
Using UV–visible spectroscopy, Spirulina sp., and S. subsalsa produced AgNPs with a distinct reddish-brown color (Fig. 2), surface plasmon variation (SPR) caused the color shift, and a prominent peak was seen at 432 and 458 nm in the spectral spectrum of Ssp. and Ss respectively (Fig. 3). SPR in the 450–500 nm spectral band is indicated for optical filtering applications in Ag–TiO2 nano cermet thin films formed by two different sputtering techniques, as the smaller silver grains spread out from the first heterogeneous layer produced the massive, flat Ag grains that make up the overlayer. The weak SPR absorption at about 700 nm is caused by these anisotropic granules (Dakka et al., 2000). It was in line with previous research, which indicated that the usual SPR peak of AgNPs was located between 440 and 450 nm (Dar et al., 2013). ZnO nanoparticles that have been biosynthesized have been effectively produced utilizing Moringa oleifera extract and have been characterized by several approaches. After annealing, pure, single-phase absorbance profiles with a fall beginning at 350 nm and an inflection point at 380 nm, which is equivalent to 3.2 eV and in good agreement with the bandgap of ZnO, are obtained (Matinise et al., 2017). ZnSnO3-NPs were shown to have UV–visible diffused reflectance, an absorption edge at 354 nm, a band gap of 3.50 eV, and possible photocatalytic activity using Aspalathus linearis natural extracts (Mayedwa et al., 2018a), and another study revealed a peak of SPR of AgNPs was noted the range of 420 nm (Bishoyi et al., 2021).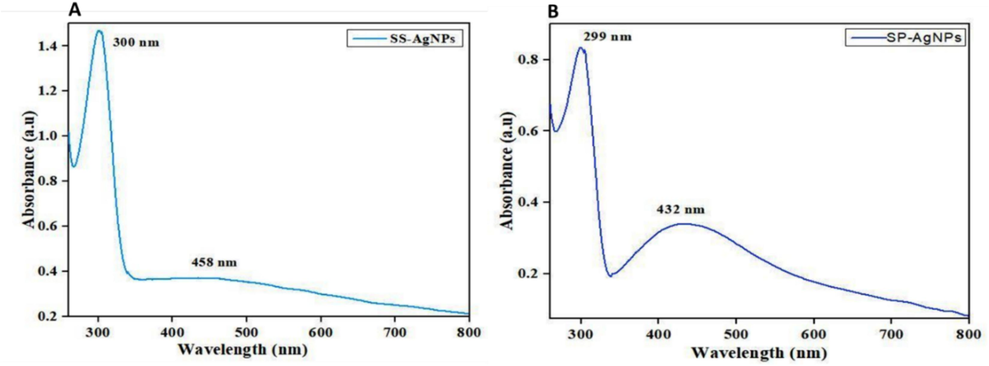
Observation of SPR absorbance peaks of (A) Ss- AgNPs and (B) Ssp.-AgNPs by UV–vis spectroscopy analysis.
3.2.2 FE-SEM of biosynthesized Ssp-AgNPs, and Ss-AgNPs
The morphology of the nanoparticles was assessed by FESEM analysis (Fig. 4). The smaller particles in the FESEM investigation had aggregated to form a larger particle-like appearance. As revealed by SEM investigation, the dispersion of silver nanoparticles on the surface grid amply illustrates the morphological regularity. SEM tests indicated that the O. princeps extract generated spherical AgNP units with diameters ranging from 100–200 nm during the manufacture of O. princeps-AgNPs (Bishoyi et al., 2021). The synthetic Spirulina-AgNPs used in this investigation had a quasi-spherical form and ranged in size from 40-50 µm.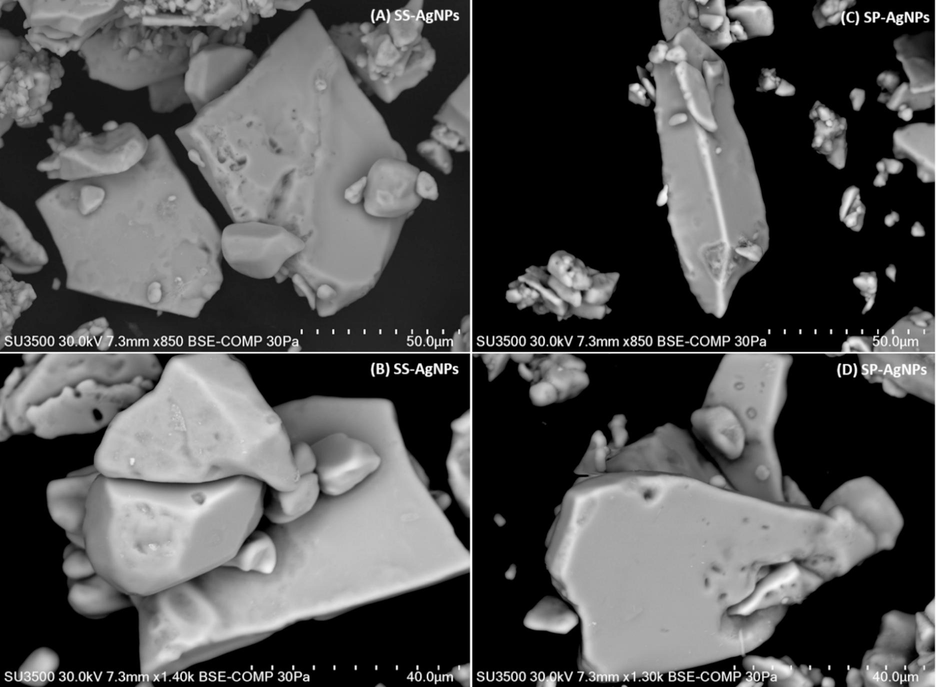
Observation of size of the particles of Ss-AgNPs (A, B), and Ssp-AgNPs, (C, D) FESEM analysis.
3.2.3 FT-IR spectrometric profile of biosynthesized Ssp-AgNPs and Ss-AgNPs
Spirulina sp. and S. subsalsa exhibit distinct peaks at 3332, 1636, and 536 cm−1 and 1634, 1634, and 536 cm−1, respectively, FTIR spectra (Fig. 5, Table 1). The Spirulina sp. and S. subsalsa peaks, respectively, correspond to the N–H group of amino acids at 3350 and 3332 cm−1. One can observe a diketone group as a peak at 1634 and 1636 cm−1. The halogen compound of C caused the peaks at 536 and 524 cm−1. (Fig. 6).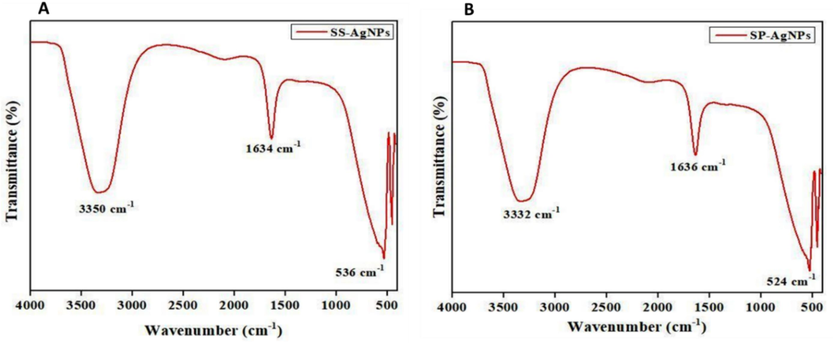
Observation of peaks of functional groups by FT-IR analysis (A) Ss-AgNPs and (B) Ssp-AgNPs.
AgNPs
Peak (cm−1)
Functional groups
Ssp- AgNPs
3332
Amino acid (N–H)
1636
Diketones
524
Halogen compound (C-I)
Ss- AgNPs
3350
Amino acid (N–H)
1634
Diketones
536
Halogen compound (C-I)
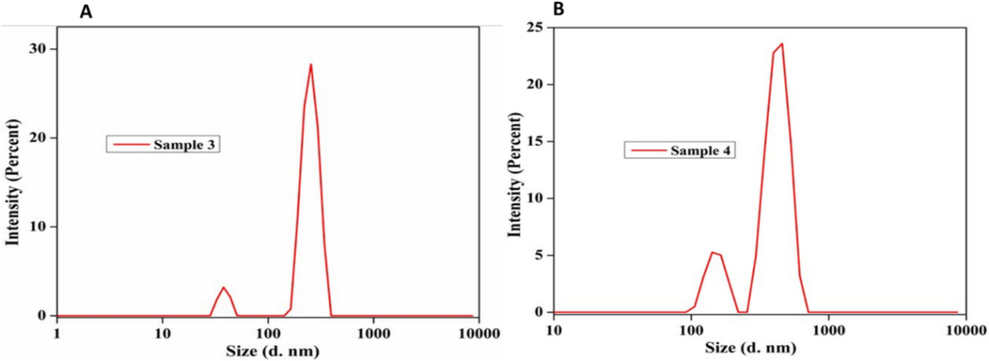
Observation of size of NPs (A) Ss- AgNPs and (B) Ssp- AgNPs by DLS analysis.
3.2.4 DLS of biosynthesized Ssp-AgNPs, and Ss-AgNPs
The DLS distribution of Spirulina AgNPs size was found to range between 30 and 60 nm, however, the previous results were with an average computed size of 26.57 nm and a maximum mean intensity of 3.02 % found to be consistent with the dispersed light intensity value of 976.11 or the mean intensity value of 16.93 (Bishoyi et al., 2021). Pedalium murex produced biogenic AgNP with an average DLS of 73.14 nm (Bishoyi et al., 2021).
3.2.5 XRD of biosynthesized Ss, and Ssp-AgNPs
Moreover, crystalline structures were generated in 0–70 theta (Θ) ranges individually in both Spirulina species, the analysis of this study was presented (Fig. 7), and the peaks list of biosynthesized AgNPs (Ss-AgNPs, and Ssp-AgNPs) indicated the crystal size of NPs showed by XRD analysis (Tables S1, and S2). The previous study of XRD analysis with values of 10.3°, 38.90°, and 63.9°, and verified the production of AgNPs. The synthesized AgNPs were generated at two different temperatures, 39.01°, and 64.72°, according to the crystalline pattern (Bishoyi et al., 2021).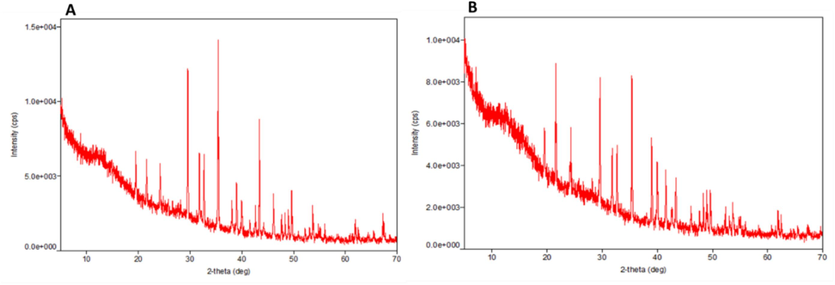
Observation of crystalline structure of (A) Ss- AgNPs and (B) Ssp- AgNPs by XRD analysis.
3.2.6 Antibacterial properties of biosynthesized Ssp-AgNPs, and Ss-AgNPs
The produced Ssp-AgNPs and Ss-AgNPs exhibited remarkable antibacterial efficacy against bacterial strains, E. coli, A. baumannii, and S. pyogenes. The MIC values for the bacterial species were 50–100 µg/ml, the MBC value ranged from 80-140 µg/ml where the ZOI of −ve control was 0 mm, + ve control (Gentamycin) ranged from 17 mm to 19 mm. The ZOI of Ssp-AgNPs showed higher potencies of killing MDR bacteria than Ss-AgNPs (Table 2, Fig. 8). However, the early study ZOI of S platensis extracts ranged from 5 to 45 mm at 200 µl/well. The mean ZOI (30 mm) in the S. platensis nanomaterial extract inhibited Gram-positive bacteria with ZOI as given, Pseudomonas aeruginosa (12 mm), Staphylococcus aureus, Enterococcus faecalis (6 mm), and Klebsiella pneumoniae (11 mm) (Awadalla et al., 2021).
AgNPs
Bacterium
ZOI of −ve control (mm)
ZOI of + ve control (mm)
ZOI of Ssp-AgNPs and Ss-AgNPs (mm)
MIC (µg/ml)
MBC (µg/ml)
Ssp-AgNPs
A. baumannii
0
19
15
80
100
Ssp −AgNPs
E. coli
0
18
14
50
80
Ssp −AgNPs
S. pyogenes
0
17
14
100
140
Ss-AgNPs
A. baumannii
0
18
14
80
120
Ss-AgNPs
E. coli
0
18
13
75
130
Ss-AgNPs
S. pyogenes
0
17
12
60
90
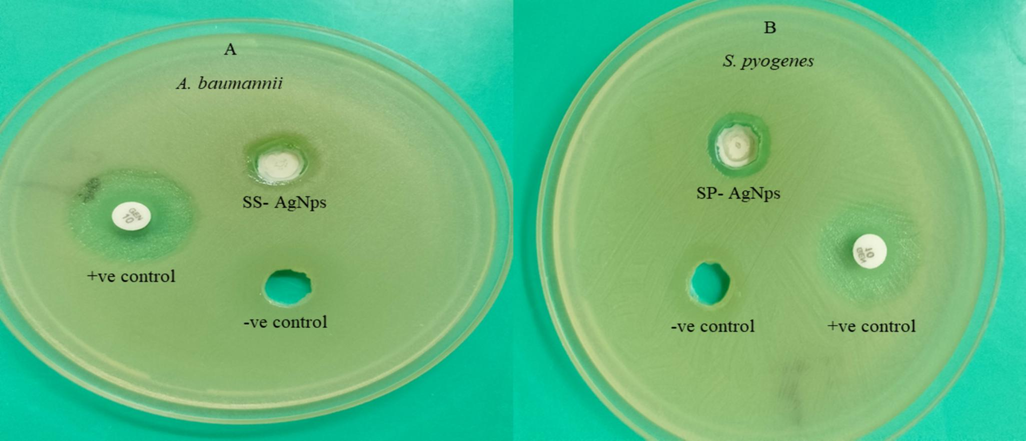
Observations of antibacterial activity, (A) Ss-AgNPs against A. baumannii, (B) Ssp-AgNPs against S. pyogenes.
4 Discussion
Cyanobacteria as microalgae, are highly sought after as naturally occurring sources of bioactive compounds with antibacterial, antiviral, anticancer, antioxidant, and anti-inflammatory qualities. They also yield several interesting compounds with a wide spectrum of biological functions (Hassan et al., 2022). Also, plant extracts, Aspalathus linearis with green-synthesized palladium and palladium oxide nanoparticles showed two distinct peaks: a broad peak including flavonoids that was centred around 348.5 nm, and a sharp peak containing the aspalathin component that was centred around 284.5 nm (Ismail et al., 2017). Pant-mediated green production of NPs of nickel oxide, palladium, and palladium oxide was particularly promising, because Aspalathus linearis natural extracts of NPs function as both a capping and reducing agent (Mayedwa et al., 2018b).
Along with the first production and viability of consistently replicating stoichiometric pure textured VO2 nano-structures, a potential vacuum sputtering technique utilizing an inverted cylindrical magnetron to produce nanostructured VO2 thin films was disclosed (Kana Kana et al., 2008). Generally nano system consists of a single dimension that is 100 nm or less, anisotropic, isotropic, and single-dimensional nanoparticles can be produced, in addition to two-dimensional systems like sheets or thin films and multilayered systems like nanocomposites (Henini et al., 2022).
The nanomaterial had the largest ZOI against Yersinia pestis, 45 mm, when compared to an organic solvent extract (Na-citrate) against Staphylococcus aureus (ZOI, 20 mm) (Awadalla et al., 2021). Instead of using prescription antimicrobials, S. platensis is an affordable antibacterial agent that can be used to combat harmful bacteria. It has been demonstrated that phycobiliproteins isolated from these species have several beneficial health effects, including boosting immune response. These pigments are widely used in the food, pharmaceutical, and cosmetics industries as natural alternatives to artificial colorants. Spirulina sp. is filamentous, multicellular, photoautotrophic biomass cyanobacteria resistant to the extreme environments seen in wetlands. It is rich in essential proteins, minerals, vitamins, and fatty acids used in many industries.
The cyanobacteria that produce oxygen share cytological similarities with Gram-negative bacteria, which are recognized for possessing phycobiliproteins in red and blue colors that are soluble in both water and chlorophyll a. Because of their chromatic adaptation mechanisms, cyanobacteria can survive in harsh environments with high salinity, low water content, heat, cold, nitrogen deficiency, photo-oxidation, osmotic stress, and ultraviolet light (Yarkent et al., 2020). Several compounds have undergone preclinical and/or clinical testing as possible anticancer medications, including the anti-microtubule medicines dolastatin 10 and curacin A (Barreca et al., 2020).
5 Conclusion
This research describes the characterization of products such as UV–vis spectroscopy results that confirmed the formation of AgNPs, and the FTIR analysis that identified the biomolecules responsible for the stability and reduction of the nanoparticles. XRD analyses were utilized to ascertain the dimensions, shape, and crystalline nature of the generated AgNPs. The significant antibacterial efficacy of AgNPs against bacteria was suggested by the potential application demonstrated by their ZOI, and MICs/MBC indicating the potential utility of medications of innovative antimicrobials. Future research efforts must focus on the in vitro assessment of these biosynthesized AgNPs and the development of practical applications were successfully synthesized by biological methods and combined with S. subsalsa and Spirulina sp. extracts. This study has shown the antibacterial activities of synthesized cyanobacterial extracts with AgNO3 against bacteria. A synergistic impact against E. coli, A. baumannii, and S. pyogenes was seen when conjugating AgNPs claimed that all conjugates with effects are responsible for increasing the efficacy of various extracts and conventional antibacterial drugs.
Funding information
MSK acknowledges the generous support from the Research supporting project (RSP2024R352) by King Saud University, Riyadh, Kingdoms of Saudi Arabia. The extramural fund was unavailable; S. Bej, as the Ph.D. research fellow (Regd. no. 218https://doi.org/1002054/2021) and the work was supported by SOA University, Bhubaneswar.
CRediT authorship contribution statement
Shuvasree Bej: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Data curation, Conceptualization. Surendra Swain: Writing – review & editing, Visualization, Formal analysis. Ajit Kumar Bishoyi: Writing – review & editing, Visualization, Conceptualization. Chita Ranjan Sahoo: Writing – review & editing, Visualization, Conceptualization. Bigyan Ranjan Jali: Writing – review & editing, Methodology. Mohd Shahnawaz Khan: Visualization, Funding acquisition. Rabindra Nath Padhy: Writing – review & editing, Visualization, Supervision, Funding acquisition.
Acknowledgments
We are grateful to Prof. Dr. S. Mishra, Dean, IMS & Sum Hospital, Bhubaneswar, Shiksha O Anusandhan University, Odisha, for the facilities. S. Bej is grateful to Prof. J. K. Nath, Dean (R & D) of SOA University for the SOA-PhD fellowship, support, and work as part of the Ph.D. thesis. No. 2181002054/2021,We are grateful to MSK(RSP2024R352) for Research supporting.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A review on biogenic synthesis of metal nanoparticles using marine algae and its applications. Environ. Res.. 2021;194–110672
- [CrossRef] [Google Scholar]
- Antimicrobial activities of Spirulina platensis extracts and nanoparticles material against some pathogenic bacterial isolates. Egypt J. Appl. Sci.. 2021;36:5-6.
- [CrossRef] [Google Scholar]
- An overview on anti-tubulin agents for the treatment of lymphoma patients. Pharmaco & Therape.. 2020;211:107552
- [CrossRef] [Google Scholar]
- Nanobioremediation: A sustainable approach for the removal of toxic pollutants from the environment. J. Hazard. Mater.. 2022;427:128033
- [CrossRef] [Google Scholar]
- Synthesis of silver nanoparticles using marine macroalgae Padina sp. and its antibacterial activity towards pathogenic bacteria. Beni-Suef Univer J Basic Appl Sci 2020:1-5.
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles with the brackish water blue-green alga Oscillatoria princeps and antibacterial assessment. Appl Nanosci.. 2021;389–98
- [CrossRef] [Google Scholar]
- Optical properties of Ag–TiO 2 nanocermet films prepared by co-sputtering and multilayer deposition techniques. Applied Optic.. 2000;16:2745-2753.
- [CrossRef] [Google Scholar]
- Enhanced antimicrobial activity of silver nanoparticles synthesized by Cryphonectria sp. evaluated singly and in combination with antibiotics. Nanomed: Nanotechnol. Bio Medi. 2013;1:105-110.
- [Google Scholar]
- Structural, optical and photocatalytic applications of biosynthesized NiO nanocrystals. Green Chem Lett Revis.. 2018;2:166-175.
- [Google Scholar]
- Antibacterial activity of Lantana camara L. against multidrug resistant pathogens from ICU patients of a teaching hospital. J Herb Med.. 2013;2:65-75.
- [CrossRef] [Google Scholar]
- Nannochloropsis extract–mediated synthesis of biogenic silver nanoparticles, characterization and in vitro assessment of antimicrobial, antioxidant and cytotoxic activities. Asia Pacif. J. Cancer Preven.. 2019;8:2353.
- [Google Scholar]
- Identification and characterization of the novel bioactive compounds from microalgae and cyanobacteria for pharmaceutical and nutraceutical applications. J. Basic Microbiol.. 2022;9:999-1029.
- [Google Scholar]
- Green palladium and palladium oxide nanoparticles synthesized via Aspalathus linearis natural extract. J Alloys Compound.. 2017;695:3632-3638.
- [CrossRef] [Google Scholar]
- Antimicrobial, antioxidant, and antiviral activities of biosynthesized silver nanoparticles by phycobiliprotein crude extract of the cyanobacteria Spirulina platensis and Nostoc linckia. Bionanosci.. 2021;11:355-370.
- [CrossRef] [Google Scholar]
- One-step synthesis of ultrasmall nanoparticles in glycerol as a promising green solvent at room temperature using omega-shaped microfluidic micromixers. Anal. Chem.. 2023;4:1-20.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using Melia azedarach leaf extract and their antifungal activities: In vitro and in vivo. Mater Chem Phy.. 2020;248:122898
- [CrossRef] [Google Scholar]
- Kannaujiya, VK., et al., 2020. Phycobiliproteins in microalgae: Occurrence, distribution, and biosynthesis. Pigment from microalgae handbook. p43-68. Doi: 10.1007/978-3-030-50971-2_3.
- Potential application of microalga Spirulina platensis as a protein source. J. Sci. Food Agri.. 2017;3:724-732.
- [CrossRef] [Google Scholar]
- Studies on the spectrometric analysis of metallic silver nanoparticles (AgNPs) using Basella alba leaf for the antibacterial activities. Environ. Res.. 2021;199:111274
- [CrossRef] [Google Scholar]
- ZnO nanoparticles via Moringa oleifera green synthesis: physical properties & mechanism of formation. Appl. Surf. Sci.. 2017;406:339-347.
- [CrossRef] [Google Scholar]
- Spirulina, food of past, present and future. HealthBiotechnol Biopharm.. 2020;3:1-20.
- [Google Scholar]
- Green synthesis of zin tin oxide (ZnSnO3) nanoparticles using Aspalathus linearis natural extracts: Structural, morphological, optical and electrochemistry study. Appl. Surf. Sci.. 2018;446:250-257.
- [CrossRef] [Google Scholar]
- Green synthesis of nickel oxide, palladium and palladium oxide synthesized via Aspalathus linearis natural extracts: physical properties and mechanism of formation. Appl. Surf. Sci.. 2018;446:266-272.
- [CrossRef] [Google Scholar]
- Analytical methods for the identification and characterization of silver nanoparticles: a brief review. Mater Today: Proceed.. 2021;47:5520-5532.
- [CrossRef] [Google Scholar]
- Nanodelivery of antioxidant herbal extracts, spices, and dietary constituents. Phytoantiox Nanotherap.. 2022;12:145-171.
- [CrossRef] [Google Scholar]
- Antibacterial efficacy of five medicinal plants against multidrug-resistant enteropathogenic bacteria infecting under-5 hospitalized children. J. Integr. Med.. 2015;1:45-57.
- [CrossRef] [Google Scholar]
- Biogenic silver nanoparticle synthesis with cyanobacterium Chroococcus minutus isolated from Baliharachandi sea-mouth, Odisha, and in vitro antibacterial activity. Saud. J. Bio. Sci.. 2020;6:1580-1586.
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles using Dunaliella salina and its antibacterial applications. Appl. Surf. Sci. Adv.. 2023;100377:1-13.
- [CrossRef] [Google Scholar]
- Analysis of the growth profile, biochemical composition and nutrient removal efficacy of Spirulina sp. NCIM 5143. Environ.Conser J.. 2023;24:269-286.
- [Google Scholar]
- Swain, S., et al., 2024. Biosynthesis and characterizations of silver nanoparticles with filamentous cyanobacterium Lyngbya sp. with in vitro antibacterial properties against MDR pathogenic bacteria. Naunyn-Schmiedeberg's Archiv Pharmacol. Doi: 10.1007/s00210-024-03235-z.
- Antioxidant and photocatalytic activity of aqueous leaf extract mediated green synthesis of silver nanoparticles using Passiflora edulis f. flavicarpa. J. Nanosci. Nanotechnol.. 2019;5:2640-2648.
- [CrossRef] [Google Scholar]
- Bioreduction potentials of dried root of Zingiber officinale for a simple green synthesis of silver nanoparticles: antibacterial studies. J. Photo. Photo. Biol.. 2017;177:62-68.
- [CrossRef] [Google Scholar]
- Potential of microalgal compounds in trending natural cosmetics: a review. Sustain. Chem. Pharmac.. 2020;17:100304
- [CrossRef] [Google Scholar]
- Effects of anionic and cationic surfactants on the surface Plasmon resonance intensity of biogenic silver nanoparticles: stability, and position of optical band. J. Mol. Liq.. 2023;385:122363
- [CrossRef] [Google Scholar]
- Nanoparticles and its biomedical applications in health and diseases: special focus on drug delivery. Environ. Sci. Pollut. Res.. 2020;68:19151
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103336.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







