Translate this page into:
Biolubricant basestocks from chemically modified ricinoleic acid
*Corresponding author. Tel.: +60 3 8921 5412; fax: +60 3 8921 5410 jumat@ukm.my (Jumat Salimon)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 20 August 2010
Abstract
This paper presents a series of chemically modified biolubricant basestocks derived from ricinoleic acid. The reactions were monitored and products were confirmed by NMR and FTIR. The synthesis protocol is carried out in three stages: (1) epoxidation of ricinoleic acid; (2) synthesis of 10,12-dihydroxy-9-acyloxystearic acid from epoxidized ricinoleic acid; (3) esterification of the acyloxystearic acid products with 2-ethylhexanol to yield 2-ethylhexyl-10,12-dihydroxy-9-acyloxystearate. The viscosity index, flash point, pour points (PP), and oxidative stability of the resulting products were measured. The resulting esters could plausibly be used as bio-based industrial materials in biolubricants, surfactants, or fuel because they have improved physicochemical properties.
Keywords
Viscosity index
Pour point
Chemical modification
Biolubricant basestock
Plant oil
1 Introduction
The use of renewable raw materials can significantly contribute to a sustainable development (Metzger, 2009), usually interpreted as ‘‘acting responsibly to meet the needs of the present without compromising the ability of future generations to meet their own needs’’ (Report of the United Nations Conference, 1992). In ages of depleting fossil oil reserves and an increasing emission of green house gases it is obvious that the utilization of renewable raw materials wherever and whenever possible is one necessary step towards a sustainable development. In particular, this can perennially provide a raw material basis for daily life products and avoid further contribution to green house effects due to CO2 emission minimization. Furthermore, the utilization of renewable raw materials, taking advantage of the synthetic potential of nature, can (in some cases) meet other principles of green chemistry, such as a built-in design for degradation or an expected lower toxicity of the resulting products (Eissen et al., 2002).
Some of the most widely applied renewable raw materials in the chemical industry for non-fuel applications are plant oils. Today plant oils are the most important renewable raw material for the chemical industry (e.g., in Germany 30% of the 2.7 million tons of renewable raw materials in 2005 were plant oils; in total approximately 10% of all resources were renewable) and are heavily used as raw materials for surfactants, cosmetic products, and lubricants (Carlsson, 2009). In addition, plant oils have been used for decades in paint formulations, as flooring materials and for coating and resin applications (Salimon et al., 2010).
Plant oils are triacylglycerides (tri-esters of glycerol with long chain fatty acids, see Fig. 1) (TAG) with varying composition of fatty acids depending on the plant, the crop, the season, and the growing conditions (Gunstone, 2004). The word ‘oil’ hereby refers to triglycerides that are liquid at room temperature. The most important parameters affecting the physical and chemical properties of such oils are the stereochemistry of the double bonds of the fatty acid chains, their degree of unsaturation as well as the length of the carbon chain of the fatty acids. The degree of unsaturation, which can be expressed by the iodine value (amount of iodine in gram that can react with double bonds present in 100 g of sample under specified conditions). In terms of fatty acid composition, linseed oil, for instance, mainly consists of linolenic (all-cis-9,12,15-octadecatrienoic acid) and linoleic acid (all-cis-9,12-octadecadienoic acid), whereas in castor oil, the most abundant fatty acid is ricinoleic acid ((9Z, 12R)-12-hydroxy-9-octadecenoic acid), providing additional natural chemical functionality for modifications, cross-linking, or polymerization. Fig. 1 summarizes the chemical composition of some industrially important plant oils. From Fig. 1, it can for instance be seen that new rapeseed oil is rich in oleic acid (R = 18:1), whereas palm kernel oil is rich in lauric acid (R = 12:0).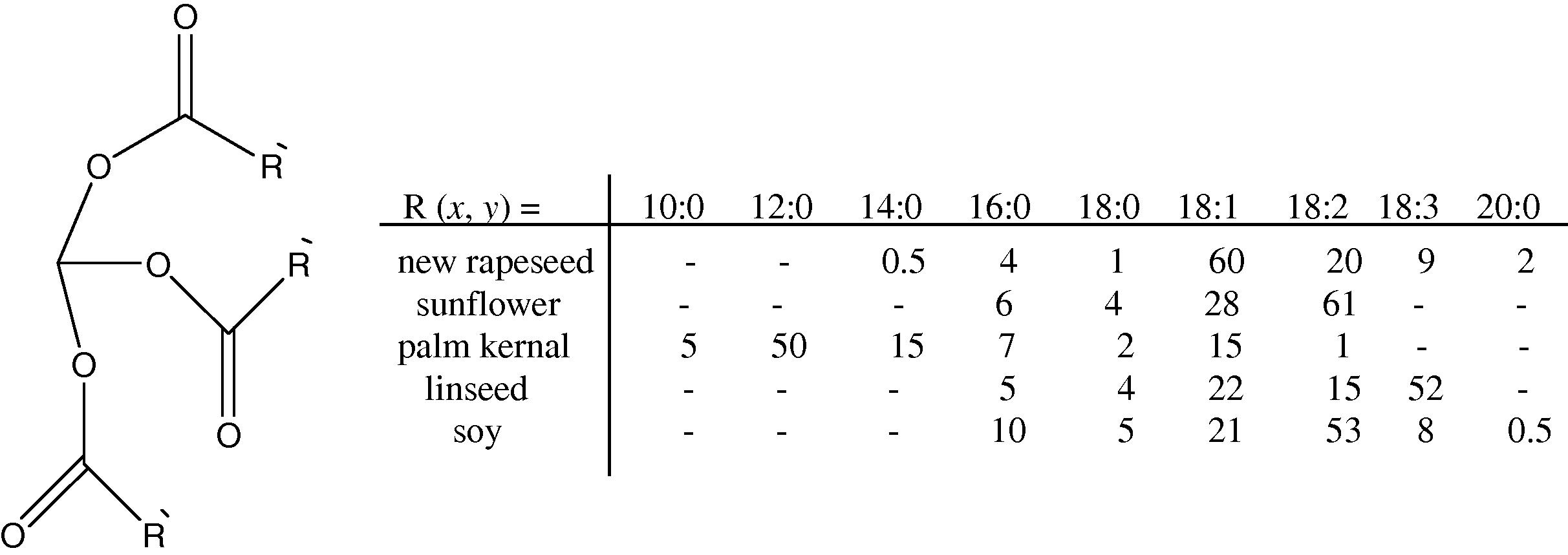
(Left) Chemical structure of triglyceride. (Right) Typical compositions of industrially important plant oils in % (R(x:y) = compositions of the fatty acids; x = chain length in carbon atoms; y = number of double bonds). (Important notes: numbers do not add to 100%; R ≠ R′.)
For a more complete overview and for reasons of easier reading and understanding, Fig. 2 displays an overview of interesting fatty acids for chemical modifications. Approximately 80% of the global oil and fat production is plant oil, whereas 20% is of animal origin (share decreasing) (Metzger and Bornscheuer, 2006). About 25% is soybean, followed by palm oil, rapeseed, and sunflower oil. Coconut and palm kernel oil (laurics) contain a high percentage of saturated C12 and C14 fatty acids (compare Fig. 1) and are most important for the production of surfactants. These commodity oils make highly pure fatty acids available that may be used for chemical conversions and for the synthesis of chemically pure compounds such as oleic acid (1) from ‘‘new sunflower,’’ linoleic acid; (2) from soybean, linolenic acid; (3) from linseed, erucic acid; (4) from rapeseed, and ricinoleic acid; (5) from castor oil (see Fig. 2) (Metzger and Bornscheuer, 2006).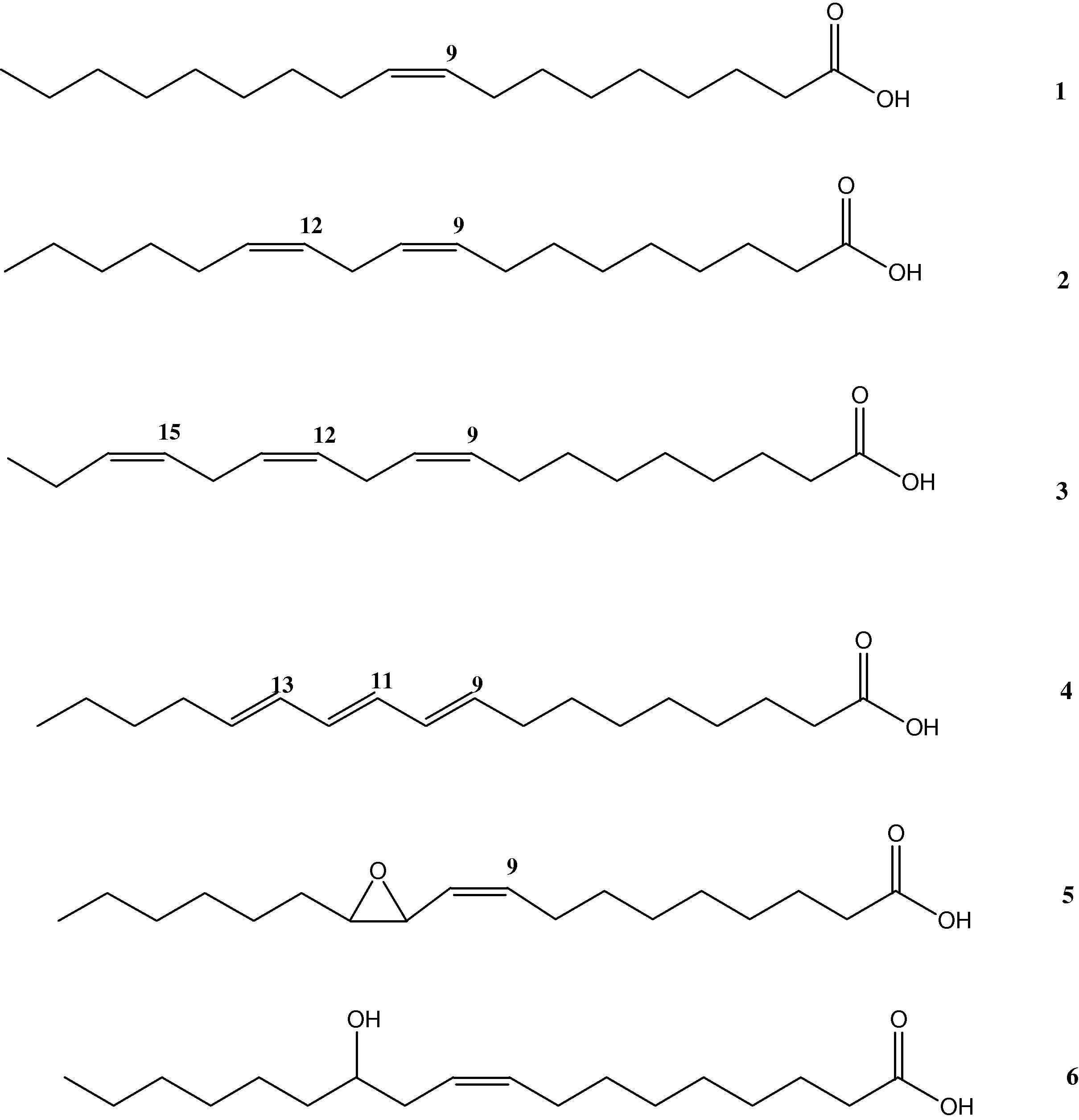
Fatty acids as starting materials for the synthesis of novel fatty compounds: (1) oleic acid, (2) linoleic acid, (3) linolenic acid, (4) α-eleostearic acid, (5) vernolic acid, (6) ricinoleic acid.
The goal of this work is to develop a chemical modification method for the preparation of biolubricant basestocks from ricinoleic acid. The synthesis, characterization, and utilization of head group manipulation together with branching strategy to improve the physicochemical properties of these basestocks are discussed within this contribution.
2 Materials and methods
2.1 Materials
Formic acid (88%) was obtained from Fisher Scientific (Pittsburgh, PA) and ricinoleic acid (92%) from Nu-Chek Prep, Inc. (Elysian, MN). All other chemicals and reagents were obtained from Aldrich Chemical (Milwaukee, WI). All materials were used without further purification. All organic extracts were dried using anhydrous magnesium sulfate (Aldrich Chemical).
2.2 Methods
2.2.1 Characterization
1H and 13C NMR spectra were recorded using a JEOL JNM-ECP 400 spectrometer operating at a frequency of 400.13 and 100.77 MHz, respectively, using a 5-mm broadband inverse Z-gradient probe in DMSO-d6 (Cambridge Isotope Laboratories, Andover, MA) as solvent. Each spectrum was Fourier-transformed, phase-corrected, and integrated using MestRe-C 2.3a (Magnetic Resonance Companion, Santiago de Compostela, Spain) software. FTIR spectra were recorded neat on a Thermo Nicolet Nexus 470 FTIR system (Madison, WI) with a Smart ARK accessory containing a 45 ZeSe trough in a scanning range of 650–4000 cm−1 for 32 scans at a spectral resolution of 4 cm−1.
2.2.2 Low temperature operability
The pour point is defined as the lowest temperature at which the sample still pours from a tilted jar. This method is routinely used to determine the low temperature flow properties of fluids. Pour point values were measured according to the ASTM D5949 method (ASTM Standard D5949) using a phase Technology Analyzer, Model PSA-70 S (Hammersmith Gate, Richmond, BC, Canada). Each sample was run in triplicate and average values rounded to the nearest whole degree are reported. For a greater degree of accuracy, PP measurements were done with a resolution of 1 °C instead of the specified 3 °C increment. Generally, materials with lower PP exhibit improved fluidity at low temperatures than those with higher PP.
2.2.3 Flash point values
The flash point is defined as the minimum temperature at which the liquid produces a sufficient concentration of vapor above it that it forms an ignitable mixture with air. The lower the flash point is, the greater the fire hazard. Flash point determination was run according to the American National Standard Method using a Tag Closed Tester (ASTM D 56-79) (ASTM Standard D 56-79). Each sample was run in triplicate and the average values rounded to the nearest whole degree are reported.
2.2.4 Viscosity index measurements
Automated multi-range viscometer tubes HV M472 obtained from Walter Herzog (Germany) were used to measure viscosity. Measurements were run in a Temp-Trol (Precision Scientific, Chicago, IL, USA) viscometer bath set at 40.0 and 100.0 °C. The viscosity and viscosity index were calculated using ASTM methods D 445-97 (ASTM Standard D 445-97) and ASTM D 2270-93 (ASTM Standard D 2270-93), respectively. Triplicate measurements were made and the average values were reported.
2.2.5 Oxidative stability
Pressurized DSC (PDSC) experiments were accomplished using a DSC 2910 thermal analyzer from TA Instruments (Newcastle, DE). Typically, a 2-μL sample, resulting in a film thickness of <1 mm, was placed in an aluminum pan hermetically sealed with a pinhole lid and oxidized in the presence of dry air (Gateway Airgas, St. Louis, MO), which was pressurized in the module at a constant pressure of 1378.95 kPa (200 psi). A 10 °C min−1 heating rate from 50 to 350 °C was used during each experiment. The oxidation onset (OT, °C) and signal maximum temperatures (SMT, °C) were calculated from a plot of heat flow (W/g) versus temperature for each experiment. Each sample was run in triplicate and average values rounded to the nearest whole degree are reported (Table 1). ERA, epoxidized ricinoleic acid; DHOSA, 10,12-dihydroxy-9-octyloxystearic acid; DHNSA, 10,12-dihydroxy-9-nonanoxystearic acid; DHLSA, 10,12-dihydroxy-9-lauroxystearic acid; DHMSA, 10,12-dihydroxy-9-myristoxystearic acid; DHPSA, 10,12-dihydroxy-9-palmitoxystearic acid; DHSSA, 10,12-dihydroxy-9-stearoxystearic acid; DHBSA, 10,12-dihydroxy-9-behenoxystearic acid; EHDHOS, ethylhexyl 10,12-dihydroxy-9-octyloxystearate; EHDHNS, ethylhexyl 10,12-dihydroxy-9-nonanoxystearate; EHDHLS, ethylhexyl 10,12-dihydroxy-9-lauroxystearate; EHDHMS, ethylhexyl 10,12-dihydroxy-9-myristoxystearate; EHDHPS, ethylhexyl 10,12-dihydroxy-9-palmitoxystearate; EHDHSS, ethylhexyl 10,12-dihydroxy-9-stearoxystearate; EHDHBS, ethylhexyl 10,12-dihydroxy-9-behenoxystearate.
Samples
Pour pointa (°C)
Flash pointa (°C)
Viscosity Indexa
OTa (°C)
SMTa (°C)
Yield (%)
ERA
9
80
67
60
228
80
DHOSA
−10
145
110
181
200
64
DHNSA
−13
189
117
170
210
75
DHLSA
−15
163
125
165
213
85
DHMSA
−17
202
146
125
245
67
DHPSA
−18
192
155
94
221
92
DHSSA
−20
264
163
87
278
90
DHBSA
−23
295
171
79
263
78
EHDHOS
−25
178
176
220
252
62
EHDHNS
−27
145
183
209
255
70
EHDHLS
−31
290
188
201
236
75
EHDHMS
−34
163
193
192
211
69
EHDHPS
−37
195
197
184
215
82
EHDHSS
−39
132
202
171
242
71
EHDHBS
−42
217
205
167
240
73
2.3 Synthesis
2.3.1 Epoxidized ricinoleic acid (ERA)
Hydrogen peroxide solution (30% in H2O, 8.0 mL) was added slowly into a stirred solution of ricinoleic acid (RA) (90%, 15 g) in formic acid (88%, 14 mL) at 4 °C (ice bath). The reaction proceeded at room temperature with vigorous stirring (900 rpm) until the formation of a powdery solid was noticed in the reaction vessel (2–5) h. The solid was collected via vacuum filtration, washed with H2O (chilled, 3× 10 mL), and placed for 12 h under high vacuum to provide epoxidized ricinoleic acid (ERA) as a white, powdery solid (14.7 g, 93%).
2.3.2 10,12-Dihydroxy-9-acyloxystearic acid (DHASA)
To a mixture of ERA (31 g), 5 g of p-toluenesulfonic acid (PTSA) and toluene, fatty acids (6 g) was added during 1.5 h in order to keep the reaction mixture temperature under 70–80 °C. The reaction mixture was subsequently heated to 90–100 °C and refluxed for 3 h. After reaction termination, the heating was stopped and the mixture was left to stand overnight at ambient room temperature. The mixture was washed with the water next day. The organic layer was dried over anhydrous magnesium sulfate and the solvent was removed using the vacuum evaporator.
2.3.3 2-Ethylhexyl 10,12-dihydroxy-9-acyloxystearate (EHDHAS)
The reaction scheme of diesters formation is shown in Fig. 3. Sulfuric acid (conc. H2SO4, 10 mol%) was added into a stirred suspension of DHASA (3.35 mmol) in the 2-ethylhexanol (3.35 mL). The suspension was heated with stirring at 60 °C for 10 h. Hexanes (5 mL) was then added, and the solution was washed with NaHCO3 (sat. aq., 1× 0.5 mL) and brine (2× 1 mL), dried (MgSO4), filtered, concentrated in vacuo and placed for 6 h under vacuum to yield the title products.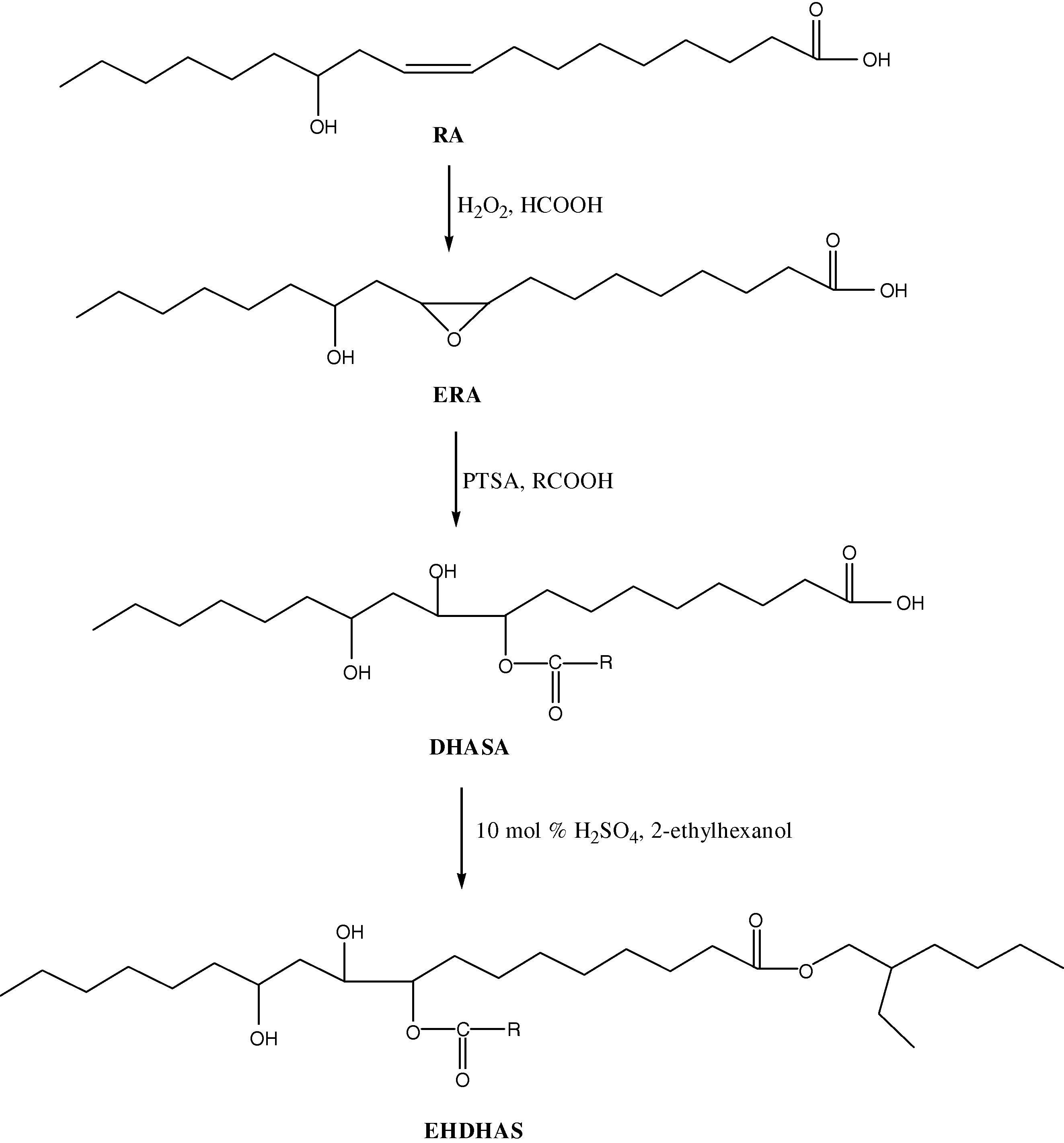
Schematic reactions of epoxidation, ring opening and esterification od ricinoleic acid derivatives (RA, ricinoleic acid; EOA, epoxidized ricinoleic acid; PTSA, p-toluenesulfonic acid; RCOOH, octanoic, nonanoic, lauric, myristic, palmitic, stearic and behenic acids; H2SO4, sulfuric acid).
3 Results and discussion
3.1 Chemical modification
The synthetic process of biolubricant basestocks consists of three steps: the first, ricinoleic acid was transferred to epoxidized ricinoleic acid with hydrogen peroxide and formic acid; then, epoxidized ricinoleic acid was transferred to mono ester derivatives (DHASA) through its reaction with different fatty acids in the presence PTSA as catalyst. In third step, DHASA compounds reacted with 2-ethylhexanol in the presence of sulfuric acid as catalyst to give diester products (EHDHAS).
Many nucleophilic reagents are known to add to an oxirane ring, resulting in ring opening. These ring-opening reactions could result in branching at the oxirane ring opening (earlier sites of unsaturation in RA). The appropriate branching groups would interfere with the formation of macro-crystalline structures during low-temperature applications and would provide enhanced fluidity to plant oils. TAGs that are hydrogenated to eliminate polyunsaturation will solidify at room temperature as a result of alignment and stacking of adjacent molecules. For this reason, it is important that there should be at least one unsaturation site available for a functionalization that will generate two branching points on the chain. The ester branching groups are quite effective for attaining the desired molecular spacing. A six-carbon chain-length ester has been observed to deliver the most desired pour point properties for some oils. These modified plant oils with chain branching are reported to have superior performance properties and are promising as biodegradable lubricant (Hwang and Erhan, 2001).
The nucleophilic attack by fatty acid molecules on the oxirane ring of ERA in the presence of PTSA resulted in the ring-opened products (DHASA), as shown in Fig. 3. The extent of the reaction was monitored by FTIR spectroscopy of small aliquots taken at 1-h intervals. As the ring-opening reaction progressed, the oxirane C–O twin bands at 830 and 846 cm−1 decreased and disappeared, while a new band start gradually to appear at 1730 cm−1. This band is assigned to the C⚌O stretching vibrations of esters (Salimon and Salih, 2010). The reaction completion was further confirmed by 1H NMR spectra of the DHASA derivatives, where the peaks at 2.42–2.84 ppm (–CH– protons of the epoxy ring) completely disappeared and additional peaks appeared in the range 3.23–3.54 ppm (protons attached to carbon of –CHOH group), 3.72-4.10 ppm (protons attached to carbon of –CHOCOR group) and 9.12–9.34 ppm (protons attached to oxygen of the O–H groups). In addition, the disappearance of epoxy carbon peaks in the range 55.11–58.23 ppm and appearance of –CHOH and –CHOCOR peaks in the range 72.51–80.69 ppm in 13C NMR spectra of DHASA confirmed the oxirane ring opening (Salimon and Salih, 2009).
In the next step, the mono ester derivatives are reacted with 2-ethylhexanol in the presence of sulfuric acid to yield diester compounds (EHDHAS). In this step, the reaction was monitored using FTIR for the disappearance of the fatty acid C⚌O stretching band in the region 1710–1715 cm−1. The epoxy absorption bands (830 and 846 cm−1), which was present in ERA spectrum, no longer exists in final diester products. The completion of reaction also has been confirmed by their respective 1H NMR spectra. Three peaks which appeared in monoesters spectra at 9.12–9.34 ppm (protons attach to the oxygen of O–H group) have disappeared completely and two peaks at 9.46–9.54 ppm (protons attach to the oxygen of O–H group) appeared in all diester products spectra. The presence of 13C NMR peaks at 173.32–176.14 ppm due to the carbonyl carbons C⚌O and 20.16–22.47 ppm for –CH2– backbone are in agreement with the diester proposed structures.
3.2 FTIR characterization of diester products
The FTIR spectra of all these products have some common peaks at about 3430–3400 (O–H stretching vibration), 2930 and 2850 (methylene asymmetric stretching), 1732 (ester carbonyl stretching), 1460 (CH2 bending vibration), 1372 (CH3 symmetric bending vibration), and 720 cm−1 (CH2 rocking vibrations), and additional peaks at 1240, 1163, and 1110 cm−1 due to stretching vibrations of the C–O group in esters (Sliverstien et al., 2005).
3.3 NMR characterization of diester products
Peaks at 3.30–3.44 and 3.55–4.18 ppm due to protons attached to carbon of –CHOH and –CHOCOR groups, respectively, are present in EHDHAS spectra. The presence of additional peaks at 2.85–3.18 ppm due to methyl protons of the –CH3– group in 1H NMR and corresponding methyl carbons at 15.23–17.90 ppm and carbonyl carbons at 173.32–176.14 ppm in 13C NMR confirm the formation of the diester products.
3.4 Physicochemical properties of synthesized compounds
Physicochemical properties of prepared compounds are summarized in Table 1. The cold flow property of plant oils is extremely poor and this limits their use at low operating temperature especially as automotive and industrial fluids. Plant oils have a tendency to form macro-crystalline structures at low temperature through uniform stacking of the ‘bend’ triglyceride backbone. Such macro-crystals restrict the easy flow of the system due to loss of kinetic energy of individual molecules during self-stacking. The ERA and synthetic esters (DHASA and EHDHAS) described above were screened for low-temperature behavior through determination of their pour point (PP).
An improvement in the cold flow behavior of diesters EHDHAS was obtained over that of their precursors DHASA. Actually there are two reasons for this behavior. The first reason is that as the chain length of the mid-chain ester is increased, a corresponding improvement in the pour point of biolubricant oil was observed. This is due to the greater ability of the longer chain esters to disrupt crystalline formation at reduced temperatures (Salimon et al., 2010). Second, the lack of one hydroxyl group means the number of hydrogen bonds decrease, which could cause the molecules to stack together
Another important fact in determining how well oil will behave as a potential biolubricant is to evaluate the oils flash point. Flash point values are a function of the apparatus design, the condition of the apparatus used, and the operational procedure carried out. The flash point can be used to determine the transportation and storage temperature requirements for biolubricants. Biolubricant producers can also use the flash point to detect potential product contamination. A biolubricants exhibiting low flash point will be suspected of contamination with a volatile products and usually require special precautions for safe handling.
From the viscosity index data of compounds ERA, DHASA, and EHDHAS (Table 1) we can see that viscosity index increases with chain length (number of carbons). A high viscosity index indicates small viscosity changes with temperature. Therefore, biolubricant oil that has a high viscosity index can be expected to undergo very little change in viscosity with temperature extremes and is considered to have a stable viscosity-temperature relation (Hwang et al., 2003).
The ability of a substance to resist oxidative degradation is another important attribute for a number of industrial materials, such as lubricants, surfactants, and fuels. Therefore, all synthesized compounds were screened for oxidation stability using PDSC through determination of OT and SMT, Table 1. PDSC is an effective method for measuring oxidation stability of oleochemicals in an accelerated mode. The OT is the temperature at which a rapid increase in the rate of oxidation is observed at a constant, high pressure (200 psi). A high OT would suggest high oxidation stability of the material. The SMT is the temperature at which maximum heat output is noted from the sample during oxidative degradation. A higher SMT does not necessarily correlate with improved oxidation stability. Both OT and SMT were calculated from a plot of heat flow (W/g) versus temperature that was generated by the sample upon degradation and, by definition, SMT > OT. In the present study, as the chain length of the mid-chain ester is decreased, a corresponding improvement in oxidation stability was observed, which is because longer chains are more susceptible to oxidative cleavage than shorter chains. These results are in agreement with other studies on synthetic esters (Randals 1999).
4 Conclusions
The process describes a systematic approach to modify chemically ricinoleic acid to yield biolubricant basestocks. Based on the results obtained, increase the chain length of the mid-chain ester had a positive influence on the low temperature properties of diesters because they create a steric barrier around the individual molecules and inhibits crystallization, resulting in lower pour point. But the trends for PP run counter to that of OT, i.e., increasing chain length is a benefit to PP, but a detriment to OT. From these results, a future study will aim to strike a balance between two opposing properties through investigation of a greater variety of mid-chain ester groups.
Acknowledgements
The authors acknowledge the Universiti Kebangsaan Malaysia for funding (“Code UKM-GUP-NBT-08-27-113” and “UKM-OUP-NBT-29-150/2010”), and the direct contributions of the support staff from the School of Chemical Sciences and Food Technology, the Faculty of Science and Technology, Universiti Kebangsaan, Malaysia.
References
- ASTM Standard D5949. Standard Test Method for Pour Point of Petroleum (Automatic Pressure Pulsing Method). ASTM, West Conshohocken, PA, USA.
- ASTM Standard D 56-79. Standard Test Method for Flash Point of Liquids with a Viscosity less than, 45 Saybolt Universal Seconds (SUS) at 37.8 °C (that don’t contain suspended solids and don’t tend to form a surface film under test).
- ASTM D 445-97. Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids. ASTM, West Conshohocken, PA, USA.
- ASTM D 2270-93. Standard Practice for Calculating Viscosity Index from Kinematic Viscosity at 40 and 100 °C. ASTM, West Conshohocken, PA, USA.
- Biochimie. 2009;91:665-670.
- Angew. Chem. Int. Ed.. 2002;41:414-436.
- The Lipid Handbook. United Kingdom: Taylor & Francis Ltd.; 2004.
- J. Am. Oil Chem. Soc.. 2001;78:1179-1184.
- J. Am. Oil Chem. Soc.. 2003;80:811-815.
- Appl. Microbiol. Biotechnol.. 2006;71:13-22.
- Eur. J. Lipid Sci. Technol.. 2009;111:865-876.
- Esters, in synthetic lubricants and high-performance functional fluids. In: Rudnick L.R., Shubkin R.L., eds. Synthetic Lubricants and High-performance Functional Fluids. New York: Marcel Dekker; 1999. p. :63-102.
- [Google Scholar]
- Report of the United Nations Conference on Environment and Development, Rio de Janeiro, 1992. Available from: <http://www.un.org/esa/sustdev/>.
- Eur. J. Sci. Res.. 2009;32:216-222.
- Eur. J. Lipid Sci. Technol.. 2010;112:519-530.
- Asian J. Chem.. 2010;22:5468-5476.
- Spectrometric Identification of Organic Compounds (seventh ed.). New York: John-Wiley; 2005.







