Translate this page into:
Biological and in silico investigation of isolated novel bioactive compound from Conocarpus lancifolius
⁎Corresponding author. maliksaadullah@gcuf.edu.pk (Malik Saadullah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
The plant Conocarpus lancifolius from Combertaceae family, has been documented to have strong medicinal potential. The objective of this research was to determine antioxidant, anti-inflammatory, and cytotoxic potential of C. lancifolius by in vitro, in vivo as well as computational studies. A forecast was made that a novel compound “lancifolian” can significantly improve inflammation, radical scavenging ability as well as cytotoxicity. For antioxidant study ABTS, DPPH, FRAP and β-carotene bleaching assays were performed. Anti-inflammatory activity was determined by carrageenan induced paw edema. Cytotoxic effects against four cancer cell lines: MCF-7, A549, COR-L23 and MDA-MB-231, were observed. Molecular docking was also performed to predict anti-inflammatory and cytotoxic properties by observing the binding affinity of lancifolian with respective receptors and validation of molecular docking was performed. Dichloromethane (DCM) and methanolic extract of C. lancifolius show strong radical scavenging ability. Different extracts showed a significant reduction in inflammation in the paw edema method. C. lancifolius extract and lancifolian showed significant cytotoxic effects against four cancer cell lines: MCF-7, A549, COR-L23 and MDA-MB-231. Subsequently, molecular docking highlighted the strong binding affinity of lancifolian with respective receptors.
Keywords
Antioxidant
Cytotoxic
Lancifolian
Molecular docking
Conocarpus lancifoilus
1 Introduction
Medicinal plants are the source of large number of secondary metabolites such as anthocyanins, terpenoids, phenolics, flavonoids, polyphenols, alkaloids and carotenoids in a different concentration and shield humans for development of various diseases. Plant extracts are used in pharmaceuticals due to the presence of these bioactive compounds. Natural products as extract or pure compound provide opportunities for drug discovery and development (Policegoudra et al., 2012). The focal of researchers on the development of medicines from natural products because synthetic medicines have side effects and contraindications (Petrovska, B. B., 2012). The medicinally important bioactive compounds are isolated from plant extract for various therapeutic purposes (M. A., 1999). Despite huge research on natural medicinal products, some phytochemicals are not fully identified even the mechanism of action of phytochemicals is also not completely understood. However, the main target of researchers is to isolate bioactive compounds for therapeutic purposes (Irshad et al., 2021). Many medicinal plants have cytotoxicity, antioxidant, anti-diabetic, anticancer, anti-inflammatory and antibacterial activities (Abdel-Hameed et al., 2012; Afsheen and Jahan, 2013; Alam et al., 2013; Greenwell and Rahman, 2015; Sato et al., 2013; Sofowora et al., 2013; Strege, 1999; Colombo Migliorero et al., 2020).
Combretaceae belongs to medicinal important plant family containing different 20 genera from which Combretum and Terminalia have been studied for isolation and characterization of phytochemicals to evaluate their different pharmacological activities (Saadullah et al., 2020). The genus Conocarpus has two species named as, C. lancifolius and C. erectus (Saleh et al., 2012). C. lancifolius belongs to an ornamental plant family and it grows usually in riverine and coastal regions of East Africa. It has been observed that this plant is also present in some regions of Pakistan (Baroon et al., 2012). On both surfaces of the leaves of C. lancifolius, trichomes and secretory ducts are present and the surface is protected by a thick cuticle layer exhibiting xerophytic characteristics. Their mature leaves are shiny and glossy in appearance. They have the leaves exhibiting xerophytic characteristics (Redha et al., 2011). It has been reported that Conocarpus genus contains different classes of secondary metabolites (Baroon et al., 2012). C. lancifolius grows in semi-arid and sandy soils environment. Secretory ducts are responsible for secretion of polyphenols, polysaccharides and epicuticular waxes.
The aim of this current study is to deal with isolation, elucidation and characterization of structure of new bioactive compound as well as determination of antioxidant, antiproliferative and anti-inflammatory activities of the novel compound. Reactive oxygen species (ROS) such as hydroxyl radical, superoxide anion radical, hypochlorous acid, hydrogen peroxide, ozone and superoxide radicals have an association with many disorders including carcinogenesis (Steer et al., 2002). ROS in the human body is produced as by-product of many metabolic reactions in the body (Navarro-Yepes et al., 2014). Many processes like activation of some factors that stimulate the transcription, phosphorylation of protein, and apoptosis are dependent and affected by suitable ROS generation and its level should be kept low inside the cells (Rajendran et al., 2014). Increased production of ROS induces harmful and unpredictable side effects on many important structural and functional components of the cell such as carbohydrates, nucleic acid, lipids and proteins (Taniyama and Griendling, 2003). Many studies in literature proved that oxidative stress is the leading cause of various disorders including metabolic and neurological disorders, cardiovascular diseases, atherosclerosis and cancer (Pizzino et al., 2017). Cancer is a worldwide health problem that may be due to exposure of carcinogenic agents such as toxins, virus, radiation and chemicals that cause uncontrolled growth of cells (Cohen et al., 2019). ROS are also responsible for induction of cancer by different mechanistic pathways such as proliferation, apoptosis and survival of cells, metabolism of energy, adhesion of cell–cell and motility of cells (Liou, G.Y. and P. Storz, 2010). Many other factors such as malnutrition, genetic variability, intake of alcohol and tobacco, lack of physical activities and some toxins are also major cause of immune suppression and development of cancer (Vineis et al., 2014). The number of cancer cases is steadily rising, driven by both rapid population growth and increased life expectancy. Projections indicate a 75 % surge in cancer cases by the year 2030 (Organization, W.H., 2016). Hence, it is crucial to explore innovative approaches for cancer treatments to alleviate patient distress and mitigate the escalating expenses associated with current costly therapies. While there is a wide array of medications targeting different cancer types, none are entirely foolproof or devoid of risks. A primary drawback of chemotherapy lies in its toxicity to healthy cells in the body, leading to significant side effects like fatigue, hair loss, anemia, and susceptibility to bruising and bleeding, among other issues (Prakash et al., 2013). Natural products and their derivatives exhibit significant promise in the creation of chemotherapeutic agents, showcasing extensive structural diversity and pharmacological and molecular attributes conducive to their advancement in this field (Pan et al., 2012). Cell lines derived from human tumors have traditionally played a crucial role in the development of novel cancer treatments. High-throughput cell-based profiling played a pivotal role across various discovery platforms but also in uncovering multiple agents later confirmed for therapeutic effectiveness. Substantial genomic diversity in human cancer and its impact on treatment response underscores the necessity to reevaluate the scope of cell line-based investigations for evaluating the efficacy of potential anticancer agents. As a result, more extensive arrays of cancer cell lines are now being utilized to uncover genomic factors influencing drug sensitivity. Recent literature has reinvigorated endeavors to employ cancer cell lines for evaluating the clinical viability of emerging investigational cancer drugs and identifying predictive biomarkers (Sharma et al., 2010). The aim of this study is to emphasize the untapped cytotoxic potential of C. lancifolius extract and isolated compound (lancifolian) against four human cancerous cell lines including MCF-7, A549, COR-L23 and MDA-MB-231.
Inflammation is the natural and immediate response of living organisms to ant kind of injury. This is defense mechanism of body to eliminate the chances of spread of infectious agent (Ofuegbe et al., 2014). There are different components of inflammation including leukocyte infiltration, granuloma formation and edema (Ofuegbe et al., 2014). The occurrence and uncontrolled progression of inflammation cause various inflammatory diseases leading to failure of body organs (Mehrzadi et al., 2021). For treatment of this adverse event, there is need of pharmacological interventions to improve the quality of healthy life (Archer et al., 2015).
Current therapeutic approaches for inflammatory disorders often involve synthetic drugs, which, despite their effectiveness, frequently come with a range of undesirable side effects and serious adverse reactions. These can encompass issues such as gastrointestinal disturbances, immunosuppression, and potential organ toxicity, making long-term use challenging for patients. The increasing recognition of these drawbacks has spurred a growing interest in herbal medicine as an alternative. Herbal remedies with anti-inflammatory properties are gaining prominence due to their perceived safety profile and the comparative absence of the adverse effects associated with many synthetic drugs. Exploring herbal alternatives becomes crucial for developing therapies that not only effectively address inflammatory conditions but also minimize the risk of debilitating side effects, offering a more holistic and potentially safer approach to long-term patient care. Numerous synthetic drugs once utilized for treating inflammatory disorders have fallen out of favor due to their associated potential side effects and serious adverse reactions, rendering them less desirable for human use. In recent decades, there has been a resurgence of interest in herbal drugs as viable alternatives for addressing various human ailments. Herbal remedies with anti-inflammatory activity have become particularly attractive due to their perceived safety profile compared to synthetic counterparts. In-depth examination of newly explored anti-inflammatory agents encompassing diverse classes of phytoconstituents. Herbal constituents undergo quite simple mechanisms that lead to fewer adverse effects. The potential interactions between herbal remedies and synthetic preparations, addressing associated adverse effects and summarizing clinical trials conducted on herbal substances proved fruitful results in anti-inflammatory activity (Beg, S., et al., 2011).
The compounds that showcase antioxidant properties slow down the oxidation process. Many medicinal plants reduce oxidative stress and ultimately shield the human from many diseases (Irshad et al., 2021). C. lancifolius extract has proven antioxidant activity as stated in the previously published paper (Saadullah et al., 2020). There are large numbers of anticancer agents from natural origins available in the markets (Olano et al., 2009). However, researchers are in a continuous search for characterization and isolation of anticancer agents from natural origins because of their high specificity towards cancer cell lines (Siddique et al., 2022). According to recent knowledge, no such report is published that investigated the anti-inflammatory effect of C. lancifolius extract to mitigate the edema of rat paw which is induced by carrageenan.
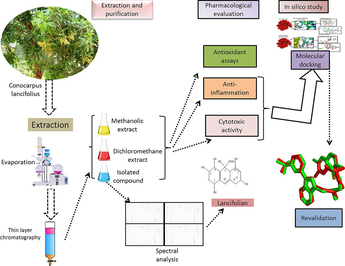
The main objectives of this study to isolate novel compounds from extract of C. lancifolius by multiple fractions that make this study more worthy. Spectral studies are used to identify the structure of novel compounds. The pharmacological activities of isolated compounds and extracts of C. lancifolius are investigated. To obtain the authentic information about the molecular aspects of this novel compound, molecular docking was performed This current project is designed by combining experimental and in silico computational analysis for evaluation of antioxidant, anti-inflammatory and cytotoxicity activity of methanol and dichloromethane extracts of C. lancifolius and lancifolian (novel isolated compound) obtained from C. lancifolius by extraction and purification for validation of our hypothesis. Antioxidant potential was evaluated by conducting in vitro test: DPPH (2,2-diphenyl-1-picrylhydrazyl) test, FRAP (ferric reducing antioxidant power) assay, ABTS (2,2′-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid) assay and β-carotene bleaching assay while anti-inflammatory effect was observed via rat paw edema induced by carrageenan. Similarly, antiproliferative activity is evaluated against human cancer cell lines. This study will be helpful for researchers to attain their attraction on evaluation of pharmacological activities of natural products.
In previous studies, lancifolius shows cytotoxicity against MRC-5 cancer cell line. Isolated novel compound lancifotarene showed cytotoxicity towards cancer cell lines including murine lymphocytic leukemia (P-388), human colon cancer (Col-2), human breast cancer (MCF-7), while no cytotoxicity was observed towards human lung cancer (Lu-1), rat normal glioma cells (ASK) and human embryonic kidney cells (Saadullah at al., 2020).
The originality of this research lies in the isolation and purification of a novel compound named lancifolian from C. lancifolius. This compound is subjected to in vitro antioxidant assays, revealing its significant free radical scavenging ability. The study also highlights the anti-inflammatory effects demonstrated by both the C. lancifolius extract and lancifolian, determined through carrageenan-induced paw edema. Additionally, the novelty extends to the observed substantial cytotoxic effects against four different cancer cell lines (MCF-7, A549, COR-L23, and MDA-MB-231). The utilization of molecular docking techniques adds another layer of innovation, predicting the anti-inflammatory and cytotoxic properties by assessing the binding affinity of lancifolian with respective receptors. The validation of these molecular docking predictions further enhances the credibility and significance of the findings, contributing to the overall originality and novelty of the research.
2 Materials and methodology
2.1 Chemicals
Chloroform (99.8 % by Sigma-Aldrich), methanol (99.9 % by Sigma-Aldrich), ethyl acetate (99.9 % by Sigma-Aldrich), dichloromethane (99.8 % by Merck-Millipore), n-hexane (99 % by GlenPure), vanillin (99 % by Thermo-Scientific), godine reagent, sulphuric acid (98 % by Thermo-Scientific), and carrageenan (commercial grade by Sigma-Aldrich). All the chemicals used in this study were of analytical grade. The chemical structures of these isolated compounds (A-G) were established with the help of spectroscopic techniques such as UV–visible and Infrared spectroscopy, Proton Nuclear Magnetic Resonance (1H NMR), 13C NMR (BB, DEPT-135, 90), Two-Dimensional Correlation Techniques (HMBC, HSQC) and Mass Spectrometry. The experimentation was conducted in H.E.J Karachi.
2.2 Collection of plant material
To conduct this study, the plant was collected in fresh condition from the two different surrounding regions of Pakistan (Lahore and Pattoki) in August 2012. After the collection of plant, this was authenticated by a taxonomist, Dr. Altaf Ahmad Dasti from Bahauddin Zakariya University Multan, Pakistan (BZU). This collected specimen is verified as C. lancifolius and also assigned the registered number (WCL-291) to this identified specimen.
2.3 Extraction
Washed the aerial parts of selected sample to eliminate impurities and air-dried them at room temperature in the shade. The dried plant material was subjected to a fine power. The simple maceration method was employed for extraction, with 150 g of dry plant powder suspended in 350 mL of dichloromethane and methanol in separated containers and labeled as CLD, and CLM. Left the containers for three days with occasional shaking. After 24 h, both materials were filtered. Following 72 h, both were subjected to rotary evaporation. Prepared extracts were subjected into air-tight labeled jars, and the percentage yields were calculated.
2.4 Screening of phytochemicals
The coarse powder of the plant material was weighed and different phytochemical tests were conducted by following the standard protocols for qualitative estimation of secondary metabolites present in the plant. For the identification of alkaloids, mayer’s, wagner’s and dragendorff’s reagents were used. Keller kiliani test was performed for confirmation of cardiac glycosides. Borntrager’s test was applied for identification of anthraquinones. Ferric chloride, catechin and gelatin test were performed for confirmation of tannins and froth test was performed for identification of saponins. Liebermann burchard test was used for identification of triterpenoids. All the tests were performed for qualitative phytochemical analysis.
2.5 Purification of lancifolian
Dichloromethanolic extract (12 gm) of C. lancifolius (CLD) was operated by column chromatography with aid of silica gel 60 of 40–63 μm (stationary phase) and mixture of chloroform and methanol used as mobile phase in the ratio as mentioned in Fig. 1 followed by alone use of methanol. The five fractions were obtained which were named as CLD-1 (1.10 gm), CLD-2 (4.27 gm), CLD-3(1.94 gm), CLD-4 (680 mg), CLD-5 (300 mg). The column chromatography was further performed to get more fractionations of 1.954 g of CLD-3 with silica gel 60 of 40–63 μm working as stationary phase and mobile phase is a mixture of ethyl acetate and hexane with ratio and sequence as shown in Fig. 1 By this operation, four more fractions were obtained named as CLD-3a + 3b (555 mg), CLD-3c (420 mg), CLD-3d (45 mg) and CLD-3e + 3f + 3 g (300 mg). The CLD-3e + 3f + 3 g (300 mg) fraction was again fractioned by column chromatography using silica gel 60 of 40–63 μm as stationary phase and mixture of chloroform and methanol was used as mobile phase. By this process, four more fractions were obtained which were named as CLD-3d-a (7 mg), CLD-3d-b (11 mg), CLD-3d-c (7 mg) and CLD-3d-d (2d mg). The 27 mg of CLD-3d-4 + 5 fraction was obtained and referred as novel compound (lancifolian). The schematic presentation of purification of dichloromethane (DCM) extract of C. lancifolius to obtain lancifolian is shown in Fig. 1.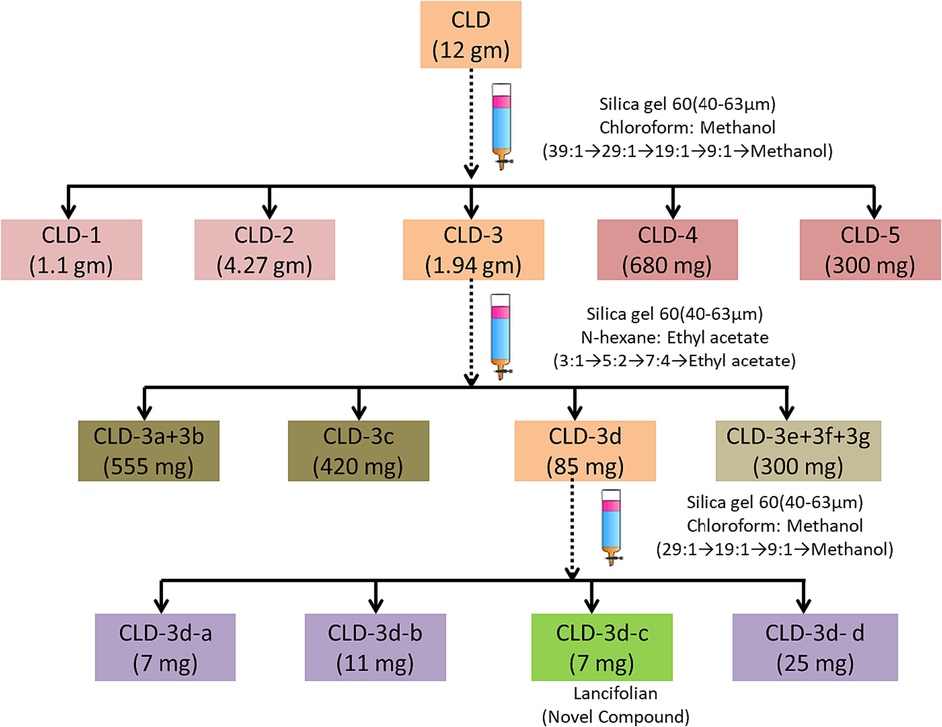
Schematic presentation for purification of novel compound (Lancifolian) from C. lancifolius extract.
2.6 Structural elucidation and spectral analysis
Column chromatography was applied for isolation of active compound from extract. Ultraviolet (UV) spectrum was measured by using Perkin elmer lambda-25 spectrophotometer while methyl alcohol (MeOH) was used as solvent. Infrared (IR) spectrum was recorded using Alpha bruker infrared instrument. At the different frequencies (300, 400, 500 and 600 MHz), proton nuclear magnetic resonance (1H NMR) spectra were recorded while deuterated methanol (CD3OD) and deuterated chloroform (CDCl3) were used as solvent to avoid any problem in the interpretation of results due to presence of proton in solvent. For this experiment, internal standard was tetramethylsilane (TMS). For measurement of 1H NMR spectra, Bruker NMR spectrometers were used. Carbon nuclear magnetic resonance (13C NMR) spectra were observed at frequency of 75, 100, 125 and 150 MHz where same solvents were used as in the measurement of 1H NMR spectra. The two dimensional NMR (2D-NMR): heteronuclear single quantum coherence (HSQC) was measured in CD3OD and CDCl3 at the different frequencies (300, 400, 500 and 600 MHz) on the same instruments as used for measurement of NMR spectra. Electron ionization mass spectrometer (EIMS) was used to record EIMS spectra at low resolution by Finnigan MAT 311 with MassPec data system. Finnigan MAT 312 mass spectrometer peak matching was used for measurement of field ionization (FI) and fields desorption (FD). High-resolution mass spectrometry (HRMS) such as Jeol JMS (HX 110) MS was used to record the HRMS spectra. Distortionless enhancement by polarization transfer (DEPT) was also used for determination of multiplicity of carbon.
2.7 In vitro antioxidant activity
For determination of antioxidant activity of DCM and methanolic extracts of C. lancifolius and lancifolian (novel compound), in vitro assays: ABTS test, DPPH test, FRAP test and β-carotene bleaching test were performed.
2.7.1 ABTS test
For investigation of antioxidant activity, ABTS test is based on the decolorization of solution due to formation of ABTS radical cation (Lee et al., 2015). The production of ABTS cation radical was as a result of the reaction between ABTS solution and potassium persulphate (1:1) and reaction mixture was stored in the dark room for period of 12 to 16 h at the room temperature. Dilution of ABTS cation radical solution was done with methanol and spectrophotometer was used to record the absorbance of reaction mixture at 734 nm. The sample was added to this prepared solution then absorbance was recorded after interval of 30 min. In each assay, blank was also run, and ascorbic acid used as standard for antioxidant activity. The absorbance of each sample was recorded three times. ABTS free radicals scavenging ability of each sample was calculated by using Eq (1).
2.7.2 DPPH test
DPPH free radical scavenging activity of samples were estimated by using previous reported method (Rajurkar et al., 2011). The samples were added in the vials containing methanolic DPPH and all the test samples were stored in the dark environment for time period of 30 min at room temperature. The absorbance of all test samples was recorded at 515 nm by spectrophotometer. Blank was also measured, and ascorbic acid was used as standard for this experiment. All the reactions were performed thrice times. DPPH free radical scavenging activity was recorded by using Eq. (2) and expressed in percentage.
2.7.3 FRAP test
The antioxidant activity of samples was measured spectrophotometrically by following reported method (Pulido et al., 2000). Preparation of FRAP reagent was done by the reaction of TPTZ (2,4,6-tripyridyl-s-triazine), ferric chloride (FeCl3), acetate buffer and hydrochloric acid (HCl). The ferric tripyridyl triazine complex (Fe3+ TPTZ) was formed when all the test samples were added in FRAP reagent as a result an intense blue color appeared. Spectrophotometer measured the absorbance of the prepared reaction mixtures at 595 nm. The results are depicted in µM Fe (II)/g. All the experiments were performed thrice times. While butylated hydroxytoluene (BHT) was used as a positive control.
2.7.4 β-carotene bleaching assay
To determine antioxidant activity of samples by β-carotene bleaching assay, the reaction mixture was prepared by mixing of β-carotene, linoleic acid and tween 20. Solvent is evaporated from this reaction mixture and then diluted with water resulted in the formation of emulsion. Sample was added in the test tube and allowed to heat in the water bath at 45 ◦C. The absorbance of all the samples was recorded at three times intervals: 0, 30 and 60 min at 470 nm. Propyl gallate was used as positive control for β-carotene bleaching assay (Rajurkar et al., 2011).
2.8 In vitro anti-inflammatory activity
2.8.1 Experimental design
30 Adult Wistar rats weighing 200–230 g were purchased to conduct the experiment and allowed to acclimatize in animal house for 14 day at standard conditions (room temperature: 25 ± 3 °C, light and dark cycle of 12 h:12 h, 50 % humidity in the environment). For experimental study on rats, all the protocols are followed provided by Bioethics committee of Bahauddin Zakariya University, Multan. 30 rats were divided into 5 groups (n = 6). The anti-inflammatory effect of dicholoromethane extract of C. lancifolius (100 and 200 mg) and isolated novel compound (10 mg) was evaluated by carrageenan induced rat paw edema. 1 % w/v carrageenan in normal saline was administered to all experimental animals except control group at a dose of 0.05 mL by sub-planter injection (Mukherjee et al., 1997). The volume of contra-lateral and injected paws was measured by plethysmometer at interval of 1 h (0, 1, 2, 3, 4, 5 h) after the administration of carrageenan. The first group is referred as control group to which only carrageenan was administered. The second group is referred as standard or reference to which indomethacin was administered at a dose of 10 mg per kg of the body weight. Third was treated with dicholoromethane extract of C. lancifolius at dose of 100 mg per kg of the body weight orally after administration of carrageenan. Dicholoromethane extract of C. lancifolius at dose of 200 mg per kg of the body weight was administered to fourth group orally after carrageenan administration. The fifth group was treated with isolated compound (10 mg) after administration of carrageenan.
2.9 In vitro anti-proliferative activity
2.9.1 Cell lines and culture conditions
For evaluation of antiproliferative activity of samples, four cell lines were used: human breast cancer ER + cells (MCF-7, ECACC N◦: 86012803), human lung adenocarcinoma cell line (A549), human Caucasian lung large cell carcinoma (COR-L23, ECACC N◦: 92031919) and triple negative breast adenocarcinoma cell line (MDA-MB-231, ECACC N◦: 92020424). All buffers, media, dyes and trypsin were sterilized before use and heated up to 37 °C. MCF-7 and Dulbecco's Modified Eagle Medium (DMEM) was used to culture A549 cells while Roswell Park Memorial Institute (RPMI) 1640 medium was used to maintain CORL-23 and MDA-MB-231 cell cultures. A549 cells cultured in DMEM/F-12 were supplemented with streptomycin/penicillin, 1 % 2 mM L-glutamine and foetal bovine serum (FBS). All cell lines were cultured in 5 % CO2 at 37 °C in a humidified environment. The cultures were trypsinized for a week using 1:30 plants 2022, 11, 1025 15 of 18 dilution of standard solution of Trypsin-EDTA. Cells viability and counts were estimated by using standard tryptan blue cell counting technique. Then, all samples were dissolved in dimethyl sulfoxide (DMSO) to obtain working solutions, samples were diluted in appropriate medium.
2.9.2 Cell viability assay
Cell viability was assessed by using a protein-staining sulforhodamine B (SRB) assay as reported earlier in the literature (Orellana et al., 2016). The protocol for this experiment involves four major steps including the preparation of sample, incubation of cells, fixation, staining with SRB and measurement of absorbance. All the selected cells were trypsinized and counted. The trypsinized cells were placed in 96-well plates of every cell line at the optima density that was estimated in a range of 5–15 × 104 to ensure the growth in the whole experimental period. The result indicated that a linear relationship is found between the cells number and absorbance at 490 nm. SRB assay was used to analyze the cultures and allowed to incubate for attachment of cells. After 24 h, all the cells were treated with series of dilution of samples to get final concentration for each sample ranging from 5 to 200 µg/mL. The final mixture that was used for treatment of cell has not DMSO solvent more than 0.5 % and the same condition was employed to solvent-control wells. Similar study has been conducted while applying MTT assay for cell viability analysis assessment and multimodal imaging (Alam et al., 2020, Alam et al., 2021).
After exposure of approximately 24 h, 40 % of ice-cold trichloroacetic acid (TCA) in 100 mL quantity was added in each well and allowed the reaction to take place at 4 °C. After period of 1 h when reaction was completed, wells were washed using double distilled water. The staining of TCA-fixed cells was done with 50 µL of SRB (0.4 % w/v) in 1 % acetic acid. Washing of well plates was done by using 1 % acetic acid and plates were allowed to air dry for overnight. To read the plates, 10 mM tris base (tris[hydroxymethyl]aminomethane) in 100 µL quantity was used to solubilize the bound dye. The absorbance was measured by Molecular Devices SpectraMax Plus Plate Reader (Molecular Devices, Celbio, Milan, Italy) at 490 nm. The survival of cells was estimated as percentage of absorbance in comparison to un-treated control cells. Taxol and vinblastine sulfate salt were used as a positive control to measure the anti-proliferative activity of samples.
2.10 Docking studies
2.10.1 Receptor preparation
Inhibition of angiogenesis through inhibition of vascular endothelial growth factor receptor 2 (VEGFR-2) has been applied in cancer therapy because of its important role in promoting cancer growth and metastasis. One of the most known angiogenic molecules is the vascular endothelial growth factor (VEGF) family members which are pivotal stimuli of physiological as well as pathological angiogenesis. Vascular endothelial growth factor (VEGF) regulates blood and lymphatic vessel development and homeostasis. VEGFR-2, a type III transmembrane receptor tyrosine kinase, is vital for angiogenesis. Several complex mechanisms are involved in the regulation of VEGFR-2 levels. Hence, blocking of VEGFR-2 or down regulation of its signaling became a main approach for the discovery of new drugs for many human angiogenesis-dependent malignancies. To date, a humanized anti-VEGF monoclonal antibody (bevacizumab) and several small molecule VEGFR-2 kinase inhibitors (sorafenib, regorafenib, sunitinib, vandetanib, pazopanib, axitinib and cabozantinib) have been approved as anti-angiogenic drugs. However, some adverse effects have been observed during their clinical use, such as bleeding complications, indicating that the development of safer VEGFR-2 inhibitors is still an active field of research (Abdullaziz et al., 2017). The primary responsibility of TNF-alpha is to initiate and regulate the body's inflammatory response. TNF-alpha serves as a key mediator by triggering various cellular responses. TNF-α participates in vasodilatation and edema formation, and leukocyte adhesion to epithelium through expression of adhesion molecules; it regulates blood coagulation and contributes to oxidative stress in sites of inflammation (Candido and Hagemann, 2013).
The 3-dimensional structure of TNF-α (PDB Id: 2AZ5) having resolution 2.10 Å responsible for inflammation and VEGFR-2 (Abdullaziz et al., 2017) (PDB Id: 4ASD, resolution 2.03 Å) responsible for cytotoxicity were obtained from the Protein Data Bank database (Berman et al., 2002). The proteins were in a complex with a ligand. MGL Tools were used to prepare the proteins (Dallakyan and Olson, 2015). Water molecules and co-crystalized ligands were removed from macromolecules, and Kollman Charges and polar hydrogens were added.
2.10.2 Ligand preparation
The experimental structure of the lancifolian and co-crystal ligands were optimized by using density functional theory (DFT) methods along with 6-311G** basis set (Obot, et al. 2015). GaussView 06 was used to create these, which were subsequently converted to PDB format (Migliorero and Castañeda, 2020).
Co-crystalized Ligand for TNF (PDB# 2AZ5).
6,7-DIMETHYL-3-[(METHYL{2-[METHYL({1-[3-(TRIFLUOROMETHYL)PHENYL]-1H-INDOL-3-YL}METHYL)AMINO]ETHYL}AMINO)METHYL]-4H-CHROMEN-4-ONE
 .
.
Co-crystalized ligand for VEGFR-2 (PDB# 4ASD) Sorafenib
 .
.
2.10.3 Molecular docking protocol
PDB data of both proteins and ligands were converted to PDBQT format, an enhanced PDB format, for molecular docking research using MGL tools. The PDBQT files that were generated saved in a config (configuration) file. In terms of macromolecules, the conformation with the lowest binding affinity was mirrored as the most stable conformation of ligand. Intermolecular interactions were shown using the Biovia Discovery.
2.10.3.1 Molecular docking protocol
Receptor Preparation: 3D structures of TNF-α (PDB Id: 2AZ5) and VEGFR-2 (PDB Id: 4ASD) were obtained from the Protein Data Bank. MGL Tools were employed for receptor preparation, involving the removal of water molecules and co-crystallized ligands, and addition of charges and polar hydrogens. Ligand Preparation: The experimental structure of the ligand Lancifolian and co-crystal ligands were optimized using density functional theory (DFT) methods with a specific basis set (6-311G**). GaussView 06 was used to create ligand structures, subsequently converted to PDB format. Molecular Docking: PDB data of both proteins and ligands were converted to PDBQT format for enhanced docking research using MGL Tools. AutoDock was used for molecular docking. The PDBQT files were saved in a configuration file. The conformation with the lowest binding affinity was selected as the most stable ligand conformation. Intermolecular Interactions: Intermolecular interactions were visualized using Biovia Discovery. The interactions involved hydrophobic forces, van der Waals forces, hydrogen bonds, electrostatic forces, alkyl interactions, π-sigma interactions, π-alkyl interactions, π-π-stacked interactions, and π-amide stacked interactions. Validation Method: Redocking of 2AZ5 and 4ASD receptors without ligands and with separated co-crystal ligands. Parameters Used: Root Mean Square Deviation (RMSD) was employed for validation. A lower RMSD value (0.5 Å) was obtained for the redocking of 2AZ5, indicating a good conformational match. Software Used: MGL Tools were utilized for the preparation of proteins and conversion of PDB data to PDBQT format. Molecular docking research was performed using AutoDock, a widely used docking software. 2D AND 3D interactions in Fig. 4 shows Docking Coordinates.
2.10.4 Docking validation
Validation of docking results was done by redocking the 2AZ5 and 4ASD receptors without ligands and with previously separated co-crystal ligands to see whether the docking values could be accounted for.
2.11 Statistical analysis
The percentage of inhibition (IC50) was calculated using non-linear regression in Graph Pad Prism version 4.0. The graph was plotted between percentage inhibition and concentration to obtain concentration–response curve. One-way analysis of variance (ANOVA) test was followed by multiple comparison Dunnett’s test which was used to determine the differences between and within groups.
3 Results
3.1 Structural elucidation
Lancifolian (7 mg) was obtained by from DCM fractions of C. lancifolius as coarse powder and appeared as colorless gummy solid having melting point 138–145 °C. Ultraviolet (UV) spectrum of lancifolian shows the absorption peaks at 222 and 230 nm (Fig. 2(a)) which depicts that conjugated system is present. IR spectrum of compound was represented in Fig. 2b. IR spectrum of lancifolian shows absorption peak at 3419.6 cm−1 for –OH. The peak at 2928.3 cm−1 is for C-H stretching (Sp2 hybridized). IR spectrum shows absorption at 2125.4 cm−1 shows carbonyl group present in the compound. C = C indicates by presence of absorption peak at 1513.1 cm−1. Absorption peaks at 1513.1 cm−1 shows that lactone moiety is present in compound. The proton NMR (1H NMR) spectra of isolated compound shows the signals at δ 5.78 (1H, singlet) and δ 4.71 (1H, dt having J = 4.2, 2.4 Hz) for alkene and alcoholic proton. The peak at δ 4.58 (1H, d having J = 6.3 Hz) shows the presence of lactone moiety in compound (Fig. 2(c)). 13C NMR spectrum (Fig. 2(d)) shows broad band and depth. The compound in 13C NMR spectra shows the presence of fourteen carbon signals: six for hydroxyl group, seven for methane and one for methylene (Fig. 2(d)). 13C NMR spectra also show the five quaternary carbon signals. In the downfield region, singlet signals at δ 189.9 indicate the presence of carbonyl carbon in compound. The signal in 13C NMR spectrum at δ167.9 indicates lactone carbons exist in compound. Alkene carbons also predicted in the structure due to presence of signals at δ 114.9, 115.9 and 130.7 cm−1. The molecular formula C14H16O9 of compound was elucidated using high-resolution electron ionization mass spectrometry (HR-EI-MS) showing peak for isolated and purified compound at 328 (m/z). Fast atomic bombardment (FAB) and electron ionization (EI) spectrum for confirmation of structure and fragmentation pattern is shown in Fig. 2e and 2f. The connectivity information of proton in two-dimensional perspective, correlation spectroscopy (COSY) and HQSC is shown in Fig. 2g and h. All mentioned physical and spectral data (Fig. 2(a–h)) confirm the identification of compound, 1,3,4,5,6,8-hexahydroxy-3,4,5,5a-tetrahydro-1H-benzo[g]isochromene-5-carboxylic acid which is named as lancifolian. Table 1 presents the summarized physical and spectral data of isolated and purified compound (lancifolian). Fig. 3 presents the structure and fragmentation pattern of lancifolian. The multiplicity of carbon is shown by DEPT tool in Table 2.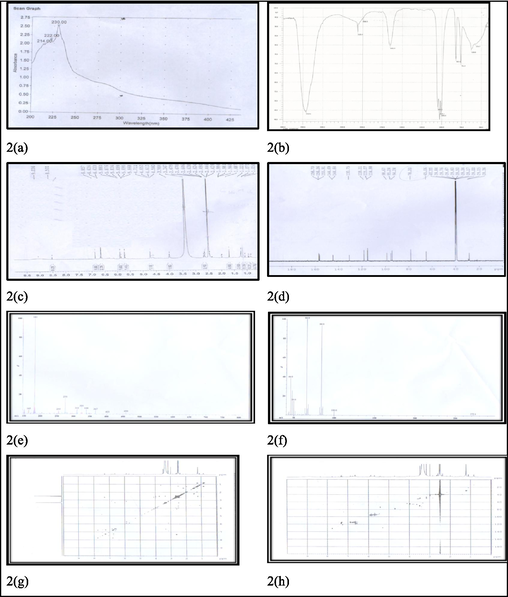
Spectrum for elucidation of lancifolian structure. 2(a): UV spectrum of lancifolian, 2(b): IR spectrum of lancifolian, 2(c): 1H NMR spectrum of lancifolian, 2(d): 13C NMR broad band (BB) spectrum of lancifolian, 2(e): FAB spectrum of lancifolian, 2(f): EI spectrum of lancifolian, 2(g): COSY spectrum of lancifolian, 2(h): HQSC spectrum of lancifolian.
Characteristics
Results
Color
Colorless gummy solid
Quantity
7 mg
Melting point
Ranging from 138 to 145 °C
[α]D25
62.0° (0.12).
Ultaviolet λmax;
Shows the maximum absorption at 230 and 222 nm.
Infrared (KBr)
Shows the peaks for different functional groups: 3419.6, 2928, 2125.4, 1652 and 1531 cm−1.
1H NMR (600 MHz)
Proton resonate at chemical shift δ 4.56 (1H, d having J = 7.5, H-1́), δ 5.79 (1H, s for 5 – OH), δ 6.72(1H, d having J = 1.4 Hz for H-4), δ 6.85(1H, d having J = 1.6 Hz, H-2), δ 6.87(1H, d having J = 1.3 Hz, H-6), δ 7.35 (1H,dd, J = 6.3, 2.7 Hz, H-4), δ 7.4 (1H, J = 6.3 Hz, H-5)
13C NMR (150 MHz)
Carbon shows chemical shift δ at 61.7, δ 70.3 (C-4″), δ 73.8 (C-2″), δ 76.8 (C-3″), δ 77.4(C-5″), δ 95.8 (C-8), δ 100.5(C-6), δ 100.7, δ 103.7 (C-3), δ 106.3, δ 114.8 (C-2″), δ 115.5 (C-5′), δ 130.3, δ 122.6, δ 166.9 (C-7), δ 167.8(C-2) and at δ 168.1.
EI-MS
(relative intensities measure) m/z 328 (72), 252 (1 8 5), 224 (20), 212 (15), 196 (20), 146 (19), 73 (14)
HR-EI-MS
(m/z): molecular formula (C14H16O9), molecular weight (MW) 328.08
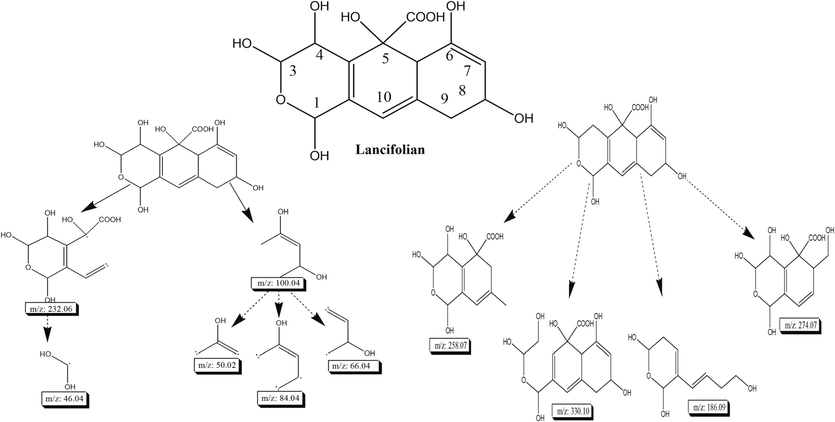
Illustrates the structure and fragmentation pattern of lancifolian, by providing a visual representation of its molecular configuration and the resulting fragments.
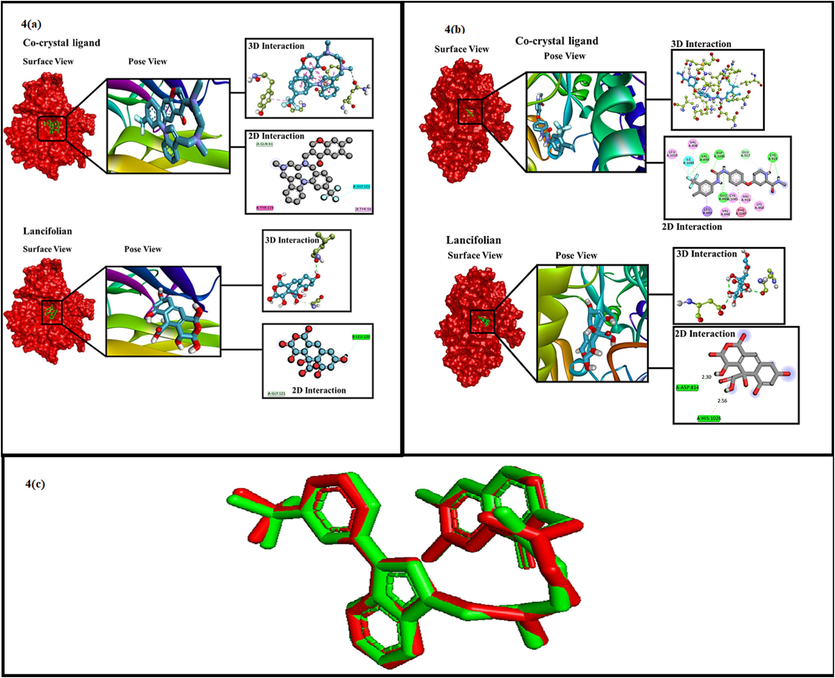
The surface view, pose view, 2D and 3D interactions study of co-crystal ligands (2AZ5 (4(a) and 4ASD 4(b)) lancifolian. The redocking process of lancifolian with crystallography (red: result of co-crystal; green: result of redocking) showing the superimposing position of the ligand (4(c).
C-No.
Multiplicity DEPT
13C NMR
1H NMR
J –value
C-1
–CH
95.4
5.26
(J = 1.4 Hz)
C-3
–CH
101.3
4.69
(J = 1.6 Hz)
C-4
–CH
70.4
4.31
(J = 2.4 Hz)
C-5
-C
79.6
C-6
-C
181.8
C-7
–CH
103.0
3.64
(J = 1.4 Hz)
C-8
–CH
64.8
3.94
(J = 2.1 Hz)
C-9
–CH2
41.7
2.27, 2.02
(J = 1.3 Hz)
C-10
–CH
126.9
2.24
(J = 2.2 Hz)
C-11
-C
132.8
C-12
-C
135.9
C-13
-C
144.8
C-14
–CH
45.5
3.23
(J = 2.3 Hz)
3.2 In vitro antioxidant activity
Diverse methods: ABTS, DPPH, FRAP assay and β-carotene bleaching assay were used to reveal the antioxidant potential of C. lancifolius. Table 3 shows the antioxidant potential of isolated compound, DCM and methanolic extract of C. lancifolius in four in vitro antioxidant assays. The results of experiment are expressed as mean standard deviation (SD) while n = 3. a presents the maximum concentration which is to be tested (500 µg/mL). b presents at the maximum concentration which is to be tested (1000 µg/mL). c present tested concentration is 2.5 mg/mL.
Sample
ABTS
DPPH
FRAP Test
β-Carotene Bleaching Test
IC50 (Unit: µg/mL)
IC50 (Unit: µg/mL)
(Unit: µM Fe(II)/g)
IC50 (Unit: µg/mL)
30 min
60 min
Lancifolian
4.8 % a
39.5 % b
4.4 ± 0.2
51.2 ± 1.7
4.6 ± 2.3
DCM extract of C. lancifolius
6.2 ± 2.4
19.4 ± 1.4
89.3 ± 2.4
13.2 ± 3.2
10.5 ± 0.6
Methanol extract of C. lancifolius
5.1 ± 2.2
21.3 ± 1.5
98.3 ± 2.6
24.2 ± 2.1
21.2 ± 0.7
Positive control
Ascorbic acid
1.6 ± 0.4
5.5 ± 0.8
Propyl gallate
1.3 ± 0.05
1.1 ± 0.04
Butylated hydroxy toluene
62.4 ± 4.4
3.3 In vitro anti-inflammatory activity
In this experimental study, the anti-inflammatory effect of tested samples on rats was estimated from 60 min after administration of carrageenan. It has been observed that paw edema was at peak at 4 h after administration of carrageenan but the second group showed a significant (p < 0.05) difference in comparison to treatment of carrageenan only. Interestingly, extract of C. lancifolius and lancifolian also exhibited same pattern for reduction of inflammation in paw from 1 to 5 h after administration of carrageenan (Table 4). Each values are expressed in Mean ± S.E.M.(Statistical error mean). Group (n = 6). Increase in paw volume presented in (mL). For calculation of results, one way ANOVA was followed by Dunnett’s test where p < 0.05, * < 0.05 & ** < 0.01compare to respective control group, IDM = Indomethacin
Dose (mg/kg p.o)
0 h
1 h
2 h
3 h
4 h
5 h
Control
1.31 ± 0.11
1.42 ± 0.12
1.49 ± 0.51
1.54 ± 0.25
1.66 ± 0.34
1.86 ± 0.030
IDM (10 mg)
1.30 ± 0.025
1.51 ± 0.010*
1.54 ± 0.019
1.59 ± 0.012*
1.38 ± 0.021*
1.29 ± 0.0215**
Dichloromethane extract of C. lancifolius (100 mg)
1.29 ± 1‘0.03
1.52 ± 0.010
1.59 ± 0.010
1.69 ± 0.019
1.74 ± 0.015
1.60 ± 0.030
Dichloromethane Methanol extract of C. lancifolius (200 mg)
1.27 ± 0.01
1.52 ± 0.010
1.60 ± 0.020
1.62 ± 0.015*
1.55 ± 0.011*
1.49 ± 0.020*
Lancifolian (10 mg)
1.28 ± 0.02
1.47 ± 0.014
1.61 ± 0.020*
1.64 ± 0.018*
1.39 ± 0.014**
1.35 ± 0.022**
3.4 In vitro anti-proliferative activity
In vitro cytotoxic activity of lancifolian, DCM and methanolic extract of C. lancifolius was screened very carefully and enlisted in Table 5. Cytotoxic activity was investigated against four selected cancer cell lines: MCF-7, A549, COR-L23 and MDA-MB-231. Data are expressed as median standard deviation (S.D) while n = 3. ** p < 0.01 vs. positive control. Abbreviations: MCF-7: human breast cancer ER + cell line; A549: human lung adenocarcinoma cell line; COR-L23: human lung large cell carcinoma cell line, MDA-MB-231: triple negative breast adenocarcinoma cell line.
Sample
MCF-7
A549
COR-L23
MDA-MB-231
Lacifolian
78.4 ± 2.5 **
>200
112.5 ± 2.3 **
82.3 ± 2.6 **
DCM extract
151.4 ± 3.2 **
>200
>200
164.3 ± 3.2 **
Methanol extract
>200
78.1 ± 2.4 **
>200
>200
Positive control
Taxol
0.06 ± 0.003
1.4 ± 0.02
Vinblastine sulfate
64.3 ± 1.5
44.2 ± 0.04
3.5 Binding energy and molecular docking interactions
We used binding energy and binding affinity interchangeably since they had the same meaning. It's vital to note that a lower binding energy indicates that ligand–protein complex is best, although binding affinity is used as a catch-all term for comparative analysis and is also greater for lower binding energies.
Amino Acid Residues and Chemical Interactions:
-
2AZ5 Interactions:
Co-crystal ligand formed interactions with specific amino acid residues, involving hydrophobic, halogen, and hydrogen bond interactions.
-
4ASD Interactions:
Sorafenib, a co-crystal ligand, exhibited interactions with amino acid residues, including H-type, halogen, and hydrophobic interactions.
-
Lancifolian Interactions:
Lancifolian showed interactions with specific amino acid residues involving H-type interactions.
All planned ligands exhibit advanced interactions with numerous residues of amino acids such as 2AZ5 and 4ASD. The interactions between small molecules (ligands) and macromolecule (protein) are hydrophobic, van der wall forces, hydrogen bond and electrostatic fore of interactions. Alkyl, π-sigma, π-alkyl, π-π-stacked and π-amide stacked interactions are included in hydrophobic interactions. In π—anion and π—cation interactions, electrostatic interactions play a significant role. Distinct forms of ligand interactions with different amino acid residues of the receptor emerge from molecular docking. All the ligands show effective binding with 2AZ5 and 4ASD receptors. Using the Discovery Studio Visualizer program, the ligands and receptor interactions are visualized. The interactions and binding of ligands with distinct amino acid residues of the protein are shown in 2D diagrams of DSV. The co-crystal ligand form total 11 interactions with 2AZ5 receptor from which 8 are hydrophobic, 2 are Halogen type and one is H-bond type interaction. Similarly, sorafenib (co-crystal with 4ASD) form total 15 interactions with 4ASD receptor from which 6 are H-type, 1 is halogen type and 8 are hydrophobic type interactions. The residue involved and distance of interaction of these are shown in Table 4 along with binding energy. The ligand lancifolian form 2H-type interactions with 2AZ5 receptor and 4ASD as shown in Table 6. Moreover, the pose view, surface view, 2D and 3D interactions of all ligands are represented in Fig. 4(a) (Interactions with Inflammatory receptor (2AZ5)) and Fig. 4(b) (Interactions with cytotoxic receptor (4ASD)).
-
Binding Energy and Affinity:
| Interacting Amino Acids of 2AZ5 | |||
|---|---|---|---|
| Ligands | Binding Energy | H-Bonds | Hydrophobic Interactions Name of residue (distance Å) |
| Co-crystal ligand | −7.9 kcal/mol | 1). GLN61(3.75 Å) | 1). GLY121(3.54 Å),2). TYR119 (5.30 Å)3). GLY121 (2.99 Å), 4). TYR59 (4.23 Å |
| Lancifolian | −7.0 kcal/mol | 1). LEU120 (2.27 Å),2). GLY121 (3.50 Å), |
|
| Ligands | Binding Energy | H-Bonds | Hydrophobic Interactions Name of residue (distance Å) |
| Co-crystal ligand(Sorafenib) | −12 kcal/mol | 1). VAL899(2.90 Å)2). ASP1046 (1.76 Å)3). GLU885 (1.92 Å)4). GLU885 (2.42 Å)5). CYS919 (2.39 Å)6). GLU917 (3.44 Å)7). ILE1044 (3.13 Å) |
1). LEU889 (3.60 Å),2). PHE1047 (4.92 Å)3). VAL898 (4.62 Å),4). LEU1019 (4.81 Å),5). VAL848 (4.64 Å),6). LYS868 (5.14 Å),7). VAL919 (4.93 Å),8). CYS1045 (4.94 Å) |
| Lancifolian | −8.00 kcal/mol | 1). HIS1026 (2.56 Å),2). ASP814 (2.30 Å), |
|
The study used binding energy and binding affinity interchangeably, with a lower binding energy indicating a better ligand–protein complex.
-
Interactions:
Various types of interactions were observed, including hydrophobic, van der Waals, hydrogen bond, and electrostatic interactions. Ligands exhibited advanced interactions with amino acid residues of 2AZ5 and 4ASD receptors.
-
Specific Interactions:
The co-crystal ligand with 2AZ5 formed 11 interactions, including hydrophobic, halogen, and hydrogen bond types.
Sorafenib (co-crystal with 4ASD) formed 15 interactions, including H-type, halogen, and hydrophobic types.
Lancifolian showed 2H-type interactions with both 2AZ5 and 4ASD receptors.
-
Visualization:
Visualizations using Discovery Studio Visualizer included pose view, surface view, 2D, and 3D interactions.
3.6 Molecular docking validation
2AZ5 was redocked to validate the findings. The parameter which is usually considered for validation of docking process is RMSD (Root Mean Square Deviation). RMSD method was used to measure the degree of variation between experimental ligand docking findings and crystallographic ligand at the identical binding site. Because the redocking ligand location is found closer to the ligand position of crystallographic, the reduced RMSD value achieved which indicates better conformation. Conversely, the greater the value of RMSD results in higher deviation which shows the higher prediction error for ligand–protein interactions. Because the RMSD value derived from the native ligand with the 2AZ5 receptor was 0.5 Å, it can be concluded that the docking approach utilized in this experimental work is legitimate and may be used as ligand with the same binding site region. Fig. 4(c) illustrates the validation findings.
4 Discussion
C. lancifolius is medicinally important plant that belongs to Combretaceae family (Ali et al., 2013). Different studies have proved that conocarpus genus possesses potential therapeutic activities such as antiprotozoal, anti-leishmanial, antibacterial and anticancer. This study focuses on the antioxidant, anti-inflammatory and antiproliferative characteristics of C. lancifolius. A novel compound, lancifolian was isolated from DCM extraction of C. lancifolius and proved as antioxidant, anti-inflammatory and anticancer agent. Purification of novel compound was done by multiple fractionations using column chromatography where stationary phase of silica gel 60 (63–200 μm) and mixture of methanol and chloroform was used as mobile phase.
Moreover, the structural elucidation and spectral analysis lancifolian was also performed. The UV and IR spectra show conjugation and existence of aromaticity in compound. IR spetra also shows the presence of hydroxyl group, carbonyl group and lactone moiety. 1H NMR spectrum shows the signals to depict the presence of protons of hydroxyl and alkene group. 13C NMR shows the presence of fourteen signals for carbon in which six for hydroxyl group, one for methylene while seven for methane group. 13C NMR also depicts five signals for quaternary carbon. Hence, structure elucidation of lancifolian was performed by HR-EI-MS and determined the molecular formula of novel compound C14H16O9 named as lancifolian.
Free radicals are species having unpaired electrons and produced in the body either exogenously (ionizing radiation, organic solvents, pesticides and air pollution) or endogenously (as a by-product of metabolism). Increased level of ROS has potential to induce tissue injury and death of cell (Chanda et al., 2009). Damage induced by ROS leads to development of various chronic diseases including neural disorders, cardiovascular disease, cognitive impairments, atherosclerosis, cancer and ulcerative colitis (Hossain et al., 2015). Antioxidant activity of phytoconstituents are due to redox potential which neutralize the free radicals species, decompose peroxides and quench singlet and triplet oxygen species (Pietta et al., 1998). Antioxidant potential of molecule is proportional to hydroxyl groups present in a molecule (Norma Francenia et al., 2019). Lancifolian has antioxidant potential due to the existence of hydroxyl group in its structure. It was evaluated by in vitro antioxidant tests (Hossain et al., 2016).
Inflammation-induced by carrageenan is mainly due to inflammatory mediators including prostaglandins, leukotriene, platelet activating factors (PAF) and others inflammatory mediators. The inflammation induced by carrageenan can be described as biphasic phenomena. In the first pahse (0 to 1 h), histamine, kinin and hydroxyl tryptamine (5-HT) releases. In the second phase (3 to 5 h) is endorsed to release of bradykinin and prostaglandin (Mehrzadi et al., 2021). 200 mg extract of C. lancifolius exhibited more anti-inflammatory effect than 100 mf extract of C. lancifolius. The results show the direct correlation of dose strength in reduction of inflammation induced by carrageenan. The extracts and isolated compound exhibited excellent results in reduction of inflammation by significantly alter the prostaglandins mediated response which was disturbed by carrageenan (Fakhar-e-Alam et al., 2023).
In vitro cytotoxic activity of lancifolian, DCM and methanolic extract of C. lancifolius was estimated. Cytotoxic activity of C. lancifolius was investigated against four specific cancer cell lines: MDA-MB-231 and MCF-7 while taxol is used as positive control and A549, COR-L23 where vinblastine sulphate is used as positive control. Lancifolian shows the best anticancer activity (IC50 = 78.4 ± 2.5) in comparison to DCM extract of C. lancifolius (IC50 = 151.4 ± 3.2) while methanolic extract does not exhibit significant anticancer activity for MCF-7 cell lines where taxol is used as positive control (IC50 = 0.06 ± 0.003). Lancifolian and DCM extract does not exhibit anticancer activity against A549 cell lines while methanolic extract exhibit anticancer activity (IC50 = 78.1 ± 2.4) where vinblastine sulfate used as positive s 82.3 ± 2.6 and 164.3 ± 3.2 respectively while methanolic extract does not show anticancer activity where taxol is considered as positive control (IC50 = 1.4 ± 0.02). For COR-L23 cell lines, only lancifolian shows anticancer activity (IC50 = 112.5 ± 2.3) while vinblastine sulphate is used as (IC50 = 44.2 ± 0.04) positive control. Almost, similar results for antiproliferative activities of Coronopus didymus leaf extract were found in literature (Muzammil et al., 2022a).
We also confirmed the binding affinity of lancifolian with receptor and determined the binding energy of lancifolian and receptor complex. In literature, site-specific docking was also performed on different reported inhibitors in T. divaricata (Muzammil et al., 2022b). The results indicated that ligand showed the best interaction with 2AZ5 and 4ASD amino acid residues of receptors. Revalidation of molecular docking was also performed (Malik et al., 2022). The results of revalidation confirmed that ligand and receptor complex have lower RMDS value indicates the position of redocking ligand is near to crystallographic ligand location.
Comparing these results with existing research, this study offers comprehensive insights into the therapeutic potential of C. lancifolius, particularly through the novel compound lancifolian. The robust experimental design detailed structural elucidation, and in-depth analysis of antioxidant, anti-inflammatory, and anticancer activities contribute to the study's strength. Future research could explore the synergistic effects of the plant extracts, conduct clinical trials, and investigate the pharmacokinetics of lancifolian for a more translational perspective.
5 Conclusion and future recommendation
This research has concluded the multicomponents from extract of C. lancifolius. The isolation and purification of novel compound (lancifolian) from C. lancifolius was done using chromatography and spectroscopic techniques. The antioxidant and cytotoxic activity of C. lancifolius extracts and isolated compound was confirmed through in vitro analysis and anti-inflammatory effect was also evaluated by conducting experiment on animal model. We confirmed the cytotoxic and anti-inflammatory activity of lancifolian by using computational tool and molecular docking. The results of our study confirmed that extracts of selected plant and isolated compound from C. lancifolius have potential to inhibit the oxidative stress, reduce the inflammation and kill the cytotoxic cells when compared with standards. Due to cytotoxic potential of lancifolian, it inhibits metastasis by causing death of cancerous cells. Lancifolian exhibited antioxidant and anti-inflammatory activity ultimately preventing the development of many diseases. Besides the significant results from in vitro analysis, molecular docking was performed to confirm the binding affinity of lancifolian with anticancer and anti-inflammatory receptors. Based on results computed by different experimental models and in silico studies, lancifolian could be recommended as best cytotoxic, anti-inflammatory and antioxidant agent. The exploration of bioactive compounds from C. lancifolius within the Combretaceae family holds immense potential for future advancements in medicine. The isolation and purification of the novel compound, lancifolian, derived from this plant, opens doors to extensive research opportunities. To further unravel its therapeutic possibilities, future investigations could focus on elucidating the specific molecular mechanisms underlying notable antioxidant effects. Clinical trials are warranted to validate the observed anti-inflammatory properties and explore the safety and efficacy of C. lancifolius. Additionally, the significant cytotoxic effects against various cancer cell lines necessitate in-depth in vivo studies for a comprehensive understanding of their anticancer potential. Optimizing extraction techniques and exploring potential synergies with existing therapeutic agents could enhance the bioavailability and efficacy of these compounds. Further validation of molecular docking predictions and the development of lancifolian derivatives could contribute to refining its application in the treatment of various diseases. Continuous collaboration with local communities for ethnobotanical insights and comprehensive long-term safety studies will be essential for harnessing the full medicinal potential of C. lancifolius and its bioactive compounds.
While the study on C. lancifolius and its bioactive compound lancifolian presents promising findings, several challenges, and limitations warrant consideration. Firstly, the in vitro assays and animal model experiments, while indicative of potential therapeutic effects, may not precisely replicate the complexities of human physiology. Additionally, though molecular docking enhances predictions, it relies on computational models and assumptions. The isolation and purification process of lancifolian, while utilizing chromatography and spectroscopic techniques, could benefit from further optimization for increased yield and purity. Furthermore, the study primarily focuses on individual components, and understanding potential synergies or interactions within the plant extracts could provide a more comprehensive picture. Future research should aim to conduct rigorous clinical trials to validate the observed effects in humans and assess long-term safety. Moreover, exploring the pharmacokinetics and pharmacodynamics of lancifolian would contribute to a more thorough understanding of its therapeutic potential. Collaborative efforts between researchers in the fields of natural products, pharmacology, and clinical medicine can enhance the translational impact of this study, facilitating the development of lancifolian as a potential therapeutic agent for various diseases.
CRediT author statement
Malik Saadullah: Conceptualization, Data curation, Writing – original draft. M. Fakhar-e-Alam: Conceptualization, Data curation, Writing – original draft. M. Atif: Formal analysis. Muhammad Asif: Writing – review & editing. Kanwal Irshad: Conceptualization, Data curation, Writing – original draft. Zulfiqar Ali: Formal analysis.
Acknowledgement
Researchers Supporting Project number (RSP2024R397), King Saud University, Riyadh, Saudi Arabia
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phytochemical studies and evaluation of antioxidant, anticancer and antimicrobial properties of Conocarpus erectus L. growing in Taif, Saudi Arabia. European. Journal of Medicinal Plants.. 2012;2(2):93.
- [Google Scholar]
- Design, synthesis, molecular docking and cytotoxic evaluation of novel 2-furybenzimidazoles as VEGFR-2 inhibitors. European Journal of Medicinal Chemistry.. 2017;136:315-329.
- [Google Scholar]
- Attenuation of chemically induced diabetes in rabbits with herbal mixture (Citrullus colocynthis and Cicer arietinum) Pakistan Veterinary Journal.. 2013;33(1)
- [Google Scholar]
- Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharmaceutical Journal.. 2013;21(2):143-152.
- [Google Scholar]
- In-vitro antibacterial activities of alkaloids extract from leaves of Conocarpus lancifolius Engl. J. Pure Appl. Microbiol.. 2013;7(3):1903-1907.
- [Google Scholar]
- Anti-inflammatory potential of probiotic Lactobacillus spp. on carrageenan induced paw edema in Wistar rats. International Journal of Biological Macromolecules.. 2015;81:530-537.
- [Google Scholar]
- Nutritional evaluation and palatability trial of ensiled Conocarpus Greenery residues. Experimental Agriculture.. 2012;48(1):138-147.
- [Google Scholar]
- In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: An overview. African Journal of Microbiology Research.. 2009;3(13):981-996.
- [Google Scholar]
- Environmental Exposures and Cancer: Using the Precautionary Principle.. 2019;ecancermedicalscience. 13
- Colombo Migliorero, M. B., S. M. Bonilla Castañeda, S. A. Fernandes, et al., 2020. Síntesis y caracterización de calix [4] areno funcionalizado con grupos sulfónicos incluido en una matriz de silice-titania.
- Small-molecule library screening by docking with PyRx. Chemical Biology: Methods and Protocols. 2015:243-250.
- [Google Scholar]
- Synthesis, Characterization, and Application of BaTiO3 Nanoparticles for Anti-Cancer Activity. J Clust Sci. 2023;34:1745-1755.
- [CrossRef] [Google Scholar]
- Medicinal plants: their use in anticancer treatment. International Journal of Pharmaceutical Sciences and Research.. 2015;6(10):4103.
- [Google Scholar]
- Optimization of Moringa Oleifera Leaf Extraction and Investigation of Anti Breast Cancer Activity with the Leaf Extract. Engineering International. 2015;3(2):97-104.
- [Google Scholar]
- Investigation of toxic effect of moringa oleifera leaf-methanol extract on breast cancer cell mcf-7. Brunei Darussalam Journal of Health.. 2016;6:75-83.
- [Google Scholar]
- Biochemical investigation of therapeutic potential of resveratrol against arsenic intoxication. Dose-Response.. 2021;19(4) 15593258211060941
- [Google Scholar]
- Lee, K. J., Y. C. Oh, W. K. Cho, et al., 2015. Antioxidant and anti-inflammatory activity determination of one hundred kinds of pure chemical compounds using offline and online screening HPLC assay. Evidence-Based Complementary and Alternative Medicine. 2015.
- Reactive oxygen species in cancer. Free Radical Research.. 2010;44(5):479-496.
- [CrossRef] [Google Scholar]
- Green synthesis of RGO-ZnO mediated Ocimum basilicum leaves extract nanocomposite for antioxidant, antibacterial, antidiabetic and photocatalytic activity. Journal of Saudi Chemical Society.. 2022;26(2):101438
- [CrossRef] [Google Scholar]
- Zingerone mitigates carrageenan-induced inflammation through antioxidant and anti-inflammatory activities. Inflammation.. 2021;44:186-193.
- [Google Scholar]
- Studies on the anti-inflammatory activity of rhizomes of Nelumbo nucifera. Planta Medica.. 1997;63(04):367-369.
- [Google Scholar]
- Antioxidant gene therapy against neuronal cell death. Pharmacology & Therapeutics.. 2014;142(2):206-230.
- [Google Scholar]
- Antioxidant Compounds and Their Antioxidant Mechanism. 2019. p. :2.
- Anti-inflammatory and analgesic activities of the methanol leaf extract of Phyllanthus amarus in some laboratory animals. Journal of Basic and Clinical Physiology and Pharmacology.. 2014;25(2):175-180.
- [Google Scholar]
- Sulforhodamine B (SRB) assay in cell culture to investigate cell proliferation. Bio-Protocol.. 2016;6(21):e1984-e.
- [Google Scholar]
- Historical review of medicinal plants' usage. Pharmacognosy Reviews.. 2012;6(11):1-5.
- [CrossRef] [Google Scholar]
- Antioxidant activity of selected medicinal plants. Journal of Agricultural and Food Chemistry.. 1998;46(11):4487-4490.
- [Google Scholar]
- Pizzino, G., N. Irrera, M. Cucinotta, et al., 2017. Oxidative stress: harms and benefits for human health. Oxidative medicine and cellular longevity. 2017.
- Phenolic content, antioxidant activity, antibacterial activity and phytochemical composition of Garcinia lancifolia. Indian Journal of Pharmaceutical Sciences.. 2012;74(3):268.
- [Google Scholar]
- Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. Journal of Agricultural and Food Chemistry.. 2000;48(8):3396-3402.
- [Google Scholar]
- Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian Journal of Pharmaceutical Sciences.. 2011;73(2):146.
- [Google Scholar]
- Leaf traits and histochemistry of trichomes of Conocarpus lancifolius a Combretaceae in semi-arid conditions. American Journal of Plant Sciences.. 2011;2(02):165.
- [Google Scholar]
- Cytotoxic and antioxidant potentials of ellagic acid derivatives from Conocarpus lancifolius (Combretaceae) Tropical Journal of Pharmaceutical Research.. 2020;19(5):1037-1080.
- [Google Scholar]
- Differential cellular localization of antioxidant enzymes in the trigeminal ganglion. Neuroscience.. 2013;248:345-358.
- [Google Scholar]
- The role and place of medicinal plants in the strategies for disease prevention. African Journal of Traditional, Complementary and Alternative Medicines.. 2013;10(5):210-229.
- [Google Scholar]
- Cardiac and vascular structure and function are related to lipid peroxidation and metabolism. Lipids.. 2002;37(3):231-236.
- [Google Scholar]
- High-performance liquid chromatographic–electrospray ionization mass spectrometric analyses for the integration of natural products with modern high-throughput screening. Journal of Chromatography b: Biomedical Sciences and Applications.. 1999;725(1):67-78.
- [Google Scholar]
- Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension.. 2003;42(6):1075-1081.
- [Google Scholar]
- Global cancer patterns: causes and prevention. The Lancet.. 2014;383(9916):549-557.
- [Google Scholar]







