Translate this page into:
Biogenic amines mediate learning success in appetitive odor conditioning in honeybees
⁎Corresponding authors at: College of Animal Sciences (College of Bee Science) Fujian Agriculture and Forestry University, Fuzhou, China. fbiuaf91@gmail.com (Muhammad Fahad Raza), susongkun@zju.edu.cn (Songkun Su)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Biogenic amines modulate the honeybees' behavioral development, especially olfactory learning behavior. Diverse behavioral protocols have been developed to investigate the olfactory learning behavior of bees to process appetitive olfaction information. Apis mellifera ligustica is a well-known eusocial insect to examine the olfactory learning behavior. This study evaluated the relationship between olfactory learning success and biogenic amines and uncovered the role of biogenic amines to regulate the olfactory learning behavior of bees.
Methods
We used high-performance liquid chromatography (HPLC) with an electrochemical detection (ECD) system to quantify neurotransmitters levels in the bee brain following olfactory learning trials. Furthermore, the bees of the control group and the dopamine flupenthixol blocker injected group were trained to evaluate the olfactory learning performance.
Results
Our finding showed that learning success was directly correlated with the levels of DA and serotonin (5-HT), furthermore, bees of the control group showed higher proboscis extension response than dopamine blocker injected group. Taken together, these findings revealed that dopamine (DA) and serotonin (5-HT) could thus act together to define optimal motivational or attentional levels and improve learning success and indicated that dopamine blocker flupenthixol has to modulate influence on the olfactory learning performance of bees.
Conclusion
The results strongly imply that biogenic amines can durably modify the learning behavior in future model insects.
Keywords
Biogenic amines
Proboscis extension response
1-nonanol
Odor
Apis mellifera
1-hexanol
1 Introduction
Learning ability is a crucial asset of the nervous system, which establishes predictive rules in a complex environment. Different forms of learning exist, among which Pavlovian conditioning or classical conditioning has gained much attention because of its universality across species. Bees remember the link between an unconditioned stimulus (US) and a conditioned stimulus (CS) in this conditioning protocol. In this form of learning, initially, bees do not elicit the proboscis to a neutral stimulus, however, it stimulates the quick response by biologically relevant stimulus. In this conditioning protocol, the pairing of the unconditioned stimulus and conditioned stimulus consequences in the acquisition of a predictive association between US and CS, and finally conditioned reaction to the CS (Rudy, 2008).
Insects as remarkable models for the testing of pavlovian conditioning protocol. In particular, honey bees, Apis mellifera, have been widely studied using Pavlovian protocol to characterize this conditioning form's behavioral, neural and molecular underpinning. Succeeding in this protocoal, odorants (CS) were offered to harnessed bees followed by sucrose solution (US) as a reward, which is sent to bee antennae and then delivered to proboscis. Sucrose solution is offered to stimulate the antennae that elicit the proboscis extension reflex (PER), which was evoked by the odorant after successful conditioning (Raza et al., 2019).
Coincident principles for CS processing and plasticity have been found in the brain of various insect species. Olfactory receptors can detect odorants positioned on the antennae and the sensory system information is further processed in consecutive stages of the olfactory pathway including mushroom bodies, the antennal lobes and lateral horns (Sandoz, 2011). Plastic changes in neural activity have been found at these different stages because of Pavlovian learning (Matsumoto et al., 2018). Yet, discrepancies exist concerning US processing in the insect brain, even when sucrose solution is used as an appetitive reward. Although biogenic-amine signaling was crucial for reward signaling (Raza and Su, 2020), the specific biogenic amine required to this end differs between insect species. Several biogenic amines are well-known for their functions in insects' behavioral regulation and development functioning. The response of neurons, sensation activation, circadian rhythms learning, and memory are controlled by several biogenic amines (Sinakevitch et al., 2018). In the honeybee, levels of the brain's amine are linked with several factors such as behavioral development, age of bees, stress, source colony, morphological development and seasonal changes (Even et al., 2012). Dopamine is specifically regulating reward signaling (Denton et al., 2021). Dopamine typically injected into the insect's brains, evokes distinct changes in behavioral and olfactory responses. Behaviorally, DA plays an important in regulating learning abilities, odor detection and discrimination (Dacks et al., 2012). While octopaminergic signaling regulates the stimulating properties of sugar solution in the brains of the honey bee and the cricket (Mizunami et al., 2009, Mizunami and Matsumoto, 2010), dopaminergic signaling plays the same role in fruit flies Drosophila melanogaster trained to establish the link the odorants with sucrose reward (Huetteroth et al., 2015). This discrepancy is even more accentuated if one considers that the significance of dopaminergic signaling in honey bee learning has been restricted to aversive-conditioning forms, where it regulates the reinforcement characteristics of punishment-like stimuli (Marchal et al., 2019).
Here we reconsidered these discrepant findings by focusing on the appetitive olfactory conditioning of honey bees and analyzing the relationship between learning success and dopamine (DA) levels. While the role of octopaminergic signaling as a substitute for sucrose has been shown repeatedly in bees (Mizunami and Matsumoto, 2010). We hypothesized that whether biogenic amines could modulate the learning success of same age honeybees. The DA levels may correlate with the predisposition to learn the appetitive association between odor and sucrose and should thus vary between learners and fail-learners. We used high-performance liquid chromatography (HPLC) with an electrochemical detection (ECD) system to measure biogenic amine levels in the bee brain following olfactory learning and found that learning success was directly correlated with the levels of DA but also of serotonin (5-HT). Both amines could thus act together to define optimal motivational and/or attentional levels and improve learning success. Subsequently, a dopamine blocker was injected to confirm the modulating influence of dopamine in the olfactory learning success of bees. Our finding proves that dopamine alters olfactory processing and regulates the odor-reward mechanism in associative learning.
2 Materials and methods
2.1 Dopamine and serotonin mediate learning success in appetitive odor conditioning in honeybees
2.1.1 Animals
Newly emerged bees (Apis mellifera) were taken from beehives in the experimental apiary of the College of Animal of sciences, Fujian Agriculture and Forestry University (26°05′9.60″ N 119°14′3.60″ E). Capped combs were obtained from three different healthy colonies and kept in an incubator. The newly emerged bees were collected every day, marked with different colors according to the day of emergence, and placed back into their original colony. After that, 12-days old bees were caught and brought to the laboratory for olfactory PER conditioning.
2.1.2 Olfactory PER conditioning
Bees were harnessed following a standard procedure (Matsumoto et al., 2012) and kept in an incubator at a temperature of 30 °C and relative humidity of 70% (±1, 70%) for one hour. Before conditioning, a drop of 30% (w/v) sugar solution reward was touched with the antennae to check for intact PER. Bees were discarded that did not show the PER. Honeybees were trained to renowned the two odors by using a differential conditioning process, Conditioned stimulus (CS + ) paired with sugar solution 30% and conditioned stimulus (CS-) without reward. The odorants used were 1-nonanol (A) and 1- hexanol (B) (Sigma Aldrich, France). They balanced their role as CS + or CS- so that two groups of bees were conditioned in parallel (A + vs. B- and A- vs. B). Each bee was subjected to three CS + and three CS- trials in a pseudorandom sequence (Matsumoto et al., 2012). Each CS + trial lasted 39 s. First, the individual bee was positioned in front of the olfactometer and clean air was delivered to the antennae for 15 sec. An odorant was then provided in 4 sec. Two sec after odor onset, sucrose solution was given for 2 sec. Therefore, the interstimulus interval was 2 sec and the US and CS ended simultaneously. Finally, clean air was given in the absence of other stimulations during 20 s to complete the 39-s trial. CS- trials followed the same dynamic with the exception that no US was offered during them. The intertrial interval was 10 min.
The PER to each odorant (conditioned response) was recorded during training. Responses were noted as “1″ (full proboscis extension) or ”0″ (no or partial PER). Animals that never responded to the CS + were not considered for the analyses. Learning success was determined based on the response in the last learning trial, i.e., learners were bees that responded correctly to the CS + and not to the CS- at the end of training (Pamir et al., 2011). The absence of discrimination after training (either because of generalization, bees responding to both CS + and the CS-, or because an absence of response to either CS) defined the fail-learners. In addition, we considered responses during acquisition to define a sub-category of learners, which we termed optimal learners. For this analysis, the response to the first CS+/CS− the presentation was not considered as it could only be random in the absence of experience with these odors. Optimal learners were, therefore, twice-responding honeybees to the CS + and did never respond to the CS- in the last four conditioning trials. Two-way ANOVA with repeated measurement was used for olfactory behavior and learning success in olfactory discrimination learning (Fig. 1a, b).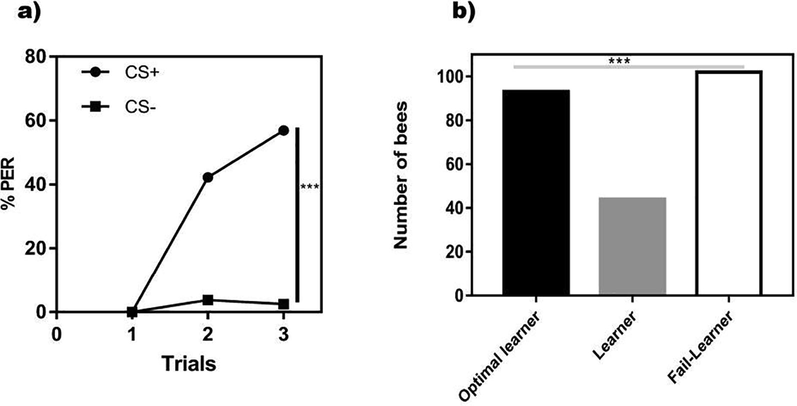
Olfactory learning and learning success in olfactory discrimination learning. a) Population responses of bees (n = 239) trained to discriminate two odorants, 1-nonanol from 1-hexanol, during three rewarded and three non-rewarded trials. No significant differences in performance were found between bees trained with 1-nonanol + vs. 1-hexanol- and bees trained with 1-nonanol- vs. 1-hexanol + so that responses of both groups were pooled and shown as a CS + vs. CS- discrimination. The % of conditioned responses (responses to the trained odorants) as a function of conditioning trials is shown. Discrimination attained at the end of the training was highly significant. b) Number of bees in the three categories defined to account for learning success after the differential conditioning shown in a). Optimal learners were bees responsive two times to the CS + and did never respond to the CS- in the last four conditioning trials. In the last two learning trials, learners were bees that responded successfully to the CS + and not to the CS-. Fail-learners were bees that did not discriminate the CS + from the CS- in the last two conditioning trials. The frequency distribution of bees between these categories varied significantly. ***: P < 0.0001.
2.1.3 Quantification of biogenic amine levels
At the end of conditioning, bees were frozen to death in liquid state nitrogen and kept at −80 °C for subsequent brain dissection and biogenic amines measurement. Brains of learner and fail-learner bees were dissected on a frozen dissecting dish in dry ice under a cold-light source. They remained frozen during the entire dissection procedure. Brains in which lost tissue pieces were discarded so that only intact brains were used for HPLC analyses. Each brain was kept individually in a 1.5 mL centrifuge tube at −80° C until analysis for comparisons of biogenic-amine levels; we randomly chose seven brains of 'optimal learners' and seven brains of 'fail- learners. We measured the concentration of the biogenic amines DA and 5-HT and 3,4-dihydroxyphenylacetic acid (DOPAC) – a DA metabolite - using high-performance liquid chromatography (HPLC) with electrochemical detection, according to Li et al. (2009). Details of the HPLC procedure are provided in the Supplementary Information. A t-test for independent samples was used to check the significance in biogenic amines analysis Fig. 2.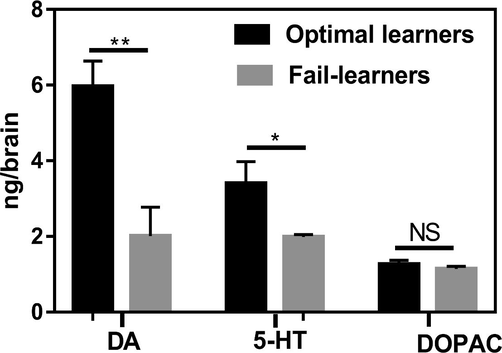
Dopamine (DA), serotonin (5-HT) and DOPAC levels (ng/brain; mean ± S.E.) measured in individual brains of optimal learners (n = 7) and fail-learners (n = 7). DA and 5-HT levels were higher in optimal learners compared to fail-learners. **: P < 0.01; *: P < 0.05. Fig. 2. illustrates that the levels of the substances measured varied between the two categories of bees considered. Levels of DA and 5-HT were higher in optimal learners than in fail-learners (DA; t-test for independent samples: t12 = 3.88, P < 0.01; 5-HT; t12 = 2.43, P < 0.05). No differences were detected for DOPAC (t12 = 0.97, P = 0.35). Thus, efficient, fast learning of the appetitive olfactory discrimination correlated with higher DA and 5-HT in the bee brain.
2.2 Effect of dopamine flupenthixol blocker in olfactory learning of honeybee
2.2.1 Animals
Apis mellifera (newly emerged) bees were collected from the experimental beehive of the College of Animal of sciences, Fujian Agriculture and Forestry University (26°05′9.60″ N 119°14′3.60″ E). Capped combs were collected from three different healthy beehives and kept in an incubator. The newly emerged bees were picked every day, marked with different colors according to the day of emergence, and placed back into their original colony. After that, 12-days old bees were caught and brought to the laboratory for olfactory PER conditioning.
2.2.2 Olfactory PER conditioning
Bees were harnessed following a standard procedure (Matsumoto et al., 2012) and kept in an incubator at a temperature of 30 °C and relative humidity of 70% (±1, 70%) for one hour. Before conditioning, a drop of 30% (w/v) sugar solution was delivered to the antennae to check for intact PER. Bees were discarded that did not show the PER.
Training of bees was performed to differentiate the two odors using a differential conditioning process, Conditioned stimulus (CS + ) linked with sugar solution 30% and conditioned stimulus (CS-) without reward. The odorants used were 1-nonanol (A) and 1- hexanol (B) (Sigma Aldrich, France). They balanced their role as CS + or CS- so that two groups of bees were conditioned in parallel (A + vs. B- and A- vs. B). Each bee was subjected to three CS + and three CS- trials in a pseudorandom sequence (Matsumoto et al., 2012). Each CS + trial lasted 39 s. First, the individual harnessed bee was positioned in front of the olfactometer, and clean air was delivered to the antennae for 15 sec. An odorant was then delivered in 4 sec. Two sec after odor onset, sucrose solution was delivered for 2 sec. Therefore, the interstimulus interval was 2 sec and the US and CS ended at the same time. Finally, clean air was delivered without other stimulations during 20 s to complete the 39-s trial. CS- trials followed the same dynamic except that no US was offered during them. The intertrial interval was 10 min.
The PER to each odorant (conditioned response) was recorded during training. Responses were noted as “1″ (full proboscis extension) or ”0″ (no or partial PER). Animals that never responded to the CS + were not considered for the analyses. Learning success was determined based on the response in the last learning trial, i.e., learners were bees that responded correctly to the conditioned stimulus with reward (CS + ) and not to the conditioned stimulus without reward CS- at the end of training (Roussel et al. 2010, Pamir et al. 2011). The absence of discrimination after training (either because of generalization, bees responding to both CS + and the CS-, or because an absence of response to either CS) defined the fail-learners. In addition, we considered responses during acquisition to define a sub-category of learners, which we termed optimal learners. For this analysis, the response to the first CS+/CS- the presentation was not considered as it could only be random in the absence of experience with these odors. Optimal learners were, therefore, two times responder bees to the CS + and did never respond to the CS- in the last four conditioning trials. To investigate the impact of flupenthixol (dopamine antagonist) on olfactory learning performance of same age bees (12-days bees) in A. mellifera. flupenthixol was injected in to head of bees for 30 min before the start of the learning evaluation (Mustard et al., 2003). Two-way ANOVA was used for group learning curves and pooled learning curves analysis.
3 Results
3.1 Dopamine and serotonin mediate learning success in appetitive odor conditioning in honeybees
3.1.1 Olfactory learning
The two bee groups of trained to differentiate 1-nonanol from 1-hexanol learned the discrimination and responded more to their respective CS + when compared with CS- in the last conditioning trial (ANOVA for repeated measurements; factor CS: 1-nonanol + vs. 1-hexanol -: F1,118 = 93.93; P < 0.0001; 1-hexanol + vs. 1-nonanol -: F1,119 = 162.45; P < 0.0001). The identity of the stimulus with reward (CS + ) and stimulus without reward (CS-) was irrelevant for olfactory cognition success as both groups did not differ from each other (Two-way factor ANOVA for repeated measurements; factor group: F1,237 = 3.25; P = 0.07), which allowed to pool their results. Fig. 1a shows the population learning performance (differentiate between the CS + and the CS-; factor CS: F1,238 = 247.33; P < 0.0001; factor Trial: F2,476 = 180.08; P < 0.0001; interaction CS × Trial: F2,476 = 155.78; P < 0.0001).
As population analyses of learning hide individual differences in learning in the case of a binomial variable such as PER (Gallistel et al., 2004), we analyzed learning success in terms of the three learning categories previously defined: optimal learners, learners and fail-learners (Fig. 1b). Our results confirm that most bees learned the task: 137 bees (i.e., 57.32 %) differentiated the CS + from the CS- in the last learning trial; 102 bees (white bar in Fig. 1b; 42.68 %) did not discriminate both stimuli and were thus considered as fail-learners. From the 137 learners, 93 could be categorized as optimal learners (black bar in Fig. 1b) and 44 as learners (gray bar in Fig. 1b). A r2 analysis showed that the frequency distribution of bees in these categories (93, 44 and 102) differed significantly from a random distribution (r2 = 24.46, df: 2, P < 0.001).
3.1.2 Quantification of biogenic amines
We then analyzed brain levels of the biogenic amines DA and 5-HT and 3,4-dihydroxyphenylacetic acid (DOPAC), a DA metabolite, using HPLC with electrochemical detection(Li et al., 2009). Bees were sacrificed immediately after conditioning to this end. We focused on optimal learners and fail-learners (n = 7 in each case) to accentuate behavioral differences and thus uncover differences in neurotransmitter contents that could appear more clearly in the point of this comparison.
3.2 Effect of dopamine flupenthixol blocker in olfactory learning of honeybee
3.2.1 Odor associating learning trials
To test the hypothesis that dopamine affects the olfactory learning behavior of twelve-day-old bees. In this experiment, we have examined the effect of dopamine antagonist flupenthixol on olfactory learning. The learning curves of control group showed statistically significant difference (Fig. 3), which showed the population learning performance (discrimination between the CS + and the CS-; factor CS: F (1, 858) = 178.3; P < 0.0001; factor Trial: F (2, 858) = 152.9; P < 0.0001; interaction CS × Trial: F (2, 858) = 49.45; P < 0.0001).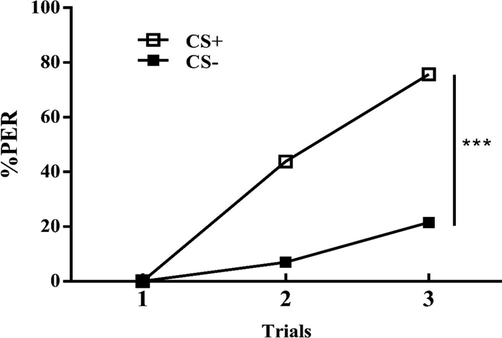
Learning curves of control group bees in an olfactory discrimination learning. Population responses of bees (n = 144) trained to discriminate two odorants, 1-nonanol from 1-hexanol during three rewarded and three non-rewarded trials and responses of both groups were pooled and revealed as a CS + vs. CS- discrimination. Discrimination attained after the training was highly significant.
The learning curves of dopamine injected group (flupenthixol) also showed significant difference during learning trials (Fig. 4.), which showed the population learning performance (differentiation between the CS + and the CS-; factor CS: F (2, 744) = 33.42; P < 0.0001; factor Trial: F (1, 744) = 21.1; P < 0.0001; interaction CS × Trial: F (2, 744) = 7.246P = 0.0008).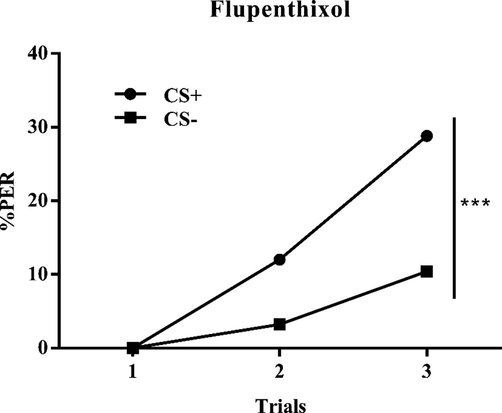
Learning curves of dopamine antagonist group bees in an olfactory discrimination learning. Population responses of bees (n = 125) trained to discriminate two odorants, 1-nonanol from 1-hexanol during three rewarded and three non-rewarded trials and responses of both groups were pooled and shown as a CS + vs. CS- discrimination. End-of-training, discrimination was highly significant.
As population analyses of learning hide individual differences in learning in the case of a binomial variable such as PER (Gallistel et al., 2004), we analyzed learning success in terms of the three learning categories previously defined: optimal learners, learners and fail-learners (Fig. 5). Our results confirm that most bees learned the task in the control group: 91 bees (i.e., 63.19 %) differentiated the CS + from the CS- in the last conditioning trial, 53 bees (36.80 %) did not discriminate both stimuli and were thus considered as fail-learners.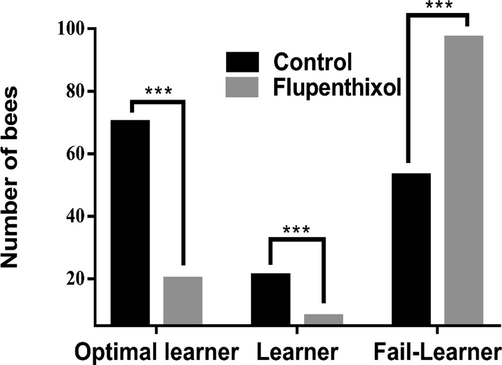
Olfactory learning performance between control and flupenthixol injected groups.
From the 91 learners, 70 could be categorized as optimal learners and 21 as learners. Our results confirm that in dopamine injected group, most bees learned the task: 28 bees (22.40 %) discriminated the CS + from the CS- in the last learning trial; 97 bees (77.60 %) did not discriminate both stimuli and were thus considered as fail-learners. From the 28 learners, 20 could be categorized as optimal learners and eight as learners.
4 Discussion
Our results show that BA, dopamine and serotonin levels are significantly elevated in the brain of optimal learners after appetitive olfactory discrimination involving a rewarded and a non-rewarded odor. These results are innovative as the traditional view of honey bee learning has related learning success to octopamine (OA) signaling, which regulates the reinforcing characteristics of sugar solution in appetitive odor conditioning (Mizunami et al., 2009, Mizunami and Matsumoto, 2010). On the contrary, DA has been relatively associated with aversive learning the bee brain (Marchal et al., 2019), so the higher levels found in our work after successful appetitive learning was unexpected. Less is known about the incidence of 5-HT on olfactory conditioning. The resolution of our measurement's techniques did not allow us to quantify OA levels reliably so that we cannot exclude that OA levels were also higher in the brain of optimal learners after our conditioning procedure. Yet, the question remains of why DA and 5-HT levels were also elevated compared to fail-learners. To reconcile the opposite views referred to the role of DA in the bee brain provided by prior works (Marchal et al., 2019) and the present one, we suggest that besides dopaminergic neurons conveying aversive signaling in the bee brain, an additional class of such neurons exist that mediate attentional processes, and thus facilitate learning (Wissink and Nehring, 2021). This would explain why optimal learners have higher DA levels, consistently with more attention to the discrimination problem. This hypothesis is supported by the demonstration of attentional procedures, like those defined in vertebrates and especially insects (Miller et al., 2011; Van Swinderen and Andretic, 2011). Transient attenuation release of dopamine in fly mutants attenuates the 20–30 Hz responsiveness to the object to be attended, and oral injection of methamphetamine, which helps to boost the dopamine release, improves this sensitivity (Andretic et al., 2005). Therefore, the higher levels of DA in the brain of optimal learners may reveal that their attentional processes were more efficient, thus leading to better discrimination learning.
The case of 5-HT may be different as various works have reported that 5-HT may exert an inhibitory effect on responsiveness in honey bees. In complete PER conditioning, in which bees learn the association of a single odor with reward, 5-HT injection reduces the achievement and recovery of sensory cognition(Menzel et al., 1999). An impairment function of serotonin signaling was recorded in different of PER learning inducing to latent inhibition, i.e. impairment in learning performance as a consequence of a non-reinforced pre-exposure to the smell to be conditioned (Fernández et al., 2012). In this situation, increased 5-HT levels were linked to latent inhibition., subsequent from repeated exposure of unrewarded CS (Fernández et al., 2012). In the differential-conditioning protocol used in our work, both rewarding and non-rewarding odor experiences had to be learned so that bees established an excitatory olfactory memory trace for the CS + and an inhibitory olfactory memory trace for the CS-. Higher levels of 5-HT in optimal learners may thus reflect the efficiency of the inhibitory processes leading to the suppression of PER responses to the CS-, which is necessary for discrimination learning. This inhibition would not be required for absolute conditioning where 5-HT would affect the only memory trace established. This interpretation is consistent with the fact that 5-HT application into the ipsilateral visual areas in the brain of bee induces long-lasting and immediate reduces the sensitivity and motion of visual antennal reflex, a common and unique movement of antennal sensitivity to both upward downward movement (stripe pattern), when the ipsilateral compound eye is stimulated. Consequently, 5-HT decreases the stripe pattern movement and background activity by lobula neurons. Phototactic sensitivity is strongly decreased by 5-HT then recovered by offering bees a mixture of Am5-HT1A receptor antagonist (prazosin) and 5-HT for two days (Thamm et al., 2010). Our first behavioral experiment showed that dopamine played an important in the olfactory learning performance of same-age bees. This study was performed to confirm dopamine's role in olfactory learning by injecting the dopamine blocker antagonists flupenthixol. We evaluated the olfactory learning performance through olfactory learning trials to discriminate the odors by proboscis extension response in twelve-day-old bees. Insects prefer to remember reward or favorable experiences and regulate the subsequent behavior depending on memory formation. Appetitive classical conditioning is the most preferred protocol to evaluate learning and memory in animals (Ichikawa et al., 2003). Our finding suggested that the dopamine blocker effect on olfactory learning performance was shown in control and flupenthixol injected groups of bees. These findings are the unconventional view of bees learning has associated with successful learning to dopamine, which supports the learning properties of odor conditioning and sucrose solution. Drosophila melanogaster (fruit fly), dopamine-mediated olfactory learning, and food reward (Giurfa, 2013). For successful olfactory conditioning, antennae are critically important in harnessed bees (Hori et al., 2006). The antennae interfere as a sensory input between sucrose reward and odor. Indeed, antennae are major organs in harnessed bees as they play a role in reward perception, olfactory sensation and gustatory (Goodman, 2003). In these olfactory learning trials, bees were trained with sucrose reward and without reward. The percentage of learning performance with sucrose rewarded was significantly higher than without reward. Sucrose is a major constituent and is preferred by bees as a reward during foraging and laboratory tests (Liao et al., 2017). The greatest sucrose preference is considered the strongest reward for feeding or eliciting PER when contacted with proboscis or antennae during the experiment (Dähn, 2020). Earlier findings reported that bees learned with a high reward concentration compared to low sucrose concentration without reward (Simcock et al., 2018). During learning trials, fruit flies learned quickly and responded with sucrose and odor pairing because sweet in taste contributes to learning and memory (Colomb et al., 2009). Thum andreas et al. (2007) reported that the fruitfly Drosophila made some learning and memory traces for future reward and learned quickly using appetitive olfactory learning (Thum et al., 2007). The desert ant Cataglyphis fortis also learns very quickly with the association of odor and sucrose reward and can remember more than 26 days (Huber and Knaden, 2018). Another study reported that ants learn quickly if associated with olfactory learning and don't forget (Piqueret et al., 2019). The olfactory learning behavior was performed on harnessed bees and provided sucrose solution (US) immediately after odor (CS) exposure. Bees showed PER after a certain time duration (interval); the efficacy of learning is evaluated by odor (CS) exposure. If sensitivity to CS (odor) alone now stimulates PER while testing the bees, that indicates that bees learned olfactory learning with reward and odor. Different appetitive methods have developed for other animals depending upon the characteristics of respective insects (Mizunami et al., 2009). Dopamine neurotransmitters in the brain are considered to play a significant role in mediating appetite reinforcement in mammals (Schultz, 2006). Brembs et al. (2002), also reported the crucial role of dopamine in mollusks (Brembs et al., 2002). The dopamine reconciled the reinforcement signals for appetitive learning in crickets and suggested that dopamine participates in honeybees' appetitive learning. In the differential-conditioning protocol used in my experiment, both rewarding and non-rewarding odor experiences had to be learned so that bees established an excitatory olfactory memory trace for the CS + and an inhibitory olfactory memory trace for the CS-. The scenario emerging from our study indicates that depressing DA levels before conditioning should lead to deficits in learning performance, particularly for differential appetitive conditioning, which requires higher levels of attention to achieve the discrimination between a rewarded and a non-rewarded stimulus (Giurfa, 2004). Diminishing 5-HT levels before conditioning would have a rather selective effect on differential but not on absolute conditioning due to the necessity of establishing an inhibitory memory trace in the former but not in the latter.
5 Conclusion
This study determined whether biogenic amines could modulate the olfactory learning behavior and modulate the learning success after learning trials. Our results reported strong evidence for the role of biogenic amines on the olfactory learning behavior of honeybees and finding the connection between biogenic amines and learning success. This study also illustrated the effect of dopamine blockers on olfactory learning behavior to validate the role of dopamine. The dopamine and serotonin level are significantly linked with learning success. Our finding provides a better overview and uncovers the neurochemical mechanism driving the olfactory behavior in eusocial insects.
Acknowledgements
The authors are thankful to Modern Agro-industry Technology Research System (No. CARS-44-KXJ4), National Natural Science Foundation of China (31772684; 31702192). The authors extend their appreciation to the Researchers Supporting Project number (RSP-2021/306), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Dopaminergic modulation of arousal in Drosophila. Curr. Biol.. 2005;15(13):1165-1175.
- [CrossRef] [Google Scholar]
- Operant reward learning in aplysia: neuronal correlates and mechanisms. Science. 2002;296(5573):1706-1709.
- [Google Scholar]
- Parametric and genetic analysis of drosophila appetitive long-term memory and sugar motivation. Genes Brain.. 2009;8(4):407-415.
- [Google Scholar]
- Olfactory modulation by dopamine in the context of aversive learning. J. Neurophysiol.. 2012;108(2):539-550.
- [Google Scholar]
- Dähn, S., 2020. Die auswirkungen des neonicotinoids thiamethoxam auf den glucosemetabolismus der honigbiene (Apis mellifera). Thesis Doctoral.
- Denton, J.A., Koludarov, I., Thompson, M., Bryk, J., Velasque, M.J.b., 2021. Apis mellifera cognition as a tool for scientific engagement.bioRxiv.
- Even, N., Devaud, J.-M., Barron, A., 2012. General stress responses in the honey bee. 3(4):1271-98.
- Latent inhibition in an insect: The role of aminergic signaling. Learn Mem.. 2012;19(12):593-597.
- [Google Scholar]
- The learning curve: implications of a quantitative analysis. Proc. Natl. Acad. Sci. U.S.A.. 2004;101(36):13124-13131.
- [CrossRef] [Google Scholar]
- Conditioning procedure and color discrimination in the honeybee Apis mellifera. Naturwissenschaften.. 2004;91(5):228-231.
- [CrossRef] [Google Scholar]
- Giurfa, M., 2013. Cognition with few neurons: Higher-order learning in insects. Trends Neurosci. 36(5):285-94. https://doi.org/pii: S0166-2236(13)00003-9.
- Form and function in the honey bee. International bee research association; 2003.
- Associative visual learning, color discrimination, and chromatic adaptation in the harnessed honeybee Apis mellifera l. J. Compar. Physiol. A. 2006;192(7):691-700.
- [Google Scholar]
- Desert ants possess distinct memories for food and nest odors. Proc. Natl. Acad. Sci.. 2018;115(41):10470-10474.
- [Google Scholar]
- Sweet taste and nutrient value subdivide rewarding dopaminergic neurons in Drosophila. Curr Biol.. 2015;25(6):751-758.
- [CrossRef] [Google Scholar]
- Importance of social stimuli for the development of learning capability in honeybees. Appl. Entomol.. 2003;38(2):203-209.
- [Google Scholar]
- Determination of monoamine neurotransmitters and their metabolites in a mouse brain microdialysate by coupling high-performance liquid chromatography with gold nanoparticle-initiated chemiluminescence. Anal Chim. Acta. 2009;645(1–2):48-55.
- [CrossRef] [Google Scholar]
- Behavioral responses of honey bees (Apis mellifera) to natural and synthetic xenobiotics in food. Sci. Rep.. 2017;7(1):1-8.
- [Google Scholar]
- Inhibitory learning of phototaxis by honeybees in a passive-avoidance task. Learn Mem.. 2019;26(10):412-423.
- [CrossRef] [Google Scholar]
- Revisiting olfactory classical conditioning of the proboscis extension response in honey bees: a step towards standardized procedures. J. Neurosci. Meths. 2012;211(1):159-167.
- [Google Scholar]
- Signaling pathways for long-term memory formation in the cricket. Front. Psychol.. 2018;9:1014.
- [CrossRef] [Google Scholar]
- Pharmacological dissociation between the reinforcing, sensitizing, and response-releasing functions of reward in honeybee classical conditioning. Behav. Neurosci.. 1999;113(4):744.
- [Google Scholar]
- Attentional switching in humans and flies: rivalry in large and miniature brains. Front. Hum. Neurosci.. 2011;18(5):188.
- [CrossRef] [Google Scholar]
- Roles of aminergic neurons in formation and recall of associative memory in crickets. Front. Behav. Neurosci.. 2010;4:172.
- [CrossRef] [Google Scholar]
- Roles of octopaminergic and dopaminergic neurons in appetitive and aversive memory recall in an insect. BMC Biol.. 2009;7(1):1-6.
- [CrossRef] [Google Scholar]
- Mustard, J.A., Blenau, W., Hamilton, I.S., Ward, V.K., Ebert, P.R., Mercer, A., 2003. Analysis of two d1-like dopamine receptors from the honey bee Apis mellifera reveals agonist-independent activity.113(1-2):67-77.
- Average group behavior does not represent individual behavior in classical conditioning of the honeybee. Learn Mem.. 2011;18(11):733-741.
- [CrossRef] [Google Scholar]
- Ants learn fast and do not forget: associative olfactory learning, memory and extinction in formica fusca. R. Soc. Open Sci.. 2019;6(6):190778
- [Google Scholar]
- Raza, M., Su, S., 2020. Differential roles for dopamine d1-like and d2-like receptors in learning and behavior of honeybee and other insects. 18(1):1317-27.
- Raza, M., Li, Z., Rizwan, M., Aqai Kalan, H., Su, S.J.A.E., Research, E., 2019. Comparison of learning and memory of eastern (Apis cerana cerana) and western honey bees (Apis mellifera l.).17(2):4971-84.
- Rudy, J.W., 2008. The neurobiology of learning and memory. Sunderland, Massachusetts, Sinauer Ass. 233(4767):941-7.
- Behavioral and neurophysiological study of olfactory perception and learning in honeybees. Front. Syst. Neurosci.. 2011;5:98.
- [CrossRef] [Google Scholar]
- Behavioral theories and the neurophysiology of reward. Ann. Rev. Psys.. 2006;23(33):10495-10502.
- [Google Scholar]
- Appetitive olfactory learning and memory in the honeybee depend on sugar reward identity. J. Ins. Phy.. 2018;106:71-77.
- [Google Scholar]
- Biogenic amines and neuromodulation of animal behavior. Front. Syst. Neuro. 2018;13(12):31.
- [Google Scholar]
- Characterization of the 5-ht1a receptor of the honeybee (Apis mellifera) and involvement of serotonin in phototactic behavior. Cell Mol. Life Sci.. 2010;67(14):2467-2479.
- [CrossRef] [Google Scholar]
- Multiple memory traces for olfactory reward learning in Drosophila. J. Neuro. 2007;27(41):11132-11138.
- [Google Scholar]
- Dopamine in Drosophila: Setting arousal thresholds in a miniature brain. Proc. R. Soc. B. 2011;278(1707):906-913.
- [CrossRef] [Google Scholar]
- Wissink, M., Nehring, V., 2021. Appetitive olfactory learning suffers in ants when octopamine or dopamine receptors are blocked.224(15):jeb242732.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.101928.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







