Translate this page into:
Bioactive compounds from Nauclea latifolia leaf extracts
⁎Corresponding author. marogba@oauife.edu.ng (M.A. Aderogba)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The leaf extracts of Nauclea latifolia (Smith) were investigated for its bioactive constituents to validate it reported ethnomedicinal uses (infectious and oxidative stress related diseases). Isolation of antioxidant compounds from the ethyl acetate fraction was achieved by a DPPH directed fractionation, which resulted in trans-3,4-dihydroxycinnamic acid (caffeic acid) (1), quercetin (2), quercetin-3-O-β-glycopyranoside (3), 3-caffeoylquinic acid (chlorogenic acid) (4), and 3,5-O-dicaffeoylquinic acid (5). The extracts and isolated compounds were evaluated for antioxidant activities using five complementary assays: total antioxidant capacity (TAC), inhibition of nitric oxide (NO) radical activity, 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity, ferric reducing antioxidant potential (FRAP) and metal chelating ability (MCA) spectrophotometrically. The total phenolic content (TPC) was also determined. Antimicrobial activities of the samples were evaluated using agar cup plate diffusion and broth microdilution method. Compound (2) was more potent in the DPPH assay [IC50 = 0.59 ± 0.04 to 21.3 ± 1.01 µg/mL] than the ascorbic acid [8.82 ± 0.23 µg/mL] used as a standard. All the isolated compounds exhibited a higher degree of inhibition of NO radical than the standard. The IC50 ranged from 5.17 ± 2.59 to 55.2 ± 0.66 µg/mL compared with the standard, ascorbic acid [IC50 = 69.3 ± 1.78 µg/mL]. In the metal chelating ability (MCA) assay, compound (5) had better activity [IC50 = 105 ± 2.73 µg/mL] than the standard, EDTA [IC50 = 122 ± 1.17 µg/mL]. Compound (2) had the best TAC and FRAP results, 10.9 ± 2.14 mg AAE/g and 1.68 ± 0.09 mg AAE/g respectively. N. latifolia extracts exhibited less antibacterial activities than the isolated compounds. The MIC of quercetin (2) against S. aureus was 0.156 mg/mL while quercetin 3-O-β-glucopyranose exhibited wider spectrum of activities with MIC in the range of 1 mg/mL for most of the bacteria strains. Also, 3,5-O-dicaffeoylquinic acid had an MIC of 1.25 mg/mL against P. aeruginosa. Compounds 4 and 5 are reported from N. latifolia for the first time.
This study concluded that some of the isolated compounds had strong antioxidant and moderate antimicrobial activities.
Keywords
Nauclea latifolia
Antimicrobial activity
Antioxidant activity
Caffeic acid
1 Introduction
Nauclea latifolia belongs to the family Rubiaceae. It is used in the treatment of a number of diseases (Okwori et al., 2008). The stem bark is used for the management of toothache, dental caries, septic mouth, malaria, diarrhea and dysentery (Nworgu et al., 2008; Okiei et al., 2011). The leaf decoction is used as a remedy for diabetes mellitus in Northern Nigeria while the bark extract has been reported effective in the treatment of wounds, coughs and gonorrhoea (Gidado et al., 2005; Madibunyi, 1995). Akubue and Mittal (1982) reported the use of the roots for the treatment of hypertension. N. latifolia extracts demonstrated antimicrobial properties against Klebsiella pneumoniae (Tona et al., 1999) and a number of other organisms like Bacillus subtilis, Escherichia coli, Salmonella enteritidis, Pseudomonas aeruginosa, (Omer et al., 1998; Hussain and Deeni, 1991). The fruit extract exhibited activity against Human Immunodeficiency Virus (Hussein et al., 1999). Nauclefine and naucletine were reported as alkaloids from the plant (Karou et al., 2011). Nauclefolinine and five known triterpenoids were also reported from the roots of N. latifolia (Ngnokam et al., 2003). The plant extracts are used in the management of infectious and oxidative stress related diseases (Gidado et al., 2005; Okiei et al., 2011). Some of the activities exhibited by N. latifolia extracts suggest the presence of antioxidant and antimicrobial components in the extracts. In other to validate this, we have investigated Nauclea latifolia leaf extracts for its antioxidant and antimicrobial secondary metabolites. We report here, the first detailed report on N. latifolia linking its secondary metabolites to its biological activities (Fig. 1).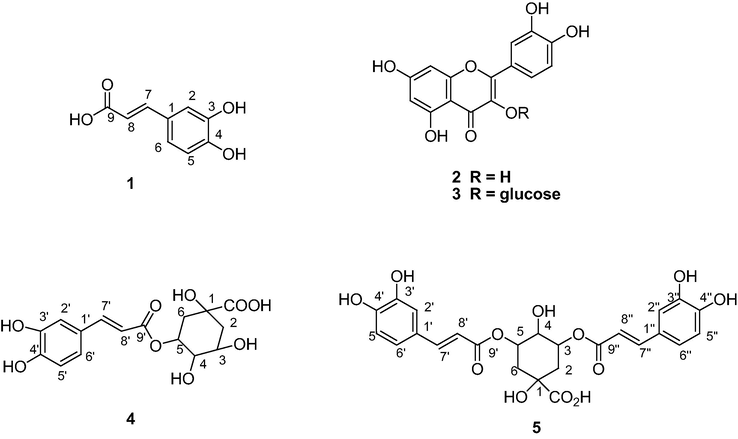
Structures of compounds (1–5) Isolated from Nauclea latifolio Leaves Extract.
2 Materials and methods
2.1 General
Stationary phase for column chromatography was either Silica gel (Merck, Germany, 0.04–0.063 mm, 230–400 mesh) or Sephadex LH-20 (Sigma-Aldrich). Thin Layer Chromatography (TLC) technique at room temperature was achieved using pre-coated plates (MERCK, silica gel 60 F254 0.2 mm) for the analyses samples. Ultraviolet lamp (254 and 366 nm) was used for spots detection. Varian (200 MHz) or Brucker (300 MHz) NMR spectra data in which the chemical shifts were determined in parts per million (ppm) were used for compounds identification. Mass spectrum (TOF MS EI+) was recorded using Waters GCT-Premier Mass Spectrometer.
2.2 Plant material
The leaves of Nauclea latifolia (Smith) were collected in June 2008, along Ile-Ife/Ilesa Bye pass, Ipetumodu, Osun State, Nigeria. Mr. G. Ibheneshbor of the IFE Herbarium, Obafemi Awolowo University, Ile-Ife, Nigeria, authenticated the plant and voucher specimen (IFE 17299) was deposited. Air-dried leaves were pulverized after two weeks.
2.2.1 Extraction of plant material
N. latifolia powdered leaves (1.0 kg) was extracted for 24 h using 80% methanol (10 L) at room temperature. Concentration of the filtrate on a rotary evaporator (Heldolph, Germany) yielded the crude extract (97 g, 9.7%). This was suspended in distilled water and partitioned successively with four solvents of varying polarities. Concentration of each of the solvent fractions afforded four solvent fractions: n-hexane (HEX), dichloromethane (DCM), ethyl acetate (EtOAc) and n-butanol (BUOH) extract respectively.
2.2.2 Qualitative antioxidant screening
Antioxidant screening of each of the solvent fractions obtained from N. latifolia was achieved by the thin layer chromatography as described by Aderogba et al. (2011). Ten μL of each sample was spotted on silica gel TLC plate. The plate was developed in ethyl acetate/methanol/water (5:0.8:0.5) and subsequently sprayed with 1,1-diphenyl-2-picrylhydrazyl (DPPH) 0.02% in MeOH (w/v) for detection of antioxidant constituents in the extracts. Bioactive compounds appeared as yellow patch on the violet background.
2.3 Isolation of antioxidant compounds from N. latifolia extract
The results of the antioxidant screening showed that the ethyl acetate fraction contained more antioxidant compounds than the remaining fractions. It was selected for isolation of its bioactive constituents.
2.3.1 Fractionation of N. latifolia ethyl acetate fraction
The sample (5.52 g) was fractionated on a silica gel column (7 cm internal diameter and 45 cm length). The column was eluted with n-hexane followed by n-hexane/ethyl acetate solvent mixture with an increasing gradient of ethyl acetate from 5% up to 100%. This was followed by ethyl acetate/methanol solvent mixture with an increasing gradient of methanol from 5% up to 100%. Flask fractions (100 mL each) were collected and analysed on TLC plates developed in (CHCl3: MeOH 9:1) solvent mixture. This afforded six fractions (1A-1F). Fraction 1D (2.34 g) was fractionated on a silica gel column (4 cm i.d., 60 cm length) using n-hexane/chloroform solvent mixture with an increasing gradient of chloroform from 5% up to 100%. This was followed with chloroform/methanol solvent mixture with an increasing gradient of methanol from 10% up to 100%. Fractions (15 mL each) collected were analysed on TLC plates using (CHCl3:MeOH, 1:1) solvent system. This yielded 15 subfractions (2A-2O). Fraction 2 M, (59 mg) was purified on a silica gel column using varying gradient of toluene and ethanol as solvent mixture. Analysis of the samples (15 mL each) collected gave fractions 3A – 3C. Purification of 3C (16 mg) on a Sephadex LH-20 column (4 cm i.d., 60 cm length) using toluene/ethanol (2:3) yielded compound 1 (9 mg). Fraction 2E (1.04 g) was purified on a Sephadex LH-20 column starting with toluene/ethanol (4:1) solvent system followed by an increasing gradient of ethanol up to 100%. Analysis of the samples collected on TLC plates gave compound 2 (163 mg, m.p. 212–214 °C, Rf 0.63 in chloroform/ethyl acetate (1:1). Fraction 2 K (125 mg) was subjected to Sephadex LH-20 column fractionation (4 cm i.d., 60 cm length) run with CHCl3/MeOH (1:1) solvent mixture. Samples collected were analysed on TLC plates using CHCl3/MeOH (1:1). Six fractions 3A-3F were obtained. Subfraction 3B (50 mg) was purified using CHCl3/MeOH (1:1) solvent mixture on a Sephadex LH-20 column. The column was isocratically eluted with CHCl3/MeOH (1:1). TLC analysis using CHCl3/MeOH (1:1) of the samples collected yielded compounds 3 (6 mg) and 4 (2.6 mg). Fraction 2O (23.7 mg) was purified using 100% MeOH on a Sephadex LH-20 column. Samples collected were analysed using CHCl3/MeOH (1:1) on TLC plates which afforded compound 5 (3.2 mg).
2.4 Quantitative antioxidant activity
N. latifolia crude extract and four solvent fractions along with the isolated compounds (1–5) were assayed for antioxidant activity using five antioxidant assays. In addition, total phenolic content (TPC) of the samples was also evaluated.
2.4.1 Total phenolic content (TPC)
The total phenolic content of each of the N. latifolia samples was determined as described by Singleton and Rossi (1965) and modified by Gulcin et al. (2003) using the Folin-Ciocalteu’s phenol reagent.
2.4.2 Total antioxidant capacity (TAC)
N. latifolia samples were evaluated for total antioxidant capacity as described by Prieto et al. (1999). It is determined by the extracts reduction of Molybdenum (VI) to Molybdenum (V). At an acidic pH, a green phosphate/molybdenum (V) complex is formed.
2.4.3 Assessment of DPPH free scavenging ability
The radical scavenging ability of each of the N. latifolia extracts and isolated compounds was determined as described by Brand-Williams et al. (1995).
2.4.4 Inhibition of nitric oxide radical
N. latifolia extracts and isolated secondary metabolites were evaluated for nitric oxide radical inhibition activity according to the method of Green et al. (1982) as described by Marcocci et al. (1994).
2.4.5 Ferric reducing antioxidant power (FRAP)
The FRAP assay was carried out in this study as described by Benzie and Strain (1999). It uses antioxidants as reductants in a redox-linked colorimetric method with absorbance measured with a spectrophotometer.
2.4.6 Metal chelating ability (ferrous ion-chelating ability) assay (MCA)
The ferrous ion-chelating (FIC) assay was carried out on N. latifolia samples as previously described (Singh and Rajini, 2004) with some modifications.
2.5 Antimicrobial assay
N. latifolia crude extract and solvent fractions were evaluated for antibacterial and antifungal activities using the agar cup-plate method as described in Ajileye et al. (2015). Antibacterial activities of the isolated secondary metabolites were evaluated by the microbroth dilution method as previously described by Zgoda and Porter (2001) and used by Ajileye et al. (2015). Streptomycin (Shijiazhuang, China) was used as a positive control at 1000–3.90625 µg/mL concentrations range. Microorganisms selected for this study were of local importance. The strains were obtained from stocks of culture collections maintained in the Pharmaceutical Microbiology Laboratory, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, Nigeria.
Microbial growth was determined as described by Ajileye et al. (2015). In this study, minimum inhibitory concentration (MIC) was defined as the lowest concentration of each of the N. latifolia samples that inhibited the growth of bacteria. Minimum bactericidal concentration (MBC) of the samples was determined by plating drops from sample concentrations that showed inhibition of growth on freshly prepared Nutrient Agar plates. At 37 °C, the lowest concentration at which growth could not be recovered, after 24 h incubation was taken as the MBC.
2.5.1 Test organisms
The strains of microorganisms used include reference and clinical isolates comprising of Gram- positive and Gram-negative bacteria and fungi strains. The Gram-negative bacteria used were Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, while the Gram-positive strains include Staphylococcus aureus ATCC 29213, Bacillus subtilis NCIB 3610, Staphylococcus aureus (MRSA) ATCC R 43300. Bacteria were maintained both in cryopreservation medium and on nutrient agar slants and fungi were maintained on Sabouraud Dextrose Agar slants at 4 °C and subcultured monthly.
3 Results and discussion
3.1 Structure elucidation of isolated compounds
A bioactivity directed fractionation was used to target the antioxidative and antimicrobial constituents of the EtOAc fraction of N. latifolia leaf extracts. Five compounds (1–5) were isolated and characterized using mass spectrometry and NMR (1D and 2D) data.
3.1.1 Characterization of Compound 1
Compound 1 (9 mg) obtained as grayish white solid. Its mass spectrum (TOF-MS-EI+) showed the molecular ion peak as the base peak at m/z = 180.0428 [M∙+], consistent with the molecular formula C9H8O2. Compound 1 was identified as caffeic acid, trans-3,4-dihydroxycinnamic acid from the NMR data. The spectroscopic data obtained are in good agreement with the literature (Lee et al., 2010), Table 2.
3.1.2 Characterization of Compound 2
Compound 2 (163 mg) was isolated as yellow powder. Its mass spectrum (TOF MS EI + ) showed the exact molecular ion peak as the base peak at m/z = 302.0428, [M+]. This is consistent with the molecular formula C15H10O7. Comparison of the NMR data with the literature identified compound 2 as a flavonol, quercetin (Boligon et al., 2009), Table 1. Compound 1 (NMR 200 MHz), Compounds 4 (NMR 300 MHz) and 5 (NMR 600 MHz).
Compound 2(d6-acetone)
Compound 3 (CD3OD)
Position
δH
J (Hz)
δC
δH
J (Hz)
δC
2
146.9
159.1
3
136.8
135.7
4
176.6
179.6
5
12.17 s
162.2
163.2
6
6.26 d,
(2.0)
99.1
6.21 d,
(1.8)
99.9
7
164.9
166.2
8
6.52 d,
(2.0)
94.5
6.39 d,
(1.8)
94.8
9
157.9
158.6
10
104.2
105.8
1ʹ
123.8
123.2
2ʹ
7.81 d,
(2.1)
115.8
7.71 d,
(2.0)
117.7
3ʹ
145.8
146.0
4ʹ
148.3
149.9
5ʹ
7.00 d,
(8.5)
116.2
6.88 d, (8.7)
116.2
6ʹ
7.70 dd,
(8.5, 2.1)
121.5
7.61 dd,
(8.7, 2.1)
123.3
1ʹʹ
5.27
(7.2)
104.4
2ʹʹ
75.8
3ʹʹ
78.4
4ʹʹ
71.3
5ʹʹ
78.5
6ʹʹ
62.9
Compound 1
Compound 4
Compound 5
Position
δH J (Hz)
δc
δH J (Hz)
δC
δH J (Hz)
δC
1
127.8
77.9
77.8
2
7.16 d, (2.2)
115.7
1.98–2.18 m,
39.1
39.0
3
145.9
4.10 (3.03)
73.2
5.12
77.4
4
146.3
3.71 (3.2, 9.9)
75.1
4.14
69.4
5
6.89 d, (8.2)
115.2
5.41
72.7
5.71
70.9
6
7.03 dd, (8.2, 2.2)
122.5
2.0
40.8
41.1
7
7.58 d, (16.0)
148.6
181.1
180.7
8
6.31 d, (16.0)
115.9
9
168.1
1ʹ
127.9
127.7
2ʹ
7.05 d, (1.8)
115.6
7.01 d, (1.9)
115.2
3ʹ
146.9
146.8
4ʹ
149.7
149.7
5ʹ
6.79 d, (8.1)
115.2
6.74 d, (8.0)
116.6
6ʹ
6.96 dd, (8.1, 1.8)
123.0
6.90 dd, (8.0, 1.9)
123.2
7ʹ
7.60 d, (15.9)
146.9
7.60 d, (15.8)
147.5
8ʹ
6.32 d, (15.9)
116.6
6.28 d, (15.8)
115.0
9ʹ
169.3
168.7
1ʹʹ
127.8
2ʹʹ
7.01 d, (1.9)
115.2
3ʹʹ
146.8
4ʹʹ
149.7
5ʹʹ
6.74 dd, (8.0)
116.6
6ʹʹ
6.90 (8.0, 1.9)
123.2
7ʹʹ
7.52 (15.9)
147.6
8ʹʹ
6.20 (15.8)
115.0
9ʹʹ
168.8
3.1.3 Characterization of Compound 3
Compound 3 (6 mg) was obtained as yellow powder. It showed a molecular ion of the aglycone as the base peak at m/z = 302.0445 [M+] which is consistent with the molecular formula C15H10O7. Compound 3 was identified as quercetin-3-O-β-glucopyranoside (isoquercitrin) from the spectra data and the literature, (Guvenalp and Demirezer, 2005; Liu et al., 2008), Table 1.
3.1.4 Characterization of Compound 4
Compound 4 was isolated as yellow powder. The 1H NMR data showed the presence of caffeoyl and quinic acid groups. The point of attachment of the caffeoyl and quinic acid groups was established by the correlation (HMBC) peak between the H-3 (1H, 5.38, m) of the quinic acid and the C⚌O (δC 169.3) carbon of C-9ʹ of caffeic acid. Compound 4 was identified as 3-caffeoylquinic acid (chlorogenic acid) by comparison of its spectra data with the literature. (Satake et al., 2007; Ahmad, 2010; Agbo et al., 2014), Table 2.
3.1.5 Characterization of Compound 5
Compound 5 (3.2 mg) was obtained as off white powder. Its structure was elucidated by the comparison of its NMR data with that of Compound 4. The proton signals in compound 5 were similar to the signals of compound 4 and there is an additional caffeoyl ester group signals attached to the quinic acid moiety. Based on the NMR data obtained and comparison with the literature (Lee et al., 2010; Peng et al., 2000), the structure was established as 3,5-O-dicaffeoylquinic acid, Table 2.
3.2 Antibacterial activities
Isolated secondary metabolites (1–5) from N. latifolia in this study were either flavonoid or phenolic acid. Various biological activities have been associated with these groups of secondary metabolites including anticancer, antimicrobial, anti-inflammatory, and antioxidant (Alcaraz et al., 2000; Pegnyemb et al., 2005).
The results obtained from antimicrobial tests are presented in Tables 3 and 4. The degree of antimicrobial activities of flavonoids and other phenolic compounds have been linked with the number of free phenolic hydroxyl groups in each molecule. The results obtained for the isolated compounds (1–5) from N. latifolia are in line with earlier studies, (Alcaraz et al., 2000, Aderogba et al, 2011). Diameter of zone of inhibition of streptomycin (1 mg/mL) for each bacteria was: E. coli ATCC 25922, 14.0 mm; P. aeruginosa ATCC 27853, 14.0 mm; S. aureus ATCC 29213, 14.0 mm; B. subtilis NCIB 3610, 10.0 mm. Acriflavin (6.3 mg/mL) inhibition for the fungi was: C. albicans, 18.0 mm, C. pseudotropicalis 21.0 mm. Extracts and fractions showed no inhibitory effect against the fungi strains, C. albicans and C. pseudotropicalis tested. **Zone of inhibition less cup size.
Agent
Organisms
Concentration (mg/mL.)
Diameter of Zone of Inhibition (mm)**
MIC (mg/mL)
Crude
B. subtilis NCIB 3610
40.0
6.0
40.0
n-Hexane
B. subtilis NCIB 3610
10
6
6.60
20
16
40
26
E. coli ATCC 25922
20
9
4.15
40
13
P. aeruginosa ATCC 27853
5
6
3.65
10
8
20
18
40
26
S. aureus (MRSA) ATCC R 43300
5
2
5.00
10
11
20
15
40
31
DCM
B. subtilis NCIB 3610
10.0–20.0
7.0
0.870
40.0
11.0
E. coli ATCC 25922
10
3.0
2.50
20
5.0
40
6.0
P. aeruginosa ATCC 27853
10
23.0
0.250
20
29.0
40
31.0
S. aureus (MRSA) ATCC R 43300
5
6.0
3.80
10
9.0
20
15.0
40
27.0
Ethyl acetate
B. subtilis NCIB 3610
20
3.0
15.10
40
11.0
E. coli ATCC 25922
20
2.0
16.60
40
11.0
P. aeruginosa ATCC 27853
10
11.0
1.50
20.0–40.0
15.0
S. aureus (MRSA) ATCC R 43300
20
7.0
11.00
40
15.0
Butanol
B. subtilis NCIB 3610
40.0
6.0
40.0
P. aeruginosa ATCC 27853
40.0
3.0
40.0
S. aureus (MRSA) ATCC R 43300
10
11.0
3.00
20
13.0
40
21.0
Compounds
Compound 1 (trans-3,4-dihydroxycinnamic acid) (caffeic acid)
ompound 2 (quercetin)
Compound 3 (quercetin 3-O-β-glycopyranoside) (isoquercitrin)
Compound 4 (3-caffeoylquinic acid) (chlorogenic acid)
Compound 5 (3,5-O-dicaffeoylquinic acid)
Streptomycin
Organisms
MIC
MBC
MIC
MBC
MIC
MBC
MIC
MBC
MIC
MBC
MIC
MBC
B. subtilis NCIB 3610
2.5
5.0
1.25
5.0
1.00
>1.0
5.0
>10.0
5.0
5.0
0.500
0.500
Cl. sporogens NCIB 532
2.5
5.0
2.5
5.0
1.00
>1.0
2.5
10.0
2.5
2.5
0.125
0.500
E. coli ATCC 25922
0.625
5.0
2.50
5.0
1.00
>1.0
2.5
10.0
5.0
>10.0
0.125
0.500
K. pneumonia (clinical)
1.25
10.0
2.5
5.0
1.00
>1.0
10.0
>10.0
5.0
5.0
0.125
0.500
P. fluorescence NCIB 3756
2.5
5.0
1.25
10.0
1.00
>1.0
5.0
10.0
5.0
10.0
0.500
0.500
P. aeruginosa ATCC 27853
2.5
10.0
1.25
2.5
>1.00
>1.0
5.0
10.0
1.25
1.25
0.500
0.500
S. aureus ATCC 29213
5.00
5.0
0.156
5.0
>1.00
>1.0
2.5
10.0
5.0
5.0
0.125
0.500
Quercetin (2) and its glycosides have been reported to demonstrate antimicrobial, anti-inflammatory, anticancer and other biological activities (Yu et al., 2007, Davis et al., 2009). The results obtained in this study, support earlier reports on antimicrobial properties of quercetin. In this study, it was the most active compound and exhibited MIC of 0.16 mg/mL against S. aureus, (Table 4).
Quercetin-3-O-β-glucopyranose (3) is a glycoside of compound (2) having glucosyl as a substituent on C-3. It exhibited an MIC of 1 mg/mL for most of the typed and clinical strains investigated and an MBC of >1.0 mg/mL. These moderate activities were reflected in both Gram-positive and Gram-negative organisms. The wide spectrum of activities of this compound may contribute to the ethnomedicinal uses of N. latifolia in the treatment of infectious diseases and may thus justify the use of the plant in folk medicine. In a previous study involved antibacterial activity of four flavonol glycosides isolated from Mentha longifolia, quercetin-3-O-glucoside demonstrated the highest activity confirming our observed activity in this study (Akroum et al., 2009).
Caffeic acid (1) is a phenolic acid. Its inhibitory activity against bacteria strains is not unexpected. Structurally, chlorogenic acid (4) is an ester obtained from caffeic acid and L-quinic acid (Boerjan et al., 2003). Chlorogenic acid had been reported from bamboo (Phyllostachys edulis) and potatoes (Clifford, 2003). This is the first report of its isolation from N. latifolia. Its MIC against the tested strains of bacteria ranged from 2.5 to 5.0 mg/mL except that of K. pneumonia which was found to be 10.0 mg/mL. The MBC was found to be 10.0 mg/mL for most of the tested strains of bacteria. The results indicated the compound exhibited weak activities. Caffeic acid obtained from Olive leaf extract demonstrated antimicrobial activities in a related study justifying our report on this compound in this study (Lee and Lee, 2010).
Compound (5) 3,5-O-dicaffeoylquinic acid had been reported from coffee (Barnes et al., 1950). There are few reports of its antimicrobial activities. This is first report of its isolation from N. latifolia and also the first detailed report of its antibacterial activities. The MIC ranged from 1.25 mg/mL to 5.0 mg/mL. The MIC and the MBC are the same for most of the organisms indicating that the compounds may exhibit not only inhibitory but also cidal effects on the organisms. The activities against P. aeruginosa is noteworthy considering the fact that this organism is known to have shown resistant to most antimicrobial agents and the spectrum of agents available to control it are few.
3.3 Antioxidant activities
Six complementary antioxidant assays were used for the quantitative antioxidant activities of the N. latifolia extracts: Total Antioxidant Capacity (TAC), Total Phenolic Contents (TPC), DPPH Free Radical Scavenging, Nitric Oxide Inhibition, Ferric Reducing Antioxidant Power (FRAP) and Metal Chelating Ability (MCA) while five antioxidant assays were evaluated for the isolated compounds excluding Total Phenolic Contents. The results of the assays are as shown in Tables 5 and 6. Of all the N. latifolia solvent fractions, EtOAc fraction was the most active in the DPPH assay with the IC50 of 5.01 ± 0.55 µg/mL (Table 5). This is in agreement with the qualitative antioxidant assay. Among the isolated compounds, results indicated that compound (2) was the most active followed by compounds (5), (4), (3) and, (1) in its capacity to scavenge free radicals in the DPPH assay. In this assay, compound (2) was more potent (IC50 = 0.59 ± 0.04 µg/mL) than ascorbic acid (8.82 ± 0.23 µg/mL). Data represents Mean ± S.D. of triplicate determinations. Total antioxidant capacity and ferric reducing antioxidant potential is expressed as mg Ascorbic acid equivalent per g (mg AAE/g) and total phenolic content is expressed as mg gallic acid equivalent per g (mg GAE/g) of the test samples. Data represents Mean ± S.D. of triplicate determinations. DPPH free radical scavenging activity, metal chelating ability and nitric oxide radical scavenging activities are expressed as IC50 (µg/mL) values. *Ascorbic acid (AA). **Ethylene diamine tetraacetic acid (EDTA).
Samples
TAC
mg AAE/g sampleTPC
mg GAE/g sampleFRAP
mg AAE/g sample
Crude
104 ± 2.69
84.5 ± 7.73
114 ± 2.06
Hexane
129 ± 15.66
31.2 ± 5.40
7.73 ± 2.34
DCM
273 ± 20.11
52.5 ± 5.02
43.6 ± 3.46
EtOAc
267 ± 9.28
345 ± 8.58
250 ± 3.48
BuOH
187 ± 18.07
250 ± 16.58
202 ± 6.73
Compound 1
2.57 ± 0.01
–
0.28 ± 0.01
Compound 2
10.9 ± 2.14
–
1.68 ± 0.09
Compound 3
2.62 ± 0.10
–
0.43 ± 0.03
Compound 4
3.52 ± 0.13
–
0.94 ± 0.21
Compound 5
4.23 ± 0.23
–
1.21 ± 0.05
Samples
DPPH
C50 (µg/mL)MCA
IC50 (µg/mL)NO
IC50 (µg/mL)
Crude
67.2 ± 3.67
337 ± 28.15
223 ± 6.28
Hexane
743 ± 27.18
315 ± 36.79
704 ± 9.71
DCM
402 ± 19.33
141 ± 9.54
2300 ± 171.67
EtOAc
5.01 ± 0.55
113 ± 10.65
36.6 ± 2.23
BuOH
9.02 ± 0.34
73.4 ± 6.36
29.22 ± 0.43
Compound 1
21.3 ± 1.01
210 ± 9.94
55.2 ± 0.66
Compound 2
0.59 ± 0.04
139 ± 2.44
37.1 ± 6.09
Compound 3
9.27 ± 0.09
154 ± 5.75
5 0.17 ± 2.59
Compound 4
5.34 ± 0.12
2298 ± 7.76
20.5 ± 1.16
Compound 5
2.71 ± 0.09
105 ± 2.73
39.6 ± 3.52
Standard
8.82 ± 0.23*
121 ± 1.17**
69.3 ± 1.78*
All the isolated compounds exhibited better inhibition of NO radical activities (IC50 ranged from 5.17 ± 2.59 to 55.17 ± 0.66 µg/mL) compared with the standard, ascorbic acid [IC50 = 69.25 ± 1.78 µg/mL], however, compound (3) exhibited the highest inhibition of NO radical activity [IC50 = 5.17 ± 2.59 µg/mL]. The metal chelating ability (MCA) of the compounds ranged from IC50 = 104.55 ± 2.73 to 229.18 ± 7.76 µg/mL. Compound (5) had better activity [IC50 = 104.55 ± 2.73 µg/mL] than the standard, EDTA [IC50 = 120.92 ± 1.17 µg/mL]. The TAC of compound (2) was the highest, 10.918 ± 2.140 mg AAE/g and FRAP of this compound (2) exhibited the highest activity 1.68 ± 0.09 mg AAE/g (Table 6). In a previous study to validate the ethnomedicinal uses of Nauclea latifolia, antioxidant activities and the phenolic contents of its extracts were determined. The extracts exhibited strong antioxidant activities and high phenolic contents. It was concluded that the antioxidant activities of Nauclea latifolia extracts and its phenolic contents contributed significantly to the ethnomedicinal uses of the plant (Awah et al., 2012). In this study, five phenolic compounds were isolated and characterized from the N. latifolia leaf extracts. All the isolated compounds (1–5) demonstrated varying degrees of antioxidant activities in support of the plant use in the management of oxidative stress related diseases (Gidado et al., 2005).
4 Conclusions
This study concluded that N. latifolia extracts and isolated compounds had strong antioxidant and weak antibacterial activities. These agents could therefore be effective in the management of oxidative stress related and infectious diseases. These findings also justified the use of N. latifolia extracts in folk medicine.
Acknowledgement
Ajayi, O.S. is grateful to the Tertiary Education Trust Fund (TETFund) Nigeria for the award of Academic Staff Training and Development Travel Grant to the Department of Chemistry, University of Botswana, Gaborone, Botswana.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antioxidant and antimicrobial activities of flavonoids glycosides from Dennettia tripetala G. baker leaf extract. Nigerian J. Natural Prod. Med.. 2011;15:49-52.
- [Google Scholar]
- Phenolic constituents from Platycerium bifurcatum and their antioxidant properties. J. Nat. Prod.. 2014;7:48-57.
- [Google Scholar]
- Antioxidant activity and phenolic compounds from Colchicum luteum Baker (Liliaceae) Afr. J. Biotechnol.. 2010;9:5762-5766.
- [Google Scholar]
- Isolation and characterization of antioxidant and antimicrobial compounds from Anacardium occidentale L. (Anacardiaceae) leaf extract. J. King Saud Univ. – Sci.. 2015;27:244-252.
- [Google Scholar]
- Antibacterial activity and acute toxicity effect of flavonoids extracted from Mentha longifolia. American-Eurasian J. Sci. Res.. 2009;4(2):93-96.
- [Google Scholar]
- Clinical evaluation of a traditional herbal practice in Nigeria: preliminary report. J. Ethnopharmacol.. 1982;6:355-359.
- [Google Scholar]
- Antibacterial activity of flavonoids against methicillin-resistant Staphylococcus aureus strains. J. Theor. Biol.. 2000;205:231-240.
- [Google Scholar]
- Free radical scavenging activity, phenolic contents and cytotoxicity of selected Nigerian medicinal plants. Food Chem.. 2012;131(4):1279-1286.
- [Google Scholar]
- Isochlorogenic acid. Isolation from coffee and structure studies. J. Am. Chem. Soc.. 1950;72:4178-4182.
- [Google Scholar]
- Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity in biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Meth. Enzymol.. 1999;299:15-27.
- [Google Scholar]
- HPLC analysis and phytoconstituents isolated from ethyl acetate fraction of Scutia buxifolia reiss. Leaves. Latin Am. J. Pharm.. 2009;28(1):121-124.
- [Google Scholar]
- Use of free radical method to evaluate antioxidant activity. LWT Food Sci. Technol.. 1995;28:25-30.
- [Google Scholar]
- The analysis and characterization of chlorogenic acids and other cinnamates. In: Santos-Buelga C., Williamson G., eds. Methods in Polyphenol Analysis. Cambridge: Royal Society of Chemistry; 2003. p. :314-337.
- [Google Scholar]
- Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol.. 2009;296:R1071-1077.
- [Google Scholar]
- Effect of Nauclea latifolia leaves aqueous extracts on blood glucose levels of normal and alloxan-induced diabetic rats. Afr. J. Biotechnol.. 2005;4:91-93.
- [Google Scholar]
- Analysis of nitrate, nitrite and 15N in biological fluids. Anal. Biochem.. 1982;126:131-136.
- [Google Scholar]
- Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem.. 2003;83:371-382.
- [Google Scholar]
- Plants in kano ethnomedicine; screening for antimicrobial activity and alkaloids. Int. J. Pharmacol.. 1991;29:51-56.
- [Google Scholar]
- Inhibitory effects of Sudanese Plants on HIV-1 replication and HIV-1 protease. Phytother. Res.. 1999;13:31-36.
- [Google Scholar]
- Sub-saharan rubiaceae: a review of their traditional uses, phytochemistry and biological activities. Pakistan J. Biol. Sci.. 2011;14:149-169.
- [Google Scholar]
- Phenolic constituents from the flower buds of Lonicera japonica and their 5-lipoxygenase inhibitory activities. Food Chem.. 2010;120:134-139.
- [Google Scholar]
- Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol.. 2010;101:3751-3755.
- [Google Scholar]
- Identification of phenolics in the fruit of emblica (Phyllanthus emblica L.) and their antioxidant activities. Food Chem.. 2008;109:909-915.
- [Google Scholar]
- Anti-hepatotoxic and trypanocidal activities of ethanolic extract of Nauclea latifolium root bark. J. Herbs Spices Med. Plants. 1995;3:23-53.
- [Google Scholar]
- The nitric oxide scavenging property of Ginkgo biloba extract EGb 761. Biochem. Biophys. Res. Commun.. 1994;201:748-755.
- [Google Scholar]
- Nauclefolinine: a new alkaloid from the roots of Nauclea latifolia. Bull. Chem. Soc. Ethiop.. 2003;17(2):173-176.
- [Google Scholar]
- Preliminary studies of blood pressure lowering effect of Nauclea latifolia in rats. Afr. J. Pharmacol.. 2008;2:37-41.
- [Google Scholar]
- Comparative studies of the antimicrobial activity of components of different polarities from the leaves of Nauclea latifolia. Res. J. Med. Plants. 2011;5:321-329.
- [Google Scholar]
- The antimicrobial potential of Nauclea latifolia. Afr. J. Biotechnol.. 2008;7:1394-1399.
- [Google Scholar]
- Sudanese plants used in folkloric medicine: screening for antimicrobial activity. Fitoterapia. 1998;69:542-545.
- [Google Scholar]
- Antimicrobial Biflavonoids from the Aerial parts of Ouratea sulcata. Phytochemistry. 2005;66:1922-1926.
- [Google Scholar]
- Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem.. 1999;269:337-341.
- [Google Scholar]
- The anti-thrombotic active constituents from Centella asiatica. Biol. Pharm. Bull.. 2007;30:935-940.
- [Google Scholar]
- Free radical scavenging activity of an aqueous extract of potato peel. Food Chem.. 2004;85:611-616.
- [Google Scholar]
- Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Oncol. Viticult.. 1965;16:144-158.
- [Google Scholar]
- Antimalarial activity of 20 crude extracts from nine african medicinal plants used in Kinshasa Congo. J. Ethnopharmacol.. 1999;68:193-203.
- [Google Scholar]
- Effects ofTriterpenoids and Flavonoids isolated from Alnus firma on HIV-1 viral enzymes. Archiv. Pharmaceut. Res.. 2007;30:820-826.
- [Google Scholar]
- A convenient microdilution method for screening natural products against bacteria and fungi. Pharm. Biol.. 2001;39:221-225.
- [Google Scholar]







