Translate this page into:
Bio-physical effects of radiofrequency electromagnetic radiation (RF-EMR) on blood parameters, spermatozoa, liver, kidney and heart of albino rats
⁎Corresponding author. eaadebayo@lautech.edu.ng (E.A. Adebayo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Current study aimed at determining the consequence of Radiofrequency (≈1800 MHz) electromagnetic radiation (RF-EMR) on the histological, hematological and histochemical properties of selected tissues of rat and to assess morphological changes associated with such exposures. Three groups of thirty rats of which two groups of twenty rats were subjected to average radiation of ≈1.40 W/cm2 at 24 m from the base of two different telecommunications network mast for 5 weeks. Histochemical, hematological and histological analyses of the rats were afterward carried out on selected tissues of the experimental animals using standard procedures. Impacts of radiation were noted in the exposed groups of rats. There was rise in white blood cells with variations in other blood parameters; the sperm motility and sperm count of the exposed rats is lower than the control; the liver of the exposed rats shows pronounced dilated sinusoids, distorted architecture, hyperchromatic nuclei, congested central vein, with change of hepatocytes structure; the heart shows gross distortion of cardiac muscular architecture with distorted irregular cardiac muscle fibres and wider interfibres spaces; kidney showing hyperchromatic nuclei, gradual loss and degeneration of flattened squamous epithelial cells lining and testis shows grossly distorted seminiferous tubules and epididymis with loss of cellular structure and an area of inflammatory changes with complete absence of spermatozoa, which may lead to low fertility. This study shows that radiofrequency electromagnetic radiation may induce morphological changes in various tissues of living systems in rat.
Keywords
RF-EMR
Telecommunication
Histological
Hematological
Histochemical
1 Introduction
The effects of radiofrequency electro-magnetic radiation (RF-EMR) on the biological functions of living organisms is an emerging field of interest with regard to environmental impacts on human health (Adebayo et al., 2015). It is documented that the radiation produces reactive oxygen species (ROS) or free radicals in biological system (Gracy et al., 1999; Strinivasan et al., 2006; Mansour, 2012). Quite a number studies have also been performed to clarify direct effects exerted by radiations on living systems which involves in vivo and in vitro experiments using different approaches (Stuchly et al., 1991; Dachà et al., 1993, Khayyat and Abou-Zaid, 2009) some of which produced a number of biological effects in cells and tested whole organisms (Goodman et al., 1995; Kwee and Raskmark, 1998; Velizarov et al., 1999; Adebayo et al., 2015).
Previous reports revealed that range of cell responses to RF-EMR have been observed including gene expression (Piacentini et al., 2008; Goodman et al., 2009), differentiation and proliferation (Schwartz et al., 2008; Foletti et al., 2009), apoptosis, alteration in ion homeostasis (Iorio et al., 2011), modulation of the membrane receptors functionality (De-Matteiet al., 2009), and free radicals generation (Simk, 2007; Di-Loreto et al., 2009), but more careful studies need to be carried out to establish the nature of effect of radiofrequency non-ionizing radiation (RF-EMR) in living systems. While physiological and histological breakthroughs have increased the assessment of the impacts of electromagnetic fields on human health (Khaki et al., 2008; Hashem and El-Shakawy, 2009), results from previous works are even slightly controversial (Lai, 2005; Behari and Rajamani, 2012; SCENIHR, 2015) and the variations were attributed to various factors (Lerchl, 2009; Lerchl and Wilhelm, 2010). The effect of such radiations, especially in Nigeria has been sparsely documented. This study, therefore aims at investigating the possible histopathological, histochemical and hematological effects of RF-EMR from telecommunication base stations located around Ladoke Akintola University of Technology Ogbomoso (LAUTECH), Nigeria on experimental rats.
2 Materials and methods
2.1 Experimental animals
A total of thirty experimental rats, both male and female of age six weeks and average weight of 150 ± 5 g were purchased from the Department of Physiology, LAUTECH Oyo state, Nigeria. The animals were housed in a standard large cage partitioned to separate males from females under controlled temperature 27 ± 2 °C, 50 ± 10% humidity and photoperiod 12 h of darkness,12 h of light, with easy access to feed and water ad-libitum. Animals were put up in a standard animal facility and wood shaving was used as beddings (Kao et al., 1999) with ethical permissions duly obtained.
2.2 Experimental protocol
The experimental albino rats were grouped into three comprising of ten rats each (5 females and 5 males) and were acclimatized for two weeks before the experiment was undertaken. Two groups were then located at 24 feet from the foot of two different telecommunications network mast (Globacom Limited “GLO” Group B and Mobile Telecommunications Network “MTN” – Group C) and a control group (A) was prevented from exposure in a standard animal house for 5 weeks. The base stations were situated in LAUTECH, Ogbomoso, Nigeria (Latitude N8°, 101 and Longitude E4°, 161). The rats were handled with care, continuously supplied with food and easy access to drinking water. Beddings of the rats were changed regularly to ensure proper sanitation.
2.3 Histological, hematological and histochemical examinations
Animals were euthanized by decapitation and various body organs including heart, kidney, liver, ovules and testes were removed by non traumatic technique. Each organ was cut into small pieces, fixed and processed (Khalil et al., 2012). Tissues were fixed in Bouin’s fluid for 24 h. About 7 μm sections were made and stained with hematoxylin-eosin (H and E). Randomly selected sections were examined under Nikon E 400 microscope and representative samples were photographed using a modified method of Markus and Sarine (2014). Hematological assay, such as packed volume cell (PCV), white blood cell (WBC), red blood cell (RBC), Hemoglobin (Hb), Lymphocyte (LYMP) and Neutrophil (NEUT) were carried out following the method of Abdolmaleki et al. (2012). The histochemical analyses (Glu- Glucose, K+- Potassium, Na+- Sodium, Cl−- Chloride, SGPT- Serum Glutamic Pyruvic Transaminase, SGOT- Serum Glutamic Oxaloacetic Transaminase, ALP- Alkaline phosphatase, T.P- Total protein, Alb- Albumin, T.Ch– Total Cholesterol) were carried out according to the methods of Julian (2000) and Singh et al. (2017). Assay on sperm motility and sperm count was carried out using Adebayo et al. (2012), while histological analysis of testis was conducted following Bahaodini et al. (2015).
2.4 Electromagnetic field source
Electromagnetic field was generated by radiofrequency sources from two telecommunication network mast stations at the various experimental sites with average antenna radiation frequency of 1800 MHz, down link frequency range of 1820–1835 MHz and 1835–1850 MHz and uplink frequency of 1725–1740 MHz and 1740–1755 MHz respectively. Rats received an average radiofrequency electromagnetic radiation of ≈1.40 mW/cm2 (Table 1) at point of location as established by Akintonwa et al. (2009). Source: Akintonwa et al. (2009).
Distance (m)
0
50
100
150
200
Average Power Density (mW/cm2)
1.40
1.40
1.30
1.25
1.25
Average Power radiated (dBm)
−55
−60
−65
−70
−75
2.5 Statistical analysis
The results of histochemical and hematological blood parameters, sperm count and sperm motility were statistically analyzed by one-way ANOVA followed by the Duncan’s Multiple Range Test using SPSS version 18.
3 Results
3.1 Histochemical and Hematological blood parameters
Table 2 showed the histochemical blood parameters. One way ANOVA test was used with significant differences at (P ≤ 0. 05) in the histochemical blood parameters of rats. The potassium content in the exposed group of rats (B and C) B: 10.33 and C: 9.50 mmol/L was recorded with significant difference (P ≤ 0. 05) from the unexposed group in A: 14.00 mmol/L. There was noticeable decrease in amount with significant difference (P ≤ 0.05) in sodium (135.00 and 136.75 mmol/L), urea (47.50 and 37.75 mg/dl) and total protein (10.20 and 6.88 g/dl) in blood of rats exposed to radiation (group B and C) when compared to that of the unexposed group of rats (A) recorded as 137 mmol/L, 51.75 mg/dl and 10.70 g/dl for sodium, urea, and total protein respectively. The result also revealed an increase in amount with significant difference in the contents of glucose and total cholesterol for exposed group of rats observed as (57.75 and 39.50 mg/dl) and 88.75 and 91.75 mg/dl group comparing with the control (group A) recorded as 28.00 mg/dl and 81.00 mg/dl glucose and total cholesterol respectively. However, alkaline phosphatase slightly increased in group B (52.75 U/L) with significant difference (P ≤ 0.05) over group C (38.50 U/L) and group A (50.00 U/L), while in albumin, there was an increased in group C (5.00 g/dl) in comparison to group B (4.58 g/dl) and A (4.75 g/dl) with significant difference (P ≤ 0.05). Although there were no significant statistical differences in the record for Chloride, Serum Glutamic Pyruvic Transaminase (SGPT), and Serum Glutamic Oxaloacetic Transaminase (SGOT), there were observable increase in the amount of SGPT and SGOT in exposed group while the amount of chloride decreases. Values = Mean ± standard error; mean followed by the same alphabets in the column are not significantly different according to Duncan’s Multiple Range Test (p ≤ 0.05). Gluc- Glucose, K+- Potassium, Na+- Sodium, Cl−- Chloride, SGPT- Serum Glutamic Pyruvic Transaminase, SGOT- Serum Glutamic Oxaloacetic Transaminase, ALP- Alkaline phosphatase, T.P- Total protein, Alb- Albumin, T.Ch- Total Cholesterol.
Sample
Gluc
mg/dlK+
mmol/LNa+
mmol/LCl−
mmol/LUrea
mg/dlSGPT
U/LSGOT
U/LALP
U/LT.P
g/dlAlb
g/dlT.Ch
mg/dl
A
28.00 ± 4.02a
14.00 ± 0.41b
137.00 ± 0.41b
97.25 ± 0.95a
51.75 ± 5.94b
296.50 ± 68.95a
509.25 ± 3.28a
50.00 ± 5.40ab
10.70 ± 0.83b
4.75 ± 0.06ab
81.00 ± 1.73a
B
57.75 ± 11.10a
10.33 ± 0.27a
135.00 ± 0.41a
95.25 ± 1.03a
47.50 ± 3.20ab
254.75 ± 58.77a
514.25 ± 6.72a
52.75 ± 1.10b
10.20 ± 0.29b
4.58 ± 0.15a
88.75 ± 3.82ab
C
39.50 ± 10.60a
9.50 ± 0.86a
136.75 ± 0.63b
76.75 ± 22.33a
37.75 ± 0.85a
287.50 ± 81.92a
516.00 ± 4.85a
38.50 ± 3.43a
6.88 ± 1.15a
5.00 ± 0.09b
91.75 ± 2.87b
The least amount of sodium (135.00), urea (37.75), alkaline phosphatase (38.50), chloride (76.75) and total protein (6.88) were observed in exposed group C while total protein (10.70), SGPT (296.50), urea (51.75), Cl− (97.35), Na+ (137.00), and K+ (14.00) were highest in group A (control).
Table 3 showed hematological blood parameters of the experimental rats. There was increase in white blood cells (WBC) of the exposed rats in group B (11450 mm3) and C (7700 mm3) compared to control in group A (5650 mm3) with significant difference (P ≤ 0.05) in group B over A and C. It was also shown that there were changes in the other blood parameters as recorded in Groups A, B, and C with no significant difference (P ≤ 0.05) for PCV, RBC, Hb, LYMP and NEUT (Table 3). Values = Mean ± standard error; mean followed by the same alphabets in the column are not significantly different according to Duncan’s Multiple Range Test (p ≤ 0.05). PVC- Packed Volume Cell, WBC- White Blood Cell, RBC- Red Blood Cell, Hb- Heamoglobin, LYMP- Lymphocyte, NEUT- Neutrophil.
Sample
PCV (%)
WBC (mm3 of blood)
RBC (million/ml)
Hb (g/dl)
Differential count (%)
LYMP
NEUT
A
47.50 ± 1.85a
5650.00 ± 1607.02a
3.53 ± 0.49a
14.88 ± 0.59a
29.50 ± 3.77a
70.50 ± 3.77a
B
48.00 ± 0.82a
11450.00 ± 1135.41b
3.39 ± 0.17a
14.43 ± 0.28a
32.25 ± 2.06a
67.75 ± 2.06a
C
47.50 ± 1.66a
7700.00 ± 450.92a
3.50 ± 0.13a
15.13 ± 0.43a
34.25 ± 1.65a
65.75 ± 1.65a
3.2 The sperm count and motility
The sperm motility and count of the rats are shown in Table 4. There was a gradual decrease in sperm count of the various group of rats exposed to telecommunication radiation in comparison with control A (65.00 million/ml) > B (55.00 million/ml) > C (40.00 million/ml). Significant variation (p ≤ 0.05) was observed in the sperm count and sperm motility. Motility in the unexposed group (A) has the highest percentage (35%), which is different from 22.5% and 20% recorded in rats in exposed group of rats in C and B respectively. Values = Mean ± standard error; mean followed by the same alphabets in the column are not significantly different according to Duncan’s Multiple Range Test (p ≤ 0.05).
Sample
Motility (%)
Count(million/ml)
A
35.00 ± 5.00b
65.00 ± 5.00b
B
20.00 ± 0.00a
55.00 ± 5.00ab
C
22.50 ± 2.50ab
40.00 ± 0.00a
3.3 Histological results
The histological results were shown from plates 1 to 21 at X400 magnification.
3.3.1 Liver
In the control group (Plate 1) the result of the histological section of the liver shows a normal central venous blood vessel from which sheets of liver cells (hepatocytes) radiate. In the exposed rats, which are from group two and three (Plates 2, 3 and 5), the histological section of the liver shows a slightly distorted architecture and dilated sinusoids, while some have pronounced dilated sinusoids and distorted architecture which might suggest that radiation predisposes the rat to infection. Slide 5 shows hyperchromatic nuclei, congested central vein, with change of hepatocytes structure which may be fat area in cytoplasm of cells (Fig. 1).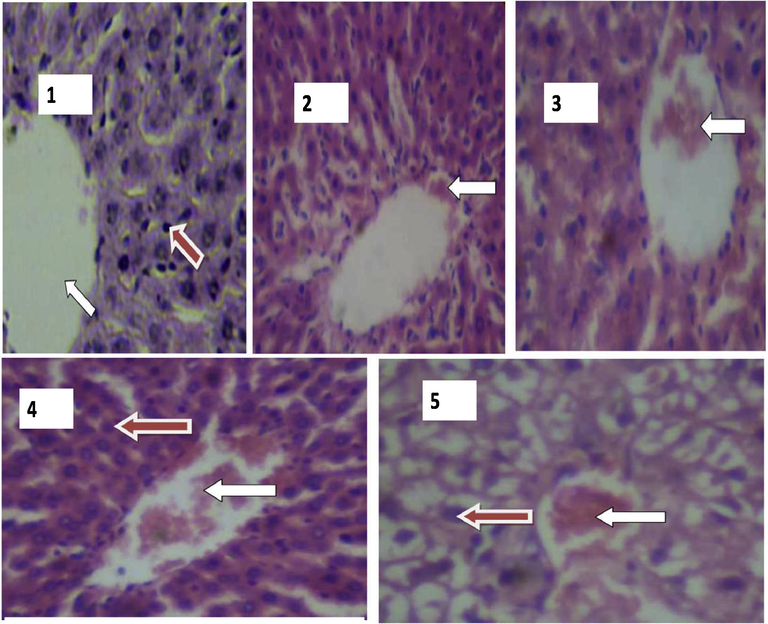
Plates showing histological results of Liver. 1: Photomicrograph of the liver showing normal morphological architecture. H & E stain, (X400). WHITE arrow indicates Central vein with mild congestion. RED arrow indicates liver cell (Hepatocyte). 2: Photomicrograph of the liver showing distorted architecture with pronounced dilated sinusoids. WHITE arrow indicates dilated sinusoid. 3: Hyperchromatic nuclei and mild congestion of the central vein (Group 2). H & E stain, (X400). WHITE arrow indicate Central vein with mild congestion. 4 & 5: Photomicrograph of the liver showing hyperchromatic nuclei, congested central vein and pronounced distortion of the cellular structure (Group 3). H & E. stain, (X400). WHITE arrow indicates congestion at the central vein. RED arrow indicates hyperchromatic liver cells (hepatocytes).
3.3.2 Heart
The photomicrograph of the heart is shown in plate 6 to 10. The result of histological section of the heart of the unexposed rat shows normal muscle fibres (plate 6), while the heart of the exposed ratsin group 2 and 3 (Plates 7 to 10) shows gross distortion of cardiac muscular architecture with distorted irregular cardiac muscle fibres and wider interfibres spaces (Fig. 2). This distortion observed in the cardiac muscle architecture could be caused by radiation, which can lead to irregular contraction of the heart muscles, wider inter-fibre space between cardiac fibre.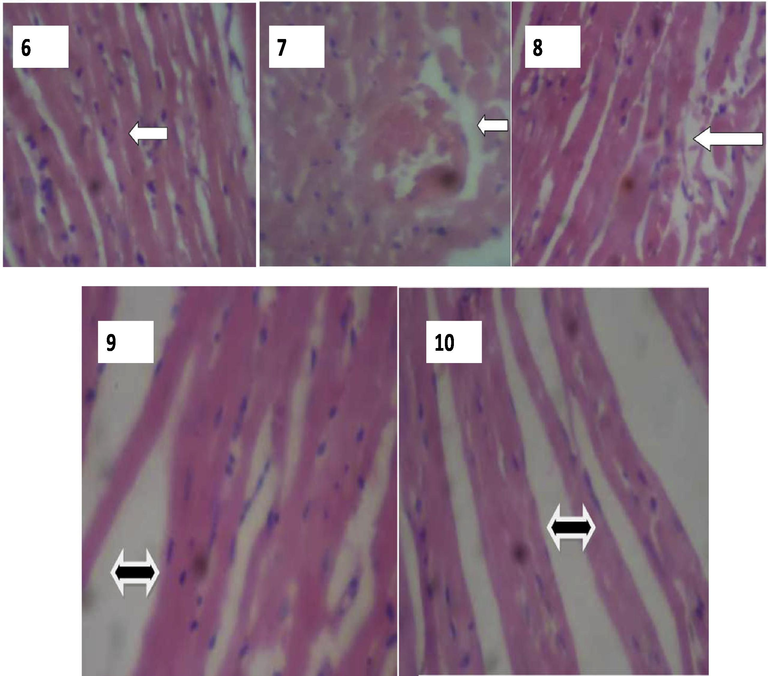
Plates showing histological results of Heart. 6: Heart showing well preserved cardiac muscle fibres (Group 1). H & E Stain, X400. WHITE arrow indicates normal cardiac muscle fibres. 7: Heart showing gross distortion of cardiac muscle architecture. (Group 2). H & E Stain, X400. WHITE arrow indicates severe distortion in the cardiac muscle fibre. 8: Heart showing distorted and irregular cardiac muscle fibres. (Group 2). H & E Stain, X400. WHITE arrow indicates severe distortion in the cardiac muscle fibre. 9: Heart showing distorted architecture of cardiac muscle fibres with wider inter fibre spaces. (Group 3). H & E Stain, X400. BLACK arrow indicates wide inter-fibre space between cardiac fibre. 10: photomicrograph of the heart showing distorted architecture of cardiac muscle fibres with pronounced wider interfibre spaces. (Group 3). H & E Stain, X400. BLACK arrow indicates wide inter-fibre space between cardiac fibre.
3.3.3 Kidney
Plates 11–15 showed photomicrograph of the kidney. The rat in control group (Plates 11 and 12) is associated with kidney showing preserved cellular structure with normal renal morphology allied with glomerulus and tubules. The histological sections of the kidney show normal appearance of the glomerulus, Bowman’s capsule, distal convoluted tubules, proximal convoluted tubules. Rat in group 2 and 3 exposed to radiation were associated with kidney showing hyperchromatic nuclei, gradual loss and degeneration of flattened squamous epithelial cells lining, wide Bowman’s space and degeneration of glomeruli with associated areas of empty and expanded bowman’s space (Plates 13–15) (Fig. 3).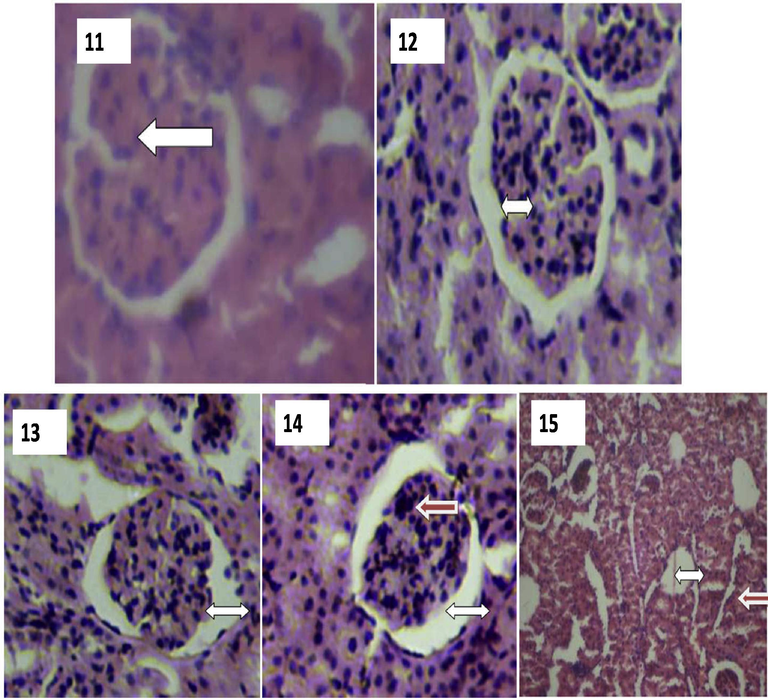
Plates showing histological results of Kidney. 11: A photomicrograph of the kidney showing well preserved cellular architecture. (Group 1). H & E Stain, X400. WHITE arrow indicates normal renal morphology (Glomerulus and tubules). 12: A photomicrograph of the kidney showing ing normal morphological appearance but with aa wide Bowman’s space. (Group 1). H & E stain, (X400). WHITE arrow indicates wide bowman’s space. 13 A photomicrograph of the kidney showing a wide Bowman’s space, hyper chromic nuclei and gradual loss of flattened squamous epithelial cells lining the Bowman’s capsule. (Group 2). H & E stain, (X400). WHITE arrow indicates wide bowman’s space. 14: A photomicrograph of the kidney showing a wide Bowman’s space, hyperchromatic nuclei and degenerated flattened squamous epithelial cells lining the Bowman’s capsule. (Group 2). H & E stain, (X400). WHITE arrow indicates wide bowman’s space. RED arrow indicates dead (necrotized) squamous eepithelial cells. 15: A photomicrograph of the kidney showing degenerating glomeruli and areas of empty Bowman’s space due to loss of glomeruli.(Group 3). H & E stain, (X400). WHITE arrow indicates wide bowman’s space. RED arrow indicates already degenerating glomerulus.
3.3.4 Testis
The testis in rat unexposed to radiation shows a well preserved architecture of seminiferous tubules and well preserve architecture of the epididymis as shown in Plate 16 and 17. In unexposed group of rat, slightly distorted epididymis of the testis and reduced stored spermatozoa in the epididymis are reported in group 2 as shown in Plate 18, while 19 shows normal seminiferous tubules with an area of inflammatory change. Plate 20 (group 3) shows grossly distorted seminiferous tubules with loss of cellular structure and an area of inflammatory changes, while plate 21 showed grossly distorted epididymis with loss of cellular structure and complete absence of spermatozoa (Fig. 4). This might have resulted from a gross damage to the somniferous tubule and which may lead to low fertility in the exposed rats.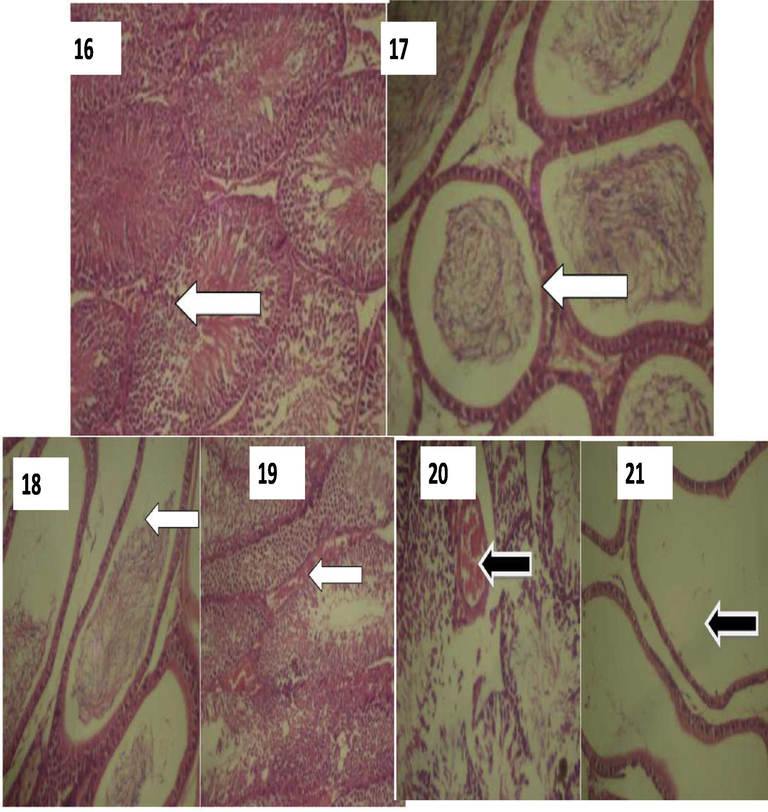
Plates showing histological results of Testes. 16: A photomicrograph of the testis showing a normal architecture of the seminiferous tubules. (Group 1). H/E stain. (X400). WHITE arrow indicates normal epididymis. 17: A photomicrograph of the testis showing a well preserved architecture of the epididymis. (Group 1). H/E stain. (X400). WHITE arrow indicates normal epididymis. 18: A photomicrograph of the testis showing reduction of stored spermatozoa in the epididymis. (Group 2). H/E stain. (X400). WHITE arrow indicates severance in the seminiferous tubule. 19: A photomicrograph of the testis showing normal seminiferous tubules with an area of inflammatory changes. (Group 2). H/E stain. (X400). WHITE arrow indicates severance in the seminiferous tubule. 20: A photomicrograph of the testis showing grossly distorted seminiferous tubules with loss of cellular structure and an area of inflammatory changes. (Group 3). H/E stain. (X400). BLACK arrow: indicates seminiferous tubules showing inflammation. 21: A photomicrograph of the testis showing grossly distorted epididymis with loss of cellular structure and complete absence of spermatozoa. (Group 3). H/E stain. (X400). BLACK arrow indicates total absence of spermatozoa (azoospermia).
4 Discussion
The histochemical analysis shows that values of blood chemical parameters of the exposed groups of rats were affected when compared with the control. Reduction in amount of sodium, potassium, urea, alkaline phosphatase (C), SGPT, albumin (B), chloride and total protein were observed in exposed group compared to control group (A). Although there were no significant statistical differences in some histochemical blood parameters like chloride, SGPT, SGOT and glucose but there were observable increase in the exposed group, while significant differences increased were obtained in total cholesterol, ALP (B) and albumin (C) in exposed group in relation with control. Till et al. (1998) reported that the rate and amount of products of biochemical reactions may be influenced by EMF radiation through free radical mechanism including direct influence on enzyme action, this may therefore account for the various differences observed in these histochemical blood products.
The increased in white blood cells of the exposed rat compared to the control could be an indicator of self-defense mechanism against exposure to foreign bodies. The increase in white blood cells of the exposed animals compared to control negates the findings of Abdolmaleki et al. (2012), while the trend in red blood cell count in this study also negates the report of Abdolmaleki et al. (2012), it is similar to the trend of report presented by Yousefi (1996). The increased white blood cell count indicates the response of the rats’ defensive system to foreign agents and the reduction in red blood cell may indicate stress in the animal system.
The liver of the exposed rats shows histological section of the liver showed a slightly distorted architecture and dilated sinusoids, while some have pronounced dilated sinusoids and distorted architecture which might suggest that radiation predisposes the rat to infection. The hyperchromatic nuclei, congested central vein, with change of hepatocytes structure which may be fat area in cytoplasm of cells. This report is similar to that of Kadhum (2015) with observed pathological effect on liver associated with exposure to EMR. The result also agreed with Usikalu et al. (2010) which reports that EMR causes histopathological damage in the liver of exposed animals. It is suggested that EMR cause cell damages by ROS and cause damages of cell membranes of liver's hepatocyte. Recurrent exposure to the EMR produced from the cell phone is capable of inducing selective tissue damage (Forgacs et al., 2004; Buckus et al., 2017). The extent of damage is however, likely to increase with time of exposure (Forgacs et al., 2006). Exposure of rats to phone radiation for three months caused severe streak proliferation of fibrosis, steatosis, diffuse degeneration and necrosis, and infiltration of a small amount of inflammatory cells in portal areas El-Bedwi et al. (2011).
The heart of the exposed rats shows gross distortion of cardiac muscular architecture with distorted irregular cardiac muscle fibres and wider interfibres spaces. This distortion observed in the cardiac muscle architecture could be caused by radiation, which can lead to irregular contraction of the heart muscles, wider inter-fibre space between cardiac fibre. Rats in exposed group to radiation were associated with kidney showing hyperchromatic nuclei, gradual loss and degeneration of flattened squamous epithelial cells lining, wide Bowman’s space and degeneration of glomeruli with associated areas of empty and expanded bowman’s space, which indicates already degenerating glomerulus. Heibashy (2010) reported that, radiation exerts damaging effect on the cells of that release enzymes from organelles. Moreover, radiation (ionising) causes alternation in enzymes ability to hydrolyse phosphate esters. Damage may result primarily from direct ionization of DNA itself or indirectly via formation of noxious products, like free radicals and free ions which react with molecules in their path (ATSDR, 1999). Studies have proposed that in-field DNA damage, after lung irradiation is produced by both the direct effects of radiation and the indirect effects of the inflammatory response, while the out-of-field impairment is possibly caused by the indirect effects of the inflammatory response alone. The exact mechanisms involved in the inflammatory response to radiation are unknown; nevertheless, it has been proposed that the generation of ROS (and RNS) after irradiation, collectively with a cyclic (and chronic) up-regulation of inflammatory cytokines and the involvement of inflammatory cells such as macrophages and neutrophils, is responsible for the damage seen in the lung after irradiation (Khan et al., 2003; Fleckenstein et al., 2007).
The results obtained from sperm count and motility of the rats shown that the sperm motility and count of irradiated rats were lesser than the control which might be as a result of the exposure. Exposed rats showed normal seminiferous tubules with an area of inflammatory change, grossly distorted seminiferous tubules with loss of cellular structure and an area of inflammatory changes, They also shows grossly distorted epididymis with loss of cellular structure and complete absence of spermatozoa. This might have resulted from a gross damage to the somniferous tubule and which may lead to low fertility in the exposed rats. The observed reduced sperm count and motility of the exposed rats in this finding is comparable to that reported by Bahaodini et al. (2015) which reported reduced sperm motility in rats exposed to EMR at 50 Hz, 24 h/day for 85 days. This report is also consistent with other findings where similar radiation from cell phone reduced sperm parameters in human semen samples (Erogul et al, 2006) and reduced percentage of motile sperm in rats exposed to mobile phone waves for 1 hr/day for 28 days (Mailankot et al., 2009). It is documented that sperm motility and count in Wistar rats decreased with increase in magnetic field strength (Mortazavi et al., 2010). Ghanbari et al. (2013) also established that EMR reduce sperm motility and viability. Several studies mentioned that EM waves have extensive range of detrimental effects on sperm parameters and the male reproductive system, and cause significant alterations in the sperm cell cycle (Kesari et al., 2011). Constant neutralization of ROS by antioxidants available in body tissues were reported (Desai et al., 2009). When the productions of ROS go above the scavenging ability of antioxidants, oxidative stress (OS) will occur (Desai et al., 2009). In 1992, Grundler et al. found that EMF increases the free radical activity in cells (Grundler et al., 1992). In the past decade, in-vivo experimental studies have shown that OS develops in response to cell phone radiation (Oktem et al., 2005; Meral et al., 2007). At the level of the testes, oxidative stress is able to disrupt the steroidogenic capacity of Leydig cells (Hales et al., 2005) and the capacity of the germinal epithelium to differentiate normal spermatozoa (Naughton et al., 2001).
It was documented that radiofrequency EMF induced tissue impairment in various organs of experimental animals including brain (Kang et al., 1997), testis (Zare et al., 2007), kidney (Khayyat and Abou-Zaid, 2009), liver (Hashem and El-Shakawy, 2009) and blood (Khayyat, 2011). This exposure have been associated with adverse effects on spermatogenesis, sertoli and leydig cells (Aydin et al., 2007; Khayyat, 2011), and liver enzymes (Rad et al., 2013). Decrease insulin production (Khaki et al., 2015) and reduction in glucose in blood stream (Al-Qudsi and Solafa, 2012) have also been reported. Our findings may therefore corroborate and give explanations to some health complications experienced by settlers living close to telecommunication mast.
5 Conclusion
From the data presented in this study, it can be observed that impact of RF-EMR from telecommunication base stations are reasonably manifested on internal organs in a similar manner observed in ionizing radiation studies. Some of the organs examined showed histological, hematological and histochemical changes which are different from normal. The study established that the reproductive organs of male rats were seriously impaired, which may have similar effect on higher mammal. It is recommended that further research to study the long term effect of RF-EMR from telecommunication on living systems in Nigeria be intensified.
References
- Effect of electromagnetic wave on blood parameters. Int. J. Heamtol., Oncol. Stem Cell Res.. 2012;6:13-16.
- [Google Scholar]
- Antimicrobial and Anti-inflammatory activities of polysaccharide from Pleurotus pulmonarius LAU 09. Afr. J. Microbiology Res.. 2012;6(13):3315-3323.
- [Google Scholar]
- Effect of radiofrequency radiation from telecommunication base stations on microbial diversity and antibiotic resistance. J. Appl. Sci. Environ. Manage.. 2015;18:669-674.
- [Google Scholar]
- The hazards of non-ionizing radiation of telecommunication mast in an urban area of lagos. Nigeria. Afri. J. of Biomed. Res.. 2009;12:31-35.
- [Google Scholar]
- Effect of electromagnetic mobile radiation on chick embryo development. Life Sci. J.. 2012;9:983-991.
- [Google Scholar]
- U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry. 1999. p. :676.
- Effect of electromagnetic field on the sperm characteristics and histopathological status of testis in rats. MedycynaWeterynaryjna. 2007;63:178-183.
- [Google Scholar]
- Bahaodini, A., Owjfard M., Tamadon A., and Jafari S.M., 2015. Low frequency electromagnetic fields long-term exposure effects on testicular histology, sperm quality and testosterone levels of male rats. http://dx.doi.org/10.1016/j.apjr.2015.06.001.
- Behari, J., Rajamani, P., 2012. Electromagnetic field exposure effect on fertility and reproduction. A manual prepared of Bioinitiative group. pp. 1–37.
- A technical approach to the evaluation of radiofrequency radiation emissions from mobile telephony base stations. Int. J. Environ. Res. Public Health.. 2017;14:244-262.
- [Google Scholar]
- Studies on the possible biological effects of 50 Hz electric and/or magnetic fields: Evaluation of some glycolytic enzymes, glycolytic flux, energy, and oxidoreductive potentials in human erythrocytes exposed in vitro to power frequency fields. Bioelectromagn. 1993;14:383-391.
- [Google Scholar]
- Adenosine analogs and electromagnetic fields inhibit prostaglandin E2 release in bovine synovial fibroblasts. Osteoarthritis Cartilage.. 2009;17:252-262.
- [Google Scholar]
- Pathophysiology of cell phone radiation: oxidative stress and carcinogenesis with focus on male reproductive system. J. Reproductive Biol. Endocrinol.. 2009;7(114):120.
- [Google Scholar]
- Fifty hertz extremely low-frequency magnetic field exposure elicits redox and trophic response in rat-cortical neurons. J. Cell. Physiol.. 2009;219:334-343.
- [Google Scholar]
- Effects of electromagnetic radiation produced by mobile phone on some vesceral organs of rat. J. Medical Sci.. 2011;11:256-260.
- [Google Scholar]
- Effects of electromagnetic radiation from a cellular phone on human sperm motility: an in vitro study. J. Arch. Med. Res.. 2006;37:840-843.
- [Google Scholar]
- New insights into the mechanism of action of amphetamines. Annu. Rev Pharmacol Toxicol.. 2007;47:681-698.
- [Google Scholar]
- Cellular ELF signals as a possible tool in informative medicine. Electromagn. Biol.Med.. 2009;28:71-79.
- [Google Scholar]
- Effect of whole-body 1800 MHz GSM-like microwave exposure on testicular steroidogenesis and histology in mice. Reprod. Toxicol.. 2006;22:111-117.
- [Google Scholar]
- Effects of whole-body 50-Hz magnetic field exposure on mouse leydig cells. Sci. World J.. 2004;4:83-90.
- [Google Scholar]
- The Effects of cell phone waves (900 MHz-GSM Band) on sperm parameters and total antioxidant capacity in rats. J. Fertility Steril.. 2013;7:21-28.
- [Google Scholar]
- Effects of electromagnetic fields on molecules and cells. Int. Rev. Cytol.. 1995;158:279-338.
- [Google Scholar]
- Extremely low frequency electromagnetic fields activate the ERK cascade, increase hsp70 protein levels and promote regeneration in Planaria. Int. J. Radiat Biol.. 2009;85:851-859.
- [Google Scholar]
- Reactive oxygen species: The unavoidable environmental insults. Mutat. Res.. 1999;428:17-23.
- [Google Scholar]
- Mechanisms of electromagnetic interaction with cellular systems. Naturwissenschaften. 1992;79:551-559.
- [Google Scholar]
- Mitochondrial function in Leydig cell steroidogenesis. Ann. N. Y. Acad. Sci.. 2005;1061:120-134.
- [Google Scholar]
- Hemato-biochemical and immunetoxicological effects of low electromagnetic field and its interaction with lead acetate in mice. Iraqi J. Vet. Sci.. 2009;23:105-114.
- [Google Scholar]
- Comparative study between dimethyl sulfoxide (DMSO), allopurinol and urate oxidase administration in nephrotoxic rats induced with gentamicin. Arab J. Nucl Sci. Appl.. 2010;43(1):281-289.
- [Google Scholar]
- Involvement of mitochondrial activity in mediating ELF-EMF stimulatory effect on human sperm motility. Bioelectromagn.. 2011;32:15-27.
- [Google Scholar]
- Laboratory Procedure Manual: Biochemistry Profile. New Mexico: Coulston Foundation Alamogordo; 2000. p. :1-57.
- Kadhum, E.H., 2015. Histological and biochemical study on the effects of electromagnetic radiation on males and females rats (Rattus norvegicus). Thesis submitted to College of Science, University of Thi-Qar. pp. 1–105.
- In-vivo study on the harmful effect of the extremely low frequency unipolar pulsating magnetic field in mice. Korean Med. Sci.. 1997;12:128-134.
- [Google Scholar]
- Early effects of ovotoxicity induced by 4-vinylcyclohexene diepoxide in rats and mice. Reprod. Toxicol.. 1999;13:67-75.
- [Google Scholar]
- Effects of radiofrequency electromagnetic wave exposure from cellular phones on the reproductive pattern in male Wistar rats. Appl. Biochem. Biotechnol.. 2011;164:546-559.
- [Google Scholar]
- A study of the effects of electromagnetic field on islets of langerhans and insulin release in rats. Crescent J. Med. Biol. Sci.. 2015;2:1-5.
- [Google Scholar]
- The effects of electromagnetic field on the microstructure of seminal vesicles in rat: a light and transmission electron microscope study. Pak. J. Biol. Sci.. 2008;11:692-701.
- [Google Scholar]
- Histological and ultrastructural analyses of male mice exposed to mobile phone radiation. J. Toxicol. Rev.. 2012;1:1-6.
- [Google Scholar]
- Biodiversity Conservation in the Thar Desert; with Emphasis on Endemic and Medicinal Plants. The Environmentalist. 2003;23:137-144.
- [Google Scholar]
- The effect of isothermal non-ionizing electromagnetic field on the liver of mice. Egypt. J. Exp. Biol. (Zool.). 2009;5:93-99.
- [Google Scholar]
- The histopathological effects of an electromagnetic field on the kidney and testis of mice. Eurasia J. Biosci.. 2011;5:103-109.
- [Google Scholar]
- Changes in cell proliferation due to environmental nonioizing radiation: 2 microwave radiation. Bioelectrochem. Bioenerg.. 1998;44:251-255.
- [Google Scholar]
- Biological effects of radio-frequency electromagnetic field. Encycl. Biomat. Biomed. Eng. 2005:1-8.
- [CrossRef] [Google Scholar]
- Comments on “Radiofrequency electromagnetic Welds (UMTS, 1,950 MHz) induce genotoxic effects in vitro in human fibroblasts but not in lymphocytes” by Schwarz et al. (Int Arch Occup Environ Health 2008: doi: 10.1007/s00420-008-0305-5) Int. Arch. Occup. Environ. Health.. 2009;82:275-278.
- [Google Scholar]
- Critical comments on DNA breakage by mobile-phone electromagnetic fields [Diem et al., Mutat Res, 583 (2005) 178–83] Mutat. Res.. 2010;697:60-65.
- [Google Scholar]
- Radio frequency electromagnetic radiation (RF-EMR) from GSM (0.9/1.8GHz) mobile phones induces oxidative stress and reduces sperm motility in rats. Clinics (Sao Paulo). 2009;64(6):561-565.
- [Google Scholar]
- The possible protective role of bone marrow transplantation against alternation induced by gamma radiations on fetal gastrointestinal tract of pregnant albino rats.The Egypt. J. Hospit. Med.. 2012;49:628-660.
- [Google Scholar]
- Markus, A.R., Sarina, M., 2014. Histopathology in Hematoxylin & Eosin stained muscle sections (MDC1A_M.1.2.004). Treat-NMD, Neuromuscular Network, pp. 1–9.
- Effects of 900- MHz electromagnetic field emitted from cellular phone on brain oxidative stress and some vitamin levels of guinea pigs. J. Brain Res.. 2007;1169:120-124.
- [Google Scholar]
- Effects of laptop computers' electromagnetic field on sperm quality. J. Reprod. Infertil.. 2010;11(4):251-258.
- [Google Scholar]
- Pathophysiology of varicoceles in male infertility. J. Human Reprod. Update. 2001;7:473-481.
- [Google Scholar]
- Oxidative damage in the kidney induced by 900-MHz-emitted mobile phone: protection by melatonin. J. Arch. Med. Res.. 2005;36:350-355.
- [Google Scholar]
- Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Cav1- channel activity. J. Cell. Physiol.. 2008;215:129-139.
- [Google Scholar]
- Effects of extremely low frequency electromagnetic fields on liver enzymes in male rats. Bull. Env. Pharmacol. Life Sci.. 2013;3:223-227.
- [Google Scholar]
- SCENIHR, 2015. Potential health effects of exposure to electromagnetic fields. 9th plenary meeting January, Luxemburg, 2015.
- Pulsed electromagnetic fields enhance BMP-2 dependent osteoblastic differentiation of human mesenchymal stem cells. J. Orthopaedic Res.. 2008;26:1250-1255.
- [Google Scholar]
- Correlation between postmenopausal period and certain hepatic enzymes in women of tertiary hospitals of Punjab. Int. J. Medical and Health Res.. 2017;3:109-112.
- [Google Scholar]
- Cell type specific redox status is responsible for diverse electromagnetic field effects. Curr. Med. Chem.. 2007;14:1141-1152.
- [Google Scholar]
- Influence of follic acid on X-irradiation induced DNA damage, Lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes. Toxicol.. 2006;228:249-259.
- [Google Scholar]
- Cancer promotion mouse-skin model by 60 Hz magnetic field: I. Experimental design and exposure system. Bioelectromagn.. 1991;12:261-271.
- [Google Scholar]
- Low level microwave exposure decreases the number of male germ cells and affect vital organs of Sprague Dawley rats. Am. J. Sci. Ind. Res.. 2010;1:410-420.
- [Google Scholar]
- The effect of radiofrequency fields on cell proliferation are nonthermal. Bioelectrochem. Bioenerg.. 1999;48:177-180.
- [Google Scholar]
- Evaluation of relationship between occupational exposure to extremely low frequency (ELF) electric and magnetic fields and hematological changes among workers at high voltage substations. Dissertation submiited to Tehran. Persian: University of Medical Sciences; 1996.
- Histological studies of the low frequency electromagnetic fields effect on liver, testes and kidney in guinea pig. World Appl. Sci. J.. 2007;2:509-511.
- [Google Scholar]







