Bimolecular interaction of zwitterionic surfactant with hen egg white lysozyme (HEWL): A biophysical study
⁎Corresponding authors. jmkhan@ksu.edu.sa (Javed Masood Khan) javedjmk@gmail.com (Javed Masood Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The interaction between Hen Egg White Lysozyme (HEWL) and 3-[(3-cholamidopropyl) dimethyl-ammonio]-1-propanesulphonate (CHAPS) was examined using biophysical techniques (spectrofluorometric, circular dichroism, and dynamic light scattering) at pH 9.0. The results obtained from multi-techniques showed that CHAPS interact with the HEWL strongly and affects protein conformations. The far-UV CD results suggest that lower as well as higher (1.0–15.0 mM) concentrations of CHAPS are inducing α-helical structure in HEWL. The near-UV CD and fluorescence data indicated that the tertiary structure of HEWL is alter in the presence of CHAPS. The ANS dye binding suggest that the exposure of HEWL tertiary structure is dependent on CHAPS concentration. As the concentrations of CHAPS is increasing the exposure of HEWL tertiary structure is also increasing and maximum exposure was found at 15.0 mM of CHAPS concentrations. The hydrodynamic radii of HEWL is also increase in the presence of sub-micellar and micellar concentrations of CHAPS because the tertiary structure of HEWL is disrupted. Overall, the results suggest that CHAPS is inducing secondary structure and disrupting tertiary structure of HEWL. This study provides detailed interaction of CHAPS with HEWL at the molecular level.
Keywords
CHAPS
Hen egg white lysozyme
Zwitterionic surfactant
pH and conformation
- Hen Egg White Lysozyme-HEWL
-
3-[(3-cholamidopropyl) dimethyl-ammonio]-1-propanesulphonate
- CHAPS
-
circular dichroism
- CD
-
Dynamic light scattering-DLS
Abbreviations
1 Introduction
Protein-surfactant interaction study has created massive interest in the last few decades because of its wide applications in drug delivery, detergents, pharmaceuticals, cosmetics, and food processing industry (Otzen 2011). The lower and higher concentrations of surfactants are known to modify the physiochemical functions and conformation of proteins (Ghosh and Dey, 2015). Sometimes surfactants interact with proteins in specific manners and provide stability to the proteins. The protein conformation dynamic is mainly governed by hydrophobic interaction and its degree of attraction of water (Hvidt and Westh, 1998). The monomeric form of surfactants interacts with proteins via electrostatic and hydrophobic interaction, leading to amyloid fibril formation (Khan et al., 2020). However, surfactants above Critical Micelle Concentration (CMC) concentration exclusively interact via hydrophobic interaction, causing denaturation in proteins and affecting the functions of enzymes and proteins (Malik 2015). Amphiphilic (cationic and anionic) surfactants have a strong tendency to interact with proteins, but anionic surfactant is more reactive (Gelamo and Tabak, 2000). Anionic surfactant, i.e., sodium dodecyl sulphate (SDS) interacts electrostatically and hydrophobically with proteins and alters the proteins structures when pH is below the isoelectric point (pI). However, anionic surfactant exclusively interacted via hydrophobic interaction when protein was present at pH above the pI (Liu et al., 2007). Zwitterionic surfactant has a lower affinity to bovine serum albumin (BSA) than other charged surfactants and cannot unfold the protein (Mateos et al., 2020).
Zwitterionic surfactants are having both positively and negatively charged head groups and have several unique properties. The cationic moiety is the part of primary, secondary, or tertiary amines or a quaternary ammonium group. However, anionic moiety comes from either carboxylic acids, sulfonic acids, sulfuric acids esters. Zwitterionic surfactants have been used in several industries like cosmetics, washing products. Most of the study focused on the interaction mechanism of cationic and anionic surfactants with protein, and the very little study was published about the zwitterionic surfactant interaction. Few studies are published in which it was found that the cocamidopropyl betaine (CAPB) is a zwitterionic surfactant binds with proteins bovine serum albumin and provide stability (Erfani et al., 2020). Zwitterionic Gemini surfactants interacted with lysozyme and form complexes and causing conformational changes (Bhat et al., 2021). In this study, we have tried to see the effect of 3-[(3-cholamidopropyl) dimethyl-ammonio]-1-propanesulphonate (CHAPS) “zwitterionic” surfactant on hen egg-white lysozyme (HEWL).
HEWL is a small 14.6 kDa globular protein. HEWL has two domains, i.e., one α-domain which possess four α-helices and one C-terminal 310 helix and one β-domains which consist of triple-stranded antiparallel β-sheets and one 310 helices (Sethuraman and Belfort, 2005). Both the domains are connected by double-stranded antiparallel β-sheet structure. The HEWL is made up of 129 amino acids and a folded structure formed by bonding four disulphide bonds (Fazili et al., 2014). HEWL aggregates cause nonneuropathic systemic amyloidosis, so its structural and functional stability is important to understand (Chiti and Dobson, 2006).
The interaction of HEWL with anionic and cationic surfactants has been studied in detail, and found that HEWL structure is modified in the presence of both the surfactants (Lad et al., 2003; Khan et al., 2019). The anionic surfactant mainly interacted electrostatically with HEWL, while the cationic surfactant interacted hydrophobically confirmed by micro-calorimetry (Chatterjee et al., 2002). Both the surfactants can interact electrostatically with proteins, and causing unfolding is dependent on the pH of the solutions (Chaturvedi et al., 2016). Interactions of CHAPS with proteins were less studied; only a few studied was published until now (Sah and Kim, 2006). It will be interesting to investigate the mode of CHAPS surfactant's interaction with HEWL and try to characterize the conformational changes. The current work aimed to examine the responses, the conformational changes, and the unfolding of HEWL in the presence of CHAPS surfactant at alkaline pH. We have identified the conformational change of HEWL at the secondary as well tertiary structure level. We have used several biophysical techniques to measure all these conformational changes. We have also characterized which forces are involved in the interactions.
2 Materials and methods
2.1 Materials
CHAPS, HEWL, Glycine, and NaOH were procured from Sigma-Aldrich (St. Louis, MO, USA). All other reagents used were of high-quality analytical grade.
3 Methods
3.1 pH measurements
The pH of solutions was measured by Mettler Toledo Seven Easy pH meter (model S20) with an Expert “Pro3 in 1” type electrode. The 20 mM glycine–NaOH and the pH 9.0 buffer were used in all the measurements. Before any measurements, the buffer was filtered through a 0.45-µm Millipore Millex-HV PVDF filter.
3.2 HEWL concentrations measurements
A stock solution of HEWL was made in 20 mM glycine-NaOH buffer of pH 9.0. The HEWL concentration was determined spectrophotometrically using a molar extinction coefficient of 2.65 dm3g−1cm−1 at 280 nm on Perkin Elmer double beam UV–Visible (Lambda 25) spectrophotometer (Seraj et al., 2018).
3.3 CD measurements
Far-UV CD spectra were recorded on a J-815 spectropolarimeter, equipped with a Peltier-type temperature controller. All the far-UV CD measurements were carried out at room temperature. Changes in the secondary structure of the HEWL were monitored in the far-UV region (200–250 nm). The HEWL concentrations were used for far-UV CD is 0.2 mg ml−1. The reference sample containing buffer and the different concentrations of CHAPS detergent was subtracted from the CD signal of HEWL treated with CHAPS.
The near-UV CD was also performed to identify the changes in the tertiary structure of HEWL (1.0 mg ml−1) in response to millimolar concentrations of CHAPS at pH 9.0. The near-UV CD spectra were scanned in the range of 250–300 nm, and every spectrum were collected on the average of two scans. CD instrument was frequently calibrated with D-10- camphor sulfonic acid. To improve the signal-to-noise ratio, two accumulations were made for each scan.
3.4 Fluorescence measurements
In intrinsic fluorescence measurements, the samples were poured in quartz cuvettes of 1 cm optical path. In all the fluorescence measurements, 1.0 mL of solution was employed into the cuvette. The HEWL (0.2 mg ml−1) was treated with different concentrations of CHAPS at pH 9.0. The emission spectra of every sample were recorded in the same buffer at pH 9.0 at room temperature. The samples were excited at 280 nm, and the emitted light was scanned in between 300 and 400 nm. The band slits for excitation and emission were set at 5 nm.
3.5 Dynamic light scattering
DLS measurements were performed on a DynaProMS800 equipped with temperature control (Proterion, Protein Solutions, and Wyatt Technology, and Santa Barbara, CA, USA) operating at a wavelength of 830 nm and a 256-channel multi tau correlator. The CHAPS and HEWL solutions stock were centrifuged at 5000 rpm for 30 min and filtered through a 0.2 μm pore size filter (Whatman). The HEWL (1.0 mg ml−1) were treated with different concentrations of CHAPS at pH 9.0 and leave for overnight. The CHAPS treated HEWL solutions were poured into 2 μl micro-cell of instruments and collected the data. The translation diffusion coefficient was calculated by Dynamics V6 software provided by the supplier. The hydrodynamic radii were calculated from the translation diffusion coefficient by Stoke's-Einstein relationship (Khan et al., 2018).
4 Results and discussion
4.1 Secondary structure modification measurements by far-UV CD
Far-UV CD spectroscopy is an absorption-based spectroscopic technique that provides information about the proteins' secondary structures (Govindarajan et al., 2021). The different secondary structure of proteins gives different far-UV CD peaks in the 180–250 nm region. The CD spectrum in the range of 180–250 nm can be examined in terms of the percent content of α-helix, β-sheet, β-turn, etc. (Khan et al., 2012). In the current study, far-UV CD spectroscopy was exploited to observe the changes persuaded by different concentrations of CHAPS on the secondary structure of HEWL at pH 9.0. From Fig. 1A, it was evident that HEWL untreated with CHAPS gives a characteristic two negative minima at 208 nm and 222 nm, which signifies the presence of α-helix. The percentage of α-helix content of pure HEWL was found to be 40%, which is very similar to published reports (Sheng et al., 2016). However, the addition of different concentrations (2.0–15.0 mM) of CHAPS, the negative ellipticity at 222 nm is increased significantly, and very little changes in ellipticity at 208 nm were found. The negative ellipticity values at 222 nm were plotted in Fig. 1B. The negative ellipticity at 222 nm was continuously increased in response to CHAPS concentration. The increase in ellipticity at 222 nm is signified that the α-helical content of HEWL is increased. The percent α-helical changes in response to different concentrations of CHAPS were tabulated in Table 1. The α-helical content of HEWL at pH 9.0 without CHAPS was found 40 percent, and in the presence of CHAPS, the content is found almost 43 percent. The overall secondary structure of HEWL is modified in the presence of CHAPS surfactant. From the far-UV CD results, it was concluded that the premicellar, as well as micellar concentrations of CHAPS, induces α-helical structure in HEWL. The possible cause of induction of α-helical content in HEWL is hydrophobic interaction. The hydrophobic part of CHAPS and non-polar amino acids of HEWL interacted with each other and caused local changes (Gull et al., 2009).
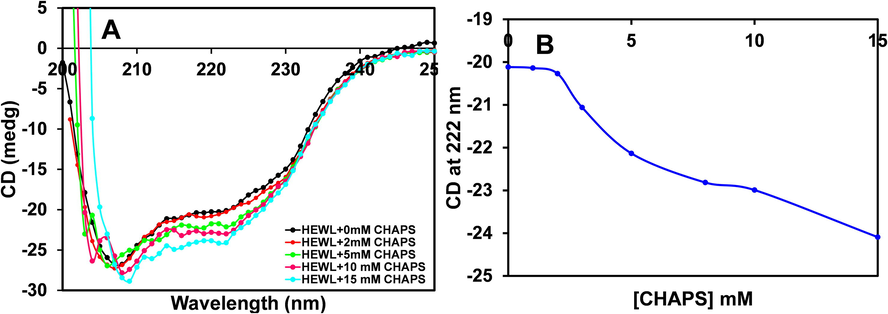
- A. Far-UV CD spectra showing changes in the secondary structure of HEWL in the presence of different concentrations of CHAPS at pH 9.0. (B) The negative ellipticity of HEWL at 222 nm was plotted against increasing concentrations of CHAPS. The HEWL concentrations was taken 0.2 mg ml−1 in all the measurements.
| S. No. | Conditions | % α-helix | % β-sheet |
|---|---|---|---|
| 1 | HEWL + pH 9.0 | 40 ± 2 | 10 ± 0.9 |
| 2 | HEWL + 1.0 mM CHAPS + pH 9.0 | 41.15 ± 2.01 | 10 ± 1.01 |
| 3 | HEWL + 2.0 mM CHAPS + pH 9.0 | 41.17 ± 2.01 | 11 ± 1.09 |
| 4 | HEWL + 5.0 mM CHAPS + pH 9.0 | 42.17 ± 1.81 | 12.09 ± 0.98 |
| 5 | HEWL + 10.0 mM CHAPS + pH 9.0 | 44.12 ± 2.10 | 12.09 ± 1.04 |
| 6 | HEWL + 15.0 mM CHAPS + pH 9.0 | 45.90 ± 2.01 | 12.02 ± 1.02 |
4.2 Tertiary structure modification measurements by Near-UV CD
The impact of CHAPS on tertiary structure conformation of HEWL was evaluated by Near-UV CD measurements. The near-UV CD peaks appear due to aromatic amino acids (Phe, Tyr and Trp) side chains, and these residues movements provide a fingerprint of tertiary structure (De Laureto et al., 2002). In Fig. 2, the near-UV CD spectra of HEWL without detergents give a positive peak between 280 and 300 nm, which is very similar to other published reports (Sharma et al., 2017). However, the HEWL is treated with 1.0 mM of CHAPS the negative ellipticity is drastically increased, and the peak position is changed. In native HEWL, the weak peak at 292 nm was recorded, but in the presence of 1.0 mM of CHAPS, the prominent peak at 292 nm has appeared. The peak at 286 nm was found to be more prominent in the presence of CHAPS. The near-UV CD data suggest that the HEWL tertiary structure was modified in the presence of CHAPS surfactant. Similarly, it was reported that the Succinylation of HEWL modified the tertiary structure (van der Veen et al., 2005).
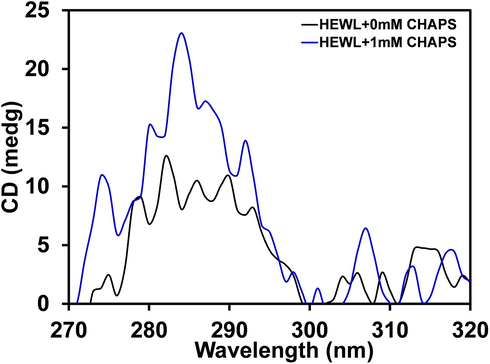
- Near-UV CD spectra of HEWL in the presence of 1.0 mM of CHAPS concentrations. The HEWL concentrations was fixed 1.0 mg ml−1 in all the measurements.
4.3 Intrinsic fluorescence measurements
Intrinsic fluorescence measurements of proteins provide important information about protein dynamics, structural changes and also tell us about the protein folding and unfolding and are sometimes used to investigate the association and dissociation of ligands (Ghosh et al., 2015). The intrinsic fluorescence spectra pattern exposed the story of the local microenvironment changes in and around the tryptophan (Trp) residue. HEWL contains six Trp residues; almost 80 % of the fluorescence in HEWL comes from residue Trp62, 108, and these residues are mostly buried in the hydrophobic core of HEWL (Ito et al., 2011). Fig. 3, shows the fluorescence spectrum of HEWL without and with different concentrations of CHAPS at pH 9.0, and all the samples were excited at 280 nm. The emission spectra of HEWL without CHAPS are showing maximum fluorescence intensity at 341 nm after excitation at 280 nm. The maximum emission at 341 nm is attributing that the HEWL is well folded and compact. Addition of 1.0–2.0 mM of CHAPS, the HELW fluorescence intensity was found to be increased and in the presence of higher concentrations (5.0–10.0 mM) of CHAPS, the fluorescence intensity slightly decreased. The possible cause of increase in florescence intensity in the presence of low CHAPS concentrations are that the monomeric concentrations of CHAPS was bind with HEWL and exposed Trp residues towards apolar environment. However, in the presence micellar concentrations of CHAPS the fluorescence intensity of HEWL was found lower due to quenching effects. The fluorescence intensity of HEWL should be more in the presence of higher concentration of CHAPS but CHAPS quenched the fluorescence intensity that is why low fluorescence intensity recorded. The intrinsic fluorescence data suggest that the HEWL tertiary structure is modified in the presence of CHAPS surfactant. The Trp residues are exposed to polar environment in the presence of both the concentrations but the fluorescence intensity was found decrease at higher concentrations because of quenching mechanism. The exposure of Trp residues were further characterized by ANS fluorescence.
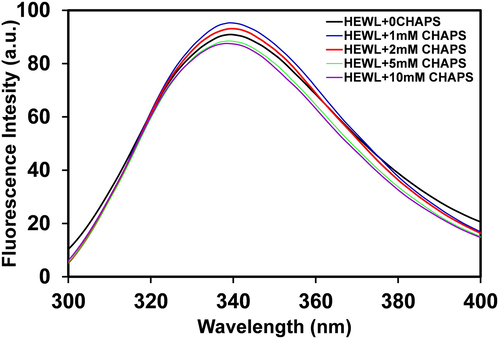
- The intrinsic fluorescence spectra of HEWL (0.2 mgml−1) were scanned in 20 mM Glycine-NaOH, pH 9.0, in the absence (black line) and in the presence of 1.0 (blue line), 2.0 (red line), 5.0 (green line), 10.0 (purple line) mM of CHAPS surfactant. The samples were incubated overnight before measurements.
4.4 8-Anilino-1-Naphthalene-Sulphonic acid (ANS) binding study
ANS is a broadly used fluorescent probe for the characterization of exposure of hydrophobic patches of protein (Kim et al., 2016). ANS has a strong affinity to partially unfolded protein and is unable to bind well folded or fully unfolded proteins because well-folded protein hydrophobic patches are buried in the core of the protein, while in the denatured protein, the hydrophobic patches are far away to ANS molecules, resultant no ANS binding (Park et al., 2011). The fluorescence intensity of ANS is increased when it is bound to the partially unfolded state of proteins (Matulis and Lovrien, 1998). The fluorescence emission spectra of HEWL without and with different concentrations of CHAPS are presented in Fig. 4. As evident from Fig. 4, the fluorescence intensity of ANS of the samples containing HEWL without surfactant is almost zero, signifying that the hydrophobic patches of HEWL are buried inside the core and HEWL is well folded. However, HEWL treated with different concentrations of CHAPS, a contentious increase in ANS fluorescence intensity was recorded. The ANS fluorescence intensity was found lower in the presence of low CHAPS concentration, and as the concentrations of CHAPS increase the fluorescence intensity increases respectively. The increase in ANS fluorescence intensity designated that the hydrophobic patches of HEWL are exposed in response to increasing concentrations of CHAPS. The exposure of hydrophobic patches leads to the unfolding of HEWL molecules. The ANS binding data signified that the HEWL tertiary structure is disrupted in the presence of low as well as higher concentrations of CHAPS. Interestingly, we can say from all the spectroscopic data that the tertiary structure of HEWL disrupted in the presence of CHAPS and gain the secondary structure in the presence of CHPAS.
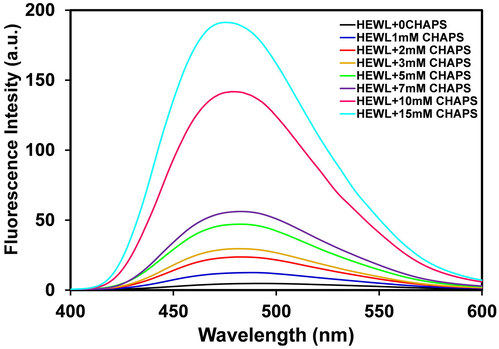
- Extrinsic fluorescence spectra of HEWL (0.2 mg mL−1) in 20 mM Glycine-NaOH buffer, pH9.0. The florescence spectra of HEWL without (black line), and with 1.0 (blue line), 2.0 (red line), 3.0 (yellow line), 5.0 (green line), 7.0 (purple line), 10.0 (magenta line), 15.0 (cyan line) mM of CHAPS concentrations.
4.5 Dynamic light scattering
DLS has been used to observe the changes in the hydrodynamic size of proteins during unfolding and refolding (Kumari et al., 2020). This has been extended here to study the changes in the hydrodynamic radius of HEWL due to the treatment of CHAPS surfactant. The changes in hydrodynamic radii of HEWL with different concentrations of CHAPS are tabulated in Table 1. Briefly, in Fig. 5, the hydrodynamic radii of HEWL at pH 9.0 is found around 2.1 nm, which is much closed to previously reported results (Khan et al., 2014). The hydrodynamic radii of HEWL increased when it was incubated with 1.0 mM of CHAPS surfactant at the same pH. The size of HEWL is increasing in response to the increasing concentrations of CHAPS, and the maximum size was found at 15.0 mM concentrations. The change in HEWL hydrodynamic radii was not found as bigger as the complete unfolded HEWL. The hydrodynamic radii changes in response to CHAPS suggest that the HEWL is not completely unfolded but only some conformational changes were seen. The DLS data is also supporting to the intrinsic and extrinsic fluorescence data. The only tertiary structure of HEWL is disrupted in the presence of CHPAS. The size of Table 2.
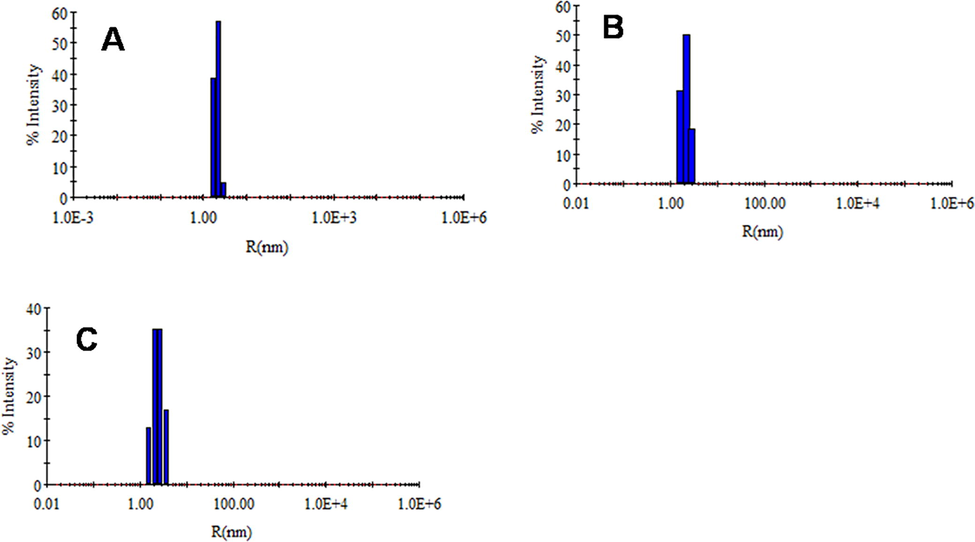
- Hydrodynamic radii of HEWL without (A) and with 1.0 (B), 15.0 (C) mM of CHAPS concentrations at 20 mM Glycine-NaOH, pH 9.0. The HEWL concentration was fixed 1.0 mg.mL−1 in all the samples.
| S. No. | Conditions | Hydrodynamic Radii (Rh) nm | Percent polydispersity (%Pd) |
|---|---|---|---|
| 1 | HEWL + pH 9.0 | 2.1 ± 0.09 | 10.0 |
| 2 | HEWL + 1.0 mM CHAPS + pH 9.0 | 2.16 ± 0.1 | 12.0 |
| 3 | HEWL + 3.0 mM CHAPS + pH 9.0 | 2.2 ± 0.12 | 11.80 |
| 4 | HEWL + 5.0 mM CHAPS + pH 9.0 | 2.4 ± 0.10 | 12.98 |
| 5 | HEWL + 10.0 mM CHAPS + pH 9.0 | 2.5 ± 0.07 | 15.0 |
| 6 | HEWL + 15.0 mM CHAPS + pH 9.0 | 2.8 ± 0.13 | 20.01 |
5 Conclusions
The result of this study revealed that CHAPS surfactant interacted marginally with HEWL at pH 9.0 and caused the conformational change. All the CHAPS concentrations are is inducing α-helical secondary structure in HEWL, and α-helical induction is dependent on CHAPS concentrations. The intrinsic and extrinsic fluorescence data suggest that the tertiary structure of HEWL is disrupted, and maximum disruption was found at 15.0 mM CHAPS concentrations. The size of HEWL is also increased due to the slight unfolding of HEWL. The results of the present study provide details of molecular interaction between protein-surfactant. The CHAPS is harming lees to the protein, so this may provide direction to widely use of this surfactant in food processing, cosmetics, pharmaceutical industries.
Acknowledgement
Authors are grateful to the Researchers Supporting Project Number (RSP-2021/360), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Exclusive behaviour of asymmetric zwitterionic gemini surfactants towards lysozyme. J. Mol. Liq.. 2021;336:116583
- [Google Scholar]
- Studies on surfactant-biopolymer interaction. I. Microcalorimetric investigation on the interaction of cetyltrimethylammonium bromide (CTAB) and sodium dodecylsulfate (SDS) with gelatin (Gn), lysozyme (Lz) and deoxyribonucleic acid (DNA) Biophys. Chem.. 2002;98(3):313-327.
- [Google Scholar]
- Comparative insight into surfactants mediated amyloidogenesis of lysozyme. Int. J. Biol. Macromol.. 2016;83:315-325.
- [Google Scholar]
- Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem.. 2006;75(1):333-366.
- [Google Scholar]
- Effect of zwitterionic betaine surfactant on interfacial behavior of bovine serum albumin (BSA) J. Mol. Liq.. 2020;318:114067.
- [CrossRef] [Google Scholar]
- Induction of amyloidogenicity in wild type HEWL by a dialdehyde: analysis involving multi-dimensional approach. Int. J. Biol. Macromol.. 2014;64:36-44.
- [Google Scholar]
- Spectroscopic studies on the interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2000;56(11):2255-2271.
- [Google Scholar]
- Binding of Fatty Acid Amide Amphiphiles to Bovine Serum Albumin: Role of Amide Hydrogen Bonding. J. Phys. Chem. B.. 2015;119(25):7804-7815.
- [Google Scholar]
- Solution behavior and interaction of pepsin with carnitine based cationic surfactant: fluorescence, circular dichroism, and calorimetric studies. J. Phys. Chem. B. 2015;119(39):12632-12643.
- [Google Scholar]
- Tannin acyl-hydrolase production by Bacillus subtilis KMS2-2: Purification, characterization, and cytotoxicity studies. J. King Saud Univ.– Sci.. 2021;33(3):101359.
- [CrossRef] [Google Scholar]
- Spectroscopic studies on the comparative interaction of cationic single-chain and gemini surfactants with human serum albumin. J. Biochem.. 2009;145(1):67-77.
- [Google Scholar]
- Different Views on the Stability of Protein Conformations and Hydrophobic Effects. Solution Chem.. 1998;27:395-402.
- [Google Scholar]
- Glycine amide shielding on the aromatic surfaces of lysozyme: Implication for suppression of protein aggregation. FEBS Lett.. 2011;585:555-560.
- [Google Scholar]
- Protonation favors aggregation of lysozyme with SDS. Soft Matter. 2014;10(15):2591.
- [CrossRef] [Google Scholar]
- An intermittent amyloid phase found in gemini (G5 and G6) surfactant induced β-sheet to α-helix transition in concanavalin A protein. J. Mol. Liq.. 2018;269:796-804.
- [Google Scholar]
- Effect of cetyltrimethylammonium bromide (CTAB) on the conformation of a hen egg white lysozyme: a spectroscopic and molecular docking study. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2019;219:313-318.
- [Google Scholar]
- SDS can be utilized as an amyloid inducer: a case study on diverse proteins. PloS one. 2012;7(1):e29694.
- [Google Scholar]
- Cationic Gemini surfactant stimulates amyloid fibril formation in bovine liver catalase at physiological pH. A biophysical study. RSC Adv.. 2020;10(71):43751-43761.
- [Google Scholar]
- Thermodynamic analysis of ANS binding to partially unfolded α-lactalbumin: correlation of endothermic to exothermic changeover with formation of authentic molten globules. J. Mol. Recognit.. 2016;29(9):446-451.
- [Google Scholar]
- Refolding of protein unfolded by gemini surfactants using β-cyclodextrin and sodium dodecyl sulfate in aqueous medium: study on role of spacer chain of surfactants. J. Mol. Liq.. 2020;300:112238
- [Google Scholar]
- The interaction between hemoglobin and two surfactants with different charges. Int. J. Biol. Macromol.. 2007;41(5):548-557.
- [Google Scholar]
- Surfactant–amino acid and surfactant–surfactant interaction in aqueous medium: a review. Appl. Biochem. Biotechnol.. 2015;176:2077-2106.
- [Google Scholar]
- Binding isotherms of surfactants used in detergent formulations to bovine serum albumin. Colloids Surf. A. 2020;598:124801.
- [CrossRef] [Google Scholar]
- 1-Anilino-8-naphthalene sulfonate anion–protein binding depends primarily on ion pair formation. Biophys. J.. 1998;74(1):422-429.
- [Google Scholar]
- Protein-surfactant interactions: a tale of many states. Biochim. Biophys. Acta. 2011;1814(5):562-591.
- [Google Scholar]
- The client protein p53 adopts a molten globule-like state in the presence of Hsp90. Nat. Struct. Mol. Biol.. 2011;18(5):537-541.
- [Google Scholar]
- Partly folded states of members of the lysozyme/lactalbumin superfamily: a comparative study by circular dichroism spectroscopy and limited proteolysis. Protein Sci.. 2002;11(12):2932-2946.
- [Google Scholar]
- Improvement of interfacial protein stability by CHAPS. Biotechnol. Lett.. 2006;28(8):567-570.
- [Google Scholar]
- Unraveling the novel effects of aroma from small molecules in preventing hen egg white lysozyme amyloid fibril formation. PLoS ONE. 2018;13(1):e0189754.
- [Google Scholar]
- Protein structural perturbation and aggregation on homogeneous surfaces. Biophys. J.. 2005;88(2):1322-1333.
- [Google Scholar]
- Comparative effect of cationic gemini surfactant and its monomeric counterpart on the conformational stability and activity of lysozyme. RSC Adv.. 2017;7(27):16763-16776.
- [Google Scholar]
- The changes of secondary structures and properties of lysozyme along with the egg storage. Int. J. Biol. Macromol.. 2016;92:600-606.
- [Google Scholar]
- Effects of Succinylation on the Structure and Thermostability of Lysozyme. J. Agric. Food Chem.. 2005;53(14):5702-5707.
- [Google Scholar]







