Translate this page into:
Bifenthrin induced toxicity in Ctenopharyngodon idella at an acute concentration: A multi-biomarkers based study
⁎Corresponding authors. sanaullah@ue.edu.pk (Sana Ullah), ghulamnabiqau@gmail.com (Ghulam Nabi), wanghekunyuan@189.cn (Kunyuan Wanghe)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
The deleterious impacts of bifenthrin on grass carp at acute concentrations were evaluated through multiple biomarkers. The appraised toxicological endpoints were behavioral inconsistencies, tissues based biochemical disruption, serum biochemical profiling, and hematotoxicity. The current study will broaden ideal toxicity biomarkers in future ecotoxicological studies and provides novel insight into bifenthrin-induced toxicities.
Abstract
Bifenthrin is a (type I; III generation; non-cyano) synthetic pyrethroid used for both agricultural and non-agricultural purposes. Due to its common use, it is widely reported from different parts of the world. Its use is on a continuous rise, which poses a significant risk to non-target organisms including fish. Therefore, the current study was undertaken to assess multiple biomarkers-based toxicological endpoints of bifenthrin-exposed Ctenopharyngodon idella, grass carp. The first part of the research evaluated the LC50 of bifenthrin against grass carp for 96 h, followed by investigating its effect on their biochemical profile [total protein; antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), glutathione peroxidase (GSH-Px), glutathione reductase (GR), and glutathione-s-transferase (GST); serum biochemical parameters including chloride, magnesium, sodium, potassium, albumin, total bilirubin, cholesterol, inorganic phosphate, total protein, and urea; DNA damage in term of tail length, %tail DNA, tail moment, and olive tail moment (OTM)], physiological disruption [whole body cortisol, Acetylcholinesterase (AChE), glucose], hematological profile [red blood cells (RBCs), white blood cells (WBCs), hemoglobin (Hb), platelets, packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular haemoglobin concentration (MCHC)], and behavioral inconsistencies (hyperactiveness, hypoactiveness, equilibrium loss, rapid swimming, adapting vertical position, jumping, etc.). At acute concentration bifenthrin exposure induced stress, evaluated in terms of reactive oxygen species (ROS) and lipid peroxidation (LPO), which led to different subtle changes in the behavioral, biochemical, and hematological profile of grass carp. The current study explicates risks posed by bifenthrin on fish with underlying the possible mechanism of toxicity and supports the necessity to investigate multiple biomarkers-based toxicological endpoints in chemical risk assessment and safety analysis using fish as a model organism. Bifenthrin was classified as a highly toxic pesticide for grass carp.
Keywords
Synthetic pyrethroids
Bifenthrin
Oxidative stress
Behavioral disruption
Biochemical profiling
DNA damage
Hematotoxicity
1 Introduction
The modern world is witnessing a swift increase in the pollution level, which is of grave concern environmentally as well as a severe menace for human and animal health. The pollutants regarded as deleterious include heavy metals, domestic sewage, and agricultural runoffs having pesticides (Ullah, 2019). These pollutants flow to aquatic bodies, both freshwater, and marine. Of these, pesticides make a major portion. Keeping in view the continuously increasing human population, there is an urgent need to address food scarcity-related issues. Zero hunger is one of the prime Sustainable Development Goals (SDGs) and Mellinium Development Goals (MDGs). At present, the agricultural productivity is elevated with different techniques and chemicals, pesticides being one of them - employed to deter, kill, and repel pests damaging crops, reducing yield, and degrading food quality (Ullah et al., 2019).
Pesticides from the agricultural fields deteriorate the water quality, which subsequently poses a threat to biodiversity and aquatic animals’ health. These pesticides keep rotating as per their use and quantity employed, however, their use is continuously increasing globally. Among these pesticides, different classes are employed against different target groups such as insecticides, fungicides, herbicides, nematicides, rodenticides, acaricides, etc. However, the use of insecticides surpasses the other classes in terms of their employability (Ullah et al., 2018). Different classes of insecticides are employed including organophosphates and synthetic pyrethroids. Owing to the acute neurotoxic effects of organophosphates on mammals, their use phased out gradually. This consequently led to an increase in the use of synthetic pyrethroids (Werner and Moran, 2008). In 2009 more than 1500 MT pyrethroids were employed as compared to 100 MT in 2000, and an increasing trend in pyrethroids use was evident according to that report (van den Berg et al., 2012).
Synthetic pyrethroids (synthetic analogous of ornamental plant Chrysanthemum cinerariaefolium) were developed in the 1970s. Initially, they were used for agricultural purposes such as to protect food, however, they were used for different non-agricultural purposes later on including lice shampoos, mosquito repellents, insects control sprays, and controlling ectoparasites of animals (Velisek et al., 2009; Brander et al., 2016a; Brander et al., 2016b). The use of synthetic pyrethroids is on a continuous rise due to their low toxic effects on birds and mammals. These are the second-highest used insecticides (Wang et al., 2017). Several studies evaluated the presence of synthetic pyrethroids in different biosphere and confirmed their presence. Some studies confirmed their presence in human breast milk and urea (Ueyama et al., 2009; Corcellas et al., 2012; Wielgomas and Piskunowicz, 2013) and aquatic organisms such as placenta and breast milk of Brazilian dolphins (Alonso et al., 2012).
Bifenthrin is a moderately hazardous (class II) compound and therefore is allowed by WHO for public use (Wang et al., 2017). Due to these characteristics, bifenthrin is widely employed. Moreover, it is highly persistent in the environment, therefore, it has been reported in agricultural fields, urban areas, house dust, floor wipes, and body tissues of humans and animals from different parts of the world (Quirós-Alcalá et al., 2011; Hladik and Kuivila, 2012; Hall and Anderson, 2014; Trunnelle et al., 2014; Weston et al., 2014; Yang et al., 2018). Bifenthrin was detected in 58% of the total collected samples in the US whereas in 78.2% of the total samples in Japan (Hladik and Kuivila, 2012; Jeppe et al., 2017).
The extensive use of bifenthrin and a continuous increase in its use is elevating the concerns regarding its fate in the environment and its serious toxic impacts on aquatic non-target animals (Phillips et al., 2012). Previous studies assessed different toxicological endpoints and biomarkers to determine bifenthrin induced toxicities including oxidative stress induction, developmental malformations, neurotoxicity, neurobehavioral toxicity, endocrine disruption, and immunotoxicity in pregnant mice (Jin et al., 2013a; Syed et al., 2016), male mice (Jin et al., 2015), rats (Liu et al., 2011; Scollon et al., 2011; Lu, 2013), and human breast cells (Zhao et al., 2010). The toxicological endpoints of bifenthrin are also studied in some model fish species including trout (Velisek et al., 2009), fathead minnow (Beggel et al., 2010), and zebrafish (Jin et al., 2013b; Bertotto et al., 2017). However, studies on bifenthrin-mediated toxicities in fish are still scanty as compared to other pyrethroids such as cypermethrin, deltamethrin, etc. and most of these studies on bifenthrin are embodied to zebrafish.
Keeping in view the limited understanding of bifenthrin mediated toxicities in Chinese carps, specifically no comprehensive studies on grass carp (Ctenopharyngodon idella), this multi-biomarkers-based study was undertaken. The current study was aimed at profiling the behavior, hematology, and biochemistry of the bifenthrin intoxicated grass carp at acute concentration. The toxicological endpoints assessed are behavioral, hematological, biochemical, and physiological via different well-established parameters including hematological indices, lipid peroxidation, reactive oxygen species, total protein contents, antioxidant enzymes, biochemical profile, whole-body cortisol, and AChE activity. This study will broaden ideal toxicity biomarkers for ecotoxicology, aquatic-toxicology and chemical risk assessment-based research, provide novel insights regarding mechanisms of toxicities of bifenthrin, and develop a general understanding of pyrethroids-induced risks on aquatic non-target animals, fish.
2 Materials and methods
2.1 Fish handling and acclimatization
Fingerlings (age of 90 days; average-sized and healthy; irrespective of their sex) of grass carp (Ctenopharyngodon idella) were transported to the lab from a local fish hatchery via the live-hauling method in a closed-system. They were then distributed in fiberglass tanks, and acclimatized for two weeks before the experiment. The fish were fed 35% basal protein (at 5% body weight; two times a day). The water was exchanged, a 12:12 h photoperiod was sustained, and to avoid stress the wastes and feed remains were siphoned off daily.
2.2 Water quality
The temperature, dissolved oxygen (DO), ammonia, pH, and hardness were assessed daily. The water quality was regularly assessed using DO meter, pH meter, and water quality checker-meter. It was made sure to keep the water quality parameters under permissible limits. Dead fish were removed to avoid deterioration of the water. The water quality was healthy such as DO ranged between 6.5 and 7.3 mg/L, temperature between 24.5℃ and 25.7℃, ammonia<0.21 ppm, pH between 6.9 and 7.4, conductivity under 290 µS/cm, and total hardness under 170 mg/L.
2.3 Experimental design
This experiment was conducted in triplicates in a semi-static system. The fish were stocked (1.5 g/L) in 6 aquaria (3 control and 3 treated groups). After each 24 h specimens were collected for biochemical and hematological examinations from each aquarium before the renewal of bifenthrin concentration and changing water (on a daily basis). MS222 was used for anesthetizing collected fish specimens (60 mg/L). The tissues including brain, gills, muscles, and liver were dissected out and stored (-20℃) for further assays/experimentations/analysis.
2.4 Test chemical
Bifenthrin was purchased from the local market and used in the current study (Table S1). Stock solution and required dilution amount were prepared, and a total of 6 concentrations (15 µg/L, 30 µg/L, 45 µg/L, 60 µg/L, 75 µg/L, and 90 µg/L) were used while evaluating the LC50, though, the acute concentration of bifenthrin was employed during the study.
2.5 Determination of LC50 concentration
After the division of the fish into different groups, they were exposed to 15, 30, 45, 60, 75, and 90 µg/L bifenthrin, based on previous experiments on bifenthrin (semi-static bioassay), employed against different species. As the chemical employed is in 10 EC formulation, so 15 µg/L corresponds to 1.5 µg/L, 30 µg/L to 3 µg/L, 45 µg/L to 4.5 µg/L, and so on. The mortality of the fish specimens after exposure to bifenthrin was observed, recorded (after each 12 h through 96 hr), and dead fish were removed instantly to avoid water quality deterioration. Probit analysis (95% CI) was employed to determine LC50.
2.6 Behavioral study
The fish in the control group and treated group after exposure to bifenthrin were observed keenly for behavioral inconsistencies, such as erratic swimming, hyperactivity, hypoactivity, sluggishness, and adapting vertical position. The fish were observed for other clinical symptoms as well, such as body color and mucus secretion.
2.7 Hematology
The hematological parameters [Red Blood Cells (RBCs), Hemoglobin (Hb), White Blood Cells (WBCs), Platelets, Packed Cell Volume (PCV), Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), and Mean Corpuscular Hemoglobin Concentration (MCHC)] were evaluated by the protocols followed by David et al. (2015).
2.8 Biochemical studies
Biochemical studies were conducted by following standard procedures and protocols. Ullah et al. (2019b) provided a detailed description of these procedures and protocols. Reactive oxygen species (ROS) were assayed by following Contreras et al. (2005), Lipid peroxidation (LPO) by following Wright et al. (1981), Total protein contents by following Lowry et al. (1951), antioxidant enzymes by following standard literature [Catalase (CAT; Chance and Maehly, 1955), Superoxide Dismutase (SOD; Kakkar et al., 1984), Glutathione Reductase (GR; Carlberg and Mannervik, 1975), Glutathione Peroxidase (GSH-Px; Mohandas et al., 1984), and Glutathione-s-transferase (GST; Habig et al., 1974)], and DNA damage (comet assay) was assessed by following Singh et al. (1988). Acetylcholine esterase (AChE) and whole-body cortisol were evaluated by following Bibi et al. (2014) and Zuberi et al. (2014), respectively. Blood biochemical profile was evaluated for albumin, urea, sodium, cholesterol, total bilirubin, total protein, inorganic phosphate, and potassium by following Qadir et al. (2014).
2.9 Statistical analysis
The data, shown as Mean ± SE, is analyzed through ANOVA followed by LSD (for multiple variance analysis in order to test the homogeneity of variance) using Statistix (V. X). The P-value < 0.05 was considered statistically significant.
3 Results
3.1 Appraisal of LC50 of bifenthrin for 96 h against grass carp
The mortalities of the fish increased linearly with an increase in the concentration of bifenthrin (Table S2). Similarly, a time-dependent increase in mortalities was observed. The fish were exposed to 15, 30, 45, 60, 75, and 90 µg/L. The Probit analysis revealed 60 µg/L of bifenthrin as LC50 against grass carp (Fig. S1). As the chemical employed is in 10 EC formulation, so 60 µg/L corresponds to 6 µg/L.
3.2 Effects of acute concentration of bifenthrin on grass carp behavior
The first occurrence of behavioral inconsistencies was observed after three hours of exposure. Table S3 shows the behavioral profile of bifenthrin intoxicated Ctenopharyngodon idella. Different behavioral inconsistencies of grass carp observed after exposure were jumping, erratic swimming, increased air gulfing, equilibrium loss, adapting vertical position, increased gill movement, body twitches, sluggishness, and becoming motionless. However, the fish in the control group was not showing such signs and there was no such behavioral disruptions in them. Another clinical symptom observed was in the form of a decrease in the intensity of body color as the bifenthrin exposed fish become pale colored.
3.3 Effects of acute concentration of bifenthrin on grass carp biochemical profile
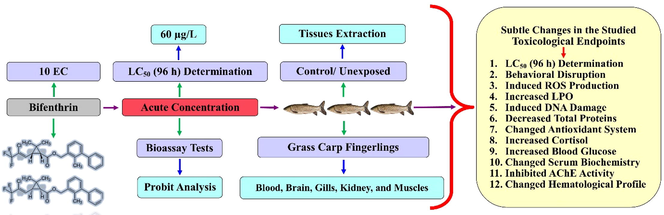
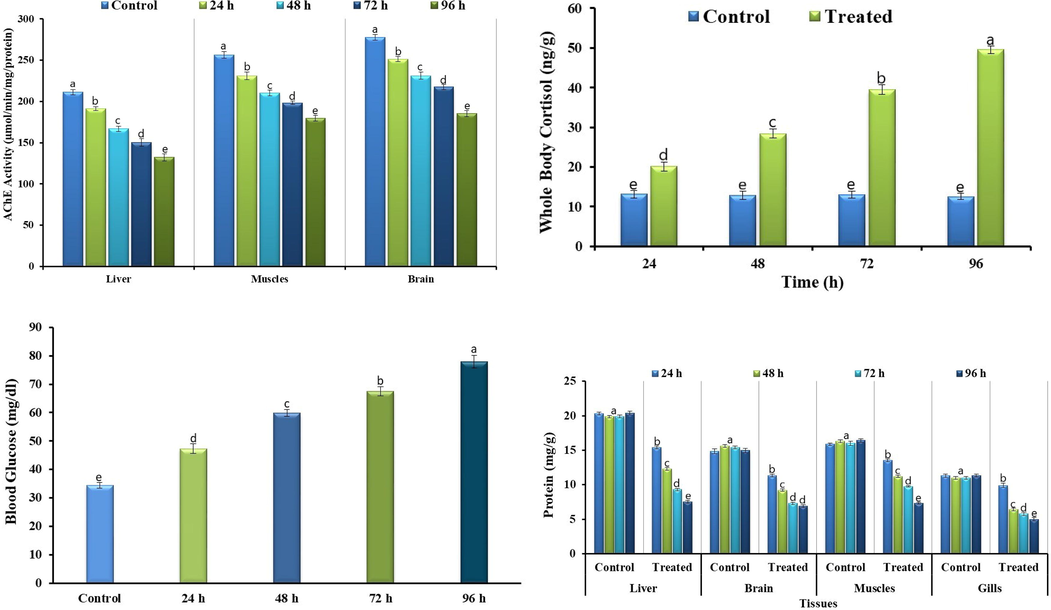
Bifenthrin exposure led to the disturbed biochemical profile of grass carp, such as it resulted in a reduction of some of the studied parameters whereas elevated the others. Bifenthrin exposure increased reactive oxygen species (ROS) production in different tissues (brain, liver, muscles, and gills) of grass carp (Fig. 1). The increased production of ROS led to the induction of lipid peroxidation (LPO) and hence a linear increase in the activity of LPO was observed (Fig. 1). At acute concentration, bifenthrin inhibited the activity of AChE (Fig. 2) in different tissues whereas the whole body cortisol got increased (Fig. 2) which subsequently led to an increase in blood glucose (Fig. 2). The exposure resulted in decreased total protein content in the studied tissues (Fig. 2) whereas increased the activities of antioxidant enzymes including catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), glutathione reductase (GR), and glutathione-S-transferase (GST) (Fig. 3). The blood biochemical profile of grass carp was disrupted after the exposure of bifenthrin in terms of total bilirubin, albumin, cholesterol, inorganic phosphate, sodium, potassium, urea, chloride, and total protein (Fig. 4). DNA damage increased in a time-dependent manner (in terms of tail length, tail DNA (%),tail moment, and olive tail moment) in peripheral blood erythrocyte after the exposure of grass carp to bifenthrin (Fig. 5).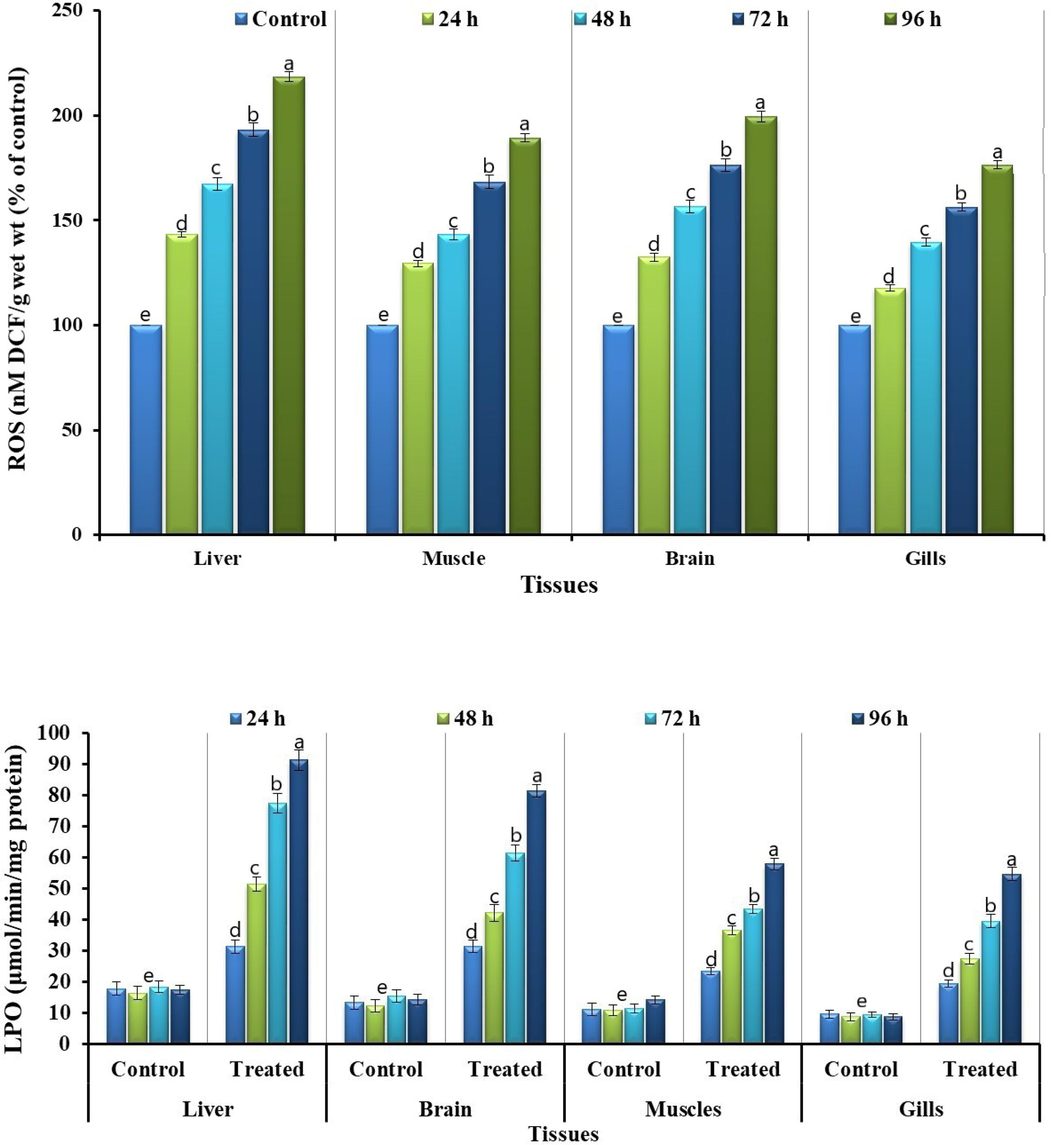
ROS profile and lipid peroxidation in bifenthrin intoxicated Ctenopharyngodon idella. Data presented as Mean ± SE (n = 6). Means with different superscripted letters are significantly different (P < 0.05).
AChE activity, whole body cortisol, blood Glucose and total protein content in bifenthrin intoxicated Ctenopharyngodon idella. Data presented as Mean ± SE (n = 6). Means with different superscripted letters are significantly different (P < 0.05).
Profile of antioxidant enzymes activities of Bifenthrin intoxicated Ctenopharyngodon idella. Data presented as Mean ± SE (n = 9). Means with different superscripted letters are significantly different (P < 0.05).
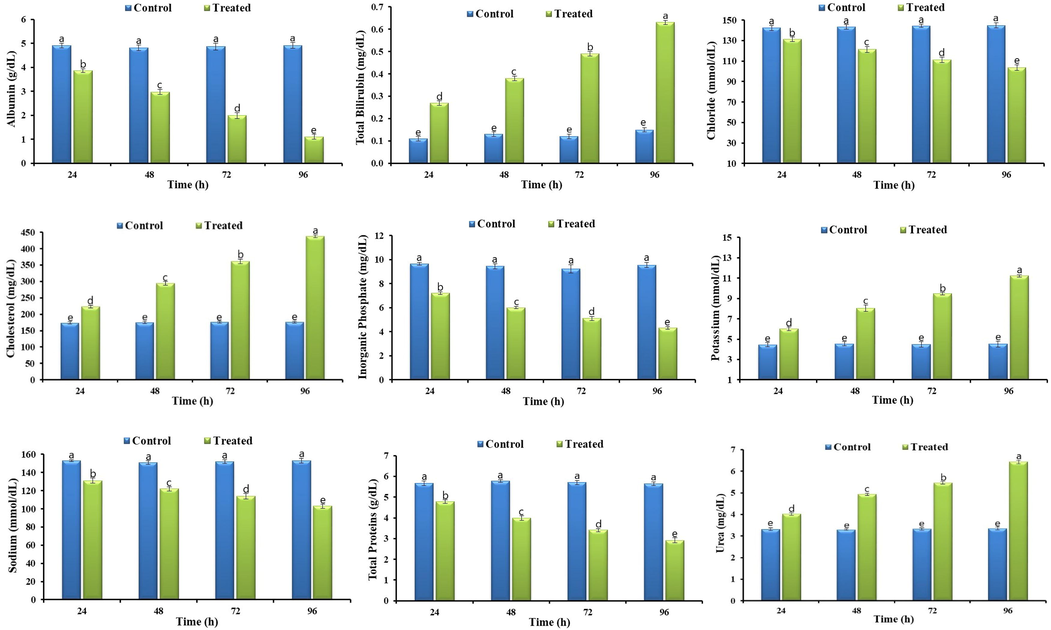
Biochemical profile of bifenthrin intoxicated Ctenopharyngodon idella. Data presented as Mean ± SE (n = 9). Means with different superscripted letters are significantly different (P < 0.05).
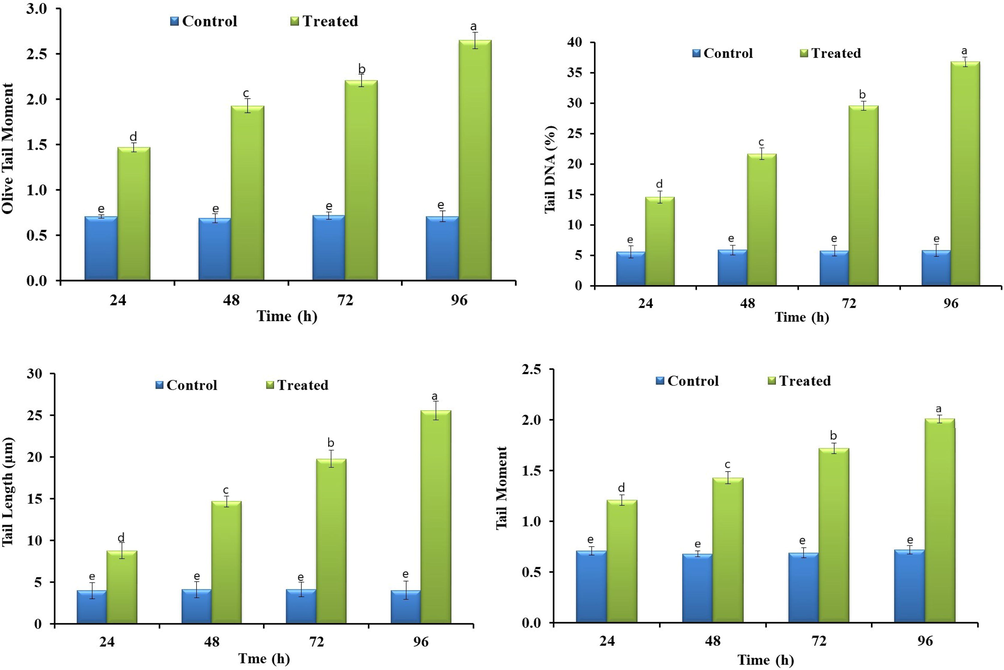
DNA damage profile in the peripheral blood erythrocyte of bifenthrin intoxicated Ctenopharyngodon idella. Data presented as Mean ± SE (n = 6). Means with different superscripted letters are significantly different (P < 0.05).
3.4 Effects of acute concentration of bifenthrin on grass carp hematology
Fig. 6 shows the hematological profile of grass carp in the control and treated group. The hematological parameters studied were red blood cells (RBCs), white blood cells (WBCs), hemoglobin (Hb), mean corpuscular volume (MCV), packed cell volume (PCV), mean corpuscular hemoglobin concentration (MCHC) and mean corpuscular hemoglobin (MCH). The bifenthrin exposure led to a disturbed hematological profile in grass carp, which revealed its hematotoxic effects. The exposure resulted in decreased RBCs, Hb, PCV, and MCHC whereas increased WBCs, platelets, MCV, and MCH.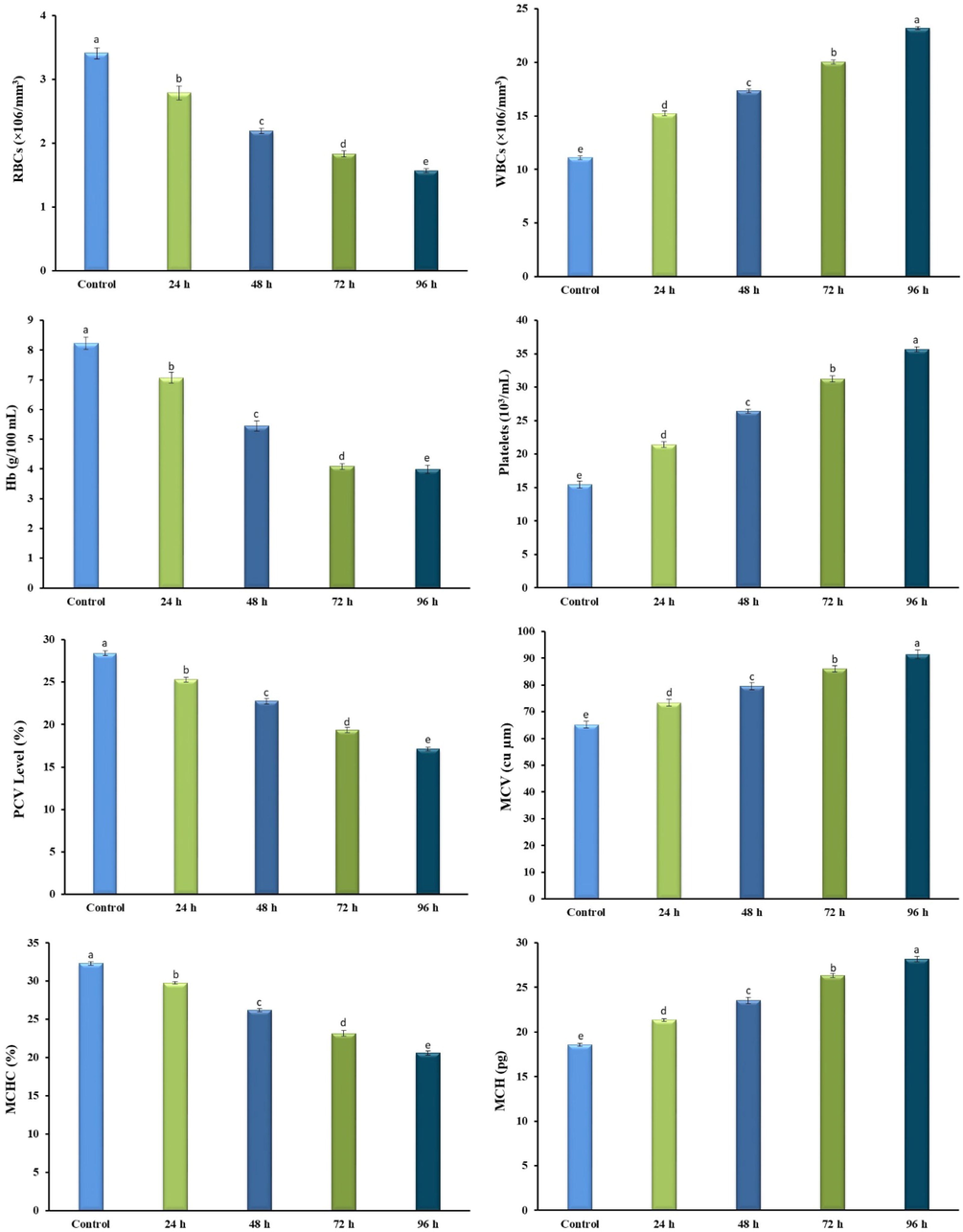
Hematological profile of Bifenthrin intoxicated Ctenopharyngodon idella. Data presented as Mean ± SE (n = 9). Means with different superscripted letters are significantly different (P < 0.05).
4 Discussion
Synthetic pyrethroids including bifenthrin leads to aquatic bodies through runoffs from agricultural fields and effluents from wastewater. Subsequently, they enter into the food chain of non-target aquatic organisms including the highly commercially valuable game fish (Ullah et al., 2018; Ullah, 2019). Bifenthrin is known to have effects on the aquatic organism at ng/L concentration (Brander et al., 2016a; Brander et al., 2016b). The toxic effects of bifenthrin are elevated by its high capability of accumulation in the tissues of these non-target organisms (Alonso et al., 2012; Corcellas et al., 2015). However, research on the ecotoxicology of bifenthrin is still scanty. The meager amount of research predicted different toxicological effects of bifenthrin on different model fish species including Cyprinus carpio (Velisek et al., 2009), Pimephales promelas (Beggel et al., 2011), Oncorhynchus mykiss (Forsgren et al., 2013), Danio rerio (Jin et al., 2013b), and Menidia beryllina (Brander et al., 2016a; Brander et al., 2016b). However, multiple biomarkers-based investigations of bifenthrin intoxicated Chinese carps is still to be conducted. Therefore, the current study was designed to determine LC50 of bifenthrin against grass carp, followed by profiling induction of stress in terms of ROS and LPO and how the induced stress leads to various biochemical and hematological toxicities.
4.1 Acute toxicity (LC50) of bifenthrin
The acute toxic concentration of the pesticides is associated with different factors such as the carrier contaminants, active ingredients, inertness of the chemical, and combination with other chemicals. The acute toxicity of any toxicant is also embodied in the physio-chemical quality of ambient water such as pH and temperature (Ullah et al., 2019b). Moreover, the age, sex, size, health, and the bodyweight of the fish also matter in this regard. However, in our study, no fluctuation in water parameters was observed and hence no association of LC50 of bifenthrin can be established with these characteristics. Similarly, the fish were of the same age, and almost the same size and body weight. Previous studies on different fish species demonstrated different concentrations of bifenthrin as LC50 against them such as 2.08 µg/L against Cyprinus carpio and 0.80 µg/L against Tilapia spp. (Liu et al., 2005), and 57.5 µg/L against Cyprinus carpio (Velisek et al., 2009). In our study, we observed 60 µg/L (corresponds to 6 µg/L) as LC50 of bifenthrin (EC10) against grass carp. The results of Velisk et al. (2009) are comparable to our study as both the studies employed EC10 of bifenthrin.
4.2 Behavioral inconsistencies after exposure to bifenthrin
Exposure of the fish to the acute concentration of bifenthrin led to different behavioral inconsistencies. These alterations might be associated with an increase in the concentration of ACh at the nerves ending and synchronous AChE inhibition. Because AChE is active at the junctions of muscle tissues, such as neural and neuromotor junctions, hence its inhibition results in neural transmission blockage and consequently leads to different behavioral disruptions including balance loss, hyper- or hypo-activity, equilibrium loss, disturbance of schooling behavior, disturbed swimming, etc. At the later stage of exposure, the fish becomes sluggish, adapts vertical position, and becomes motionless due to weaken muscles on account of numbed receptors of nicotine ACh (David et al., 2013).
The bifenthrin-exposed grass carp turned pale and their body color intensity decreased. This might be linked with the increased amount of mucus secretion under stress. The mucus secretion was evident after bifenthrin exposure and was first observed after 24 h, however, a linear increasing trend in mucus secretion was observed from 24 h through 96 h. Subsequently, this leads to a hypoxic condition, and the oxygen uptake capacity of the exposed fish is reduced considerably. This ultimately resulted in an increased breathing rate and frequent surface visits for fresh air. Several researchers documented similar behavioral inconsistencies in different fish species after exposure to different synthetic pyrethroids including deltamethrin exposed Hypophthalmichthys molitrix (Ullah et al., 2018), cypermethrin exposed Tor putitora (Ullah et al., 2014), and Cyprinus carpio exposed to bifenthrin (Velisek et al., 2009).
4.3 Oxidative stress in terms of LPO and ROS
Previous studies on synthetic pyrethroids revealed their capability of inducing stress via enhanced ROS production and subsequent damage to other components of the cells or tissues including proteins, enzymes, DNA, etc. (Ullah et al., 2018; Ullah et al., 2019a; Ullah et al., 2019b). Hence, we included ROS in our study as a key biomarker of stress induction. The exposure of grass carp led to enhanced production of ROS. ROS overproduction in turn results in disrupted membranes, make these membranes leaky, disturbed physiological functions, and ultimately leading to cell death. An increase in ROS production during our study was coupled with an increase in lipid peroxidation. The highest amount of ROS and LPO in the liver tissue suggested its central role in bifenthrin detoxification. The increased ROS production and LPO level are corresponding to the increase in the activities of antioxidant enzymes as they stabilize the overproduction of ROS and provide a primary line of defense (Ullah, 2019).
4.4 Biochemical disruption in brain, liver, gills, and muscles tissues
Grass carps exposed to bifenthrin displayed different biochemical disruptions. Different biomarkers based disrupted biochemical profile was studied in brain, liver, gills, and muscles of grass carp, such as total protein contents. Protein performs key functions, such as making structural and functional components of the cells such as enzymes, nitrogen metabolism, and is used as an energy source under stress. Keeping in view the limited carbohydrates amount in fish, protein is employed as an energy source under stress to cope with the increased energy demand. Bifenthrin exposure led to decreased total protein contents, which might be attributed to protein degradation for fulfilling increased energy demand. A decrease in protein contents in the studied tissues might also be due to damage incurred after bifenthrin exposure or weakening of the protein synthesis machinery due to immense bifenthrin-induced stress.
The antioxidant enzyme system provides a primary line of defense to cope with the enhanced production of ROS. ROS production highly influences the activities of the antioxidant system. Therefore, the activities of antioxidant enzymes are assessed in chemical risk assessment-based studies as an immediate sign of stress induction and intoxication (Ullah et al., 2021). The current study revealed that bifenthrin exposure increased the activities of the antioxidant enzymes, which might be due to enhanced production of free radicals. For instance, CAT is responsible to reduce hydrogen peroxide to water and oxygen. So, in response to the enhanced production of hydrogen peroxide, the activity of CAT enzymes increased to deter oxidative stress and maintain homeostasis (Shadegan and Banaee, 2018). Similarly, SOD detoxifies superoxide radicals whereas glutathione-based pathways regulate ROS production, reduce or end oxidative stress as these enzymes eliminate or inhibit free radicals' bio-accumulation (Ullah et al., 2014).
DNA damage was assessed in peripheral blood erythrocytes through comet assay, which is a widely employed technique because of its simplicity, versatility, sensitiveness, and reliable results. A linear increase in DNA damage in terms of tail length, % tail DNA, tail moment, and the olive tail moment were observed with time in bifenthrin intoxicated grass carps, which might be due to the clastogenic effect of bifenthrin as observed for other synthetic pyrethroids such as deltamethrin (Ullah et al., 2019b). As bifenthrin leads to increased ROS production and increased LPO, hence, the excessive ROS and free radicals induced DNA damage. ROS breaks DNA through hydrogen peroxide ions or hydroxyl ions and subsequently, the resulting DNA has oxidized bases and is damaged (Ullah et al., 2016). Moreover, the cellular lesions induced after bifenthrin exposure might result in the weakening and failure of the antioxidant enzyme system, which was supposed to neutralize free radicals/ROS.
4.5 Serum biochemical disturbance
Serum biochemistry is employed as a sensitive biomarker in ecotoxicological studies as blood biochemical profile provides key information regarding the internal environment of the exposed model animal (Ullah et al., 2018). We observed that bifenthrin intoxication led to a significantly disturbed biochemical profile. The alterations observed might be to cope with stress or physiological dysfunction due to stress. For instance, decreased sodium indicates its flow to nerves where it leads to AChE inhibition (corresponding to the inhibition of AChE observed) whereas the increased potassium and decreased chloride might be attributed to unstable membrane permeability. The disturbance in these biomarkers might also be due to injury of kidneys and gills as they both are involved in ion regulation between fish and ambient water. Similarly, the decrease in protein and albumin might be linked with the injured kidney and liver of the exposed fish. Previous studies revealed different Histomorphological changes and lesions after exposure to different synthetic pyrethroids such as Velisek et al. (2009) observed histopathological damage in the gills and liver tissues of Cyprinus carpio exposed to LC50 of bifenthrin. Moreover, the increase in the level of urea and cholesterol might be linked with dysfunction of gills and liver, respectively (Fırat et al., 2011; Hassan et al., 2018).
4.6 AChE inhibition
The inhibition or reduction in AChE activities produces nerve impulses and makes them penetrable to sodium. Allowing sodium inflow in a higher concentration delay sodium channel closure and and hence sodium penetrates in a higher amount (Ullah, 2019). As observed that exposure to bifenthrin led to an increased amount of sodium in serum. The inflow of sodium at a higher rate led to multiple nerve impulses that in turn resulted in ACh release and its accumulation at a higher rate, and subsequently, the activities of AChE were inhibited in different tissues. The highest amount of AChE inhibition was observed in the brain, which might led to behavioral inconsistencies including erratic swimming and equilibrium loss. Similarly, the activity of AChE was inhibited in the muscle tissues, which might lead to the altered swimming pattern of the exposed grass carp. The results are congruent with different studies on different synthetic pyrethroids (Ullah et al., 2019b) and bifenthrin exposed fish (Velisek et al., 2009).
4.7 Glucose and cortisol
Glucose level is widely assessed in ecotoxicological studies based on its sensitiveness as a biomarker. The current study leads to increased blood glucose levels. In other words, bifenthrin exposure made grass carp hyperglycemic. The increase in blood glucose might be attributed to disturbed or disrupted glycogenolysis or gluconeogenesis after bifenthrin exposure. It might also be associated with membrane lesions/ injuries and pouring out of glucose into the blood. Furthermore, metabolism is hugely affected by stress, therefore, hyperglycemic hormones including catecholamine and glucocorticoids are released in a higher amount, and the degraded glucose and glycogen leaked out to the bloodstream (Uddin et al., 2018).
Cortisol hormone is widely assessed in chemical risks assessment and ecotoxicology/ aquatic-toxicology. This excellent biomarker is employed to evaluate alterations in the functions of the hypothalamo-pituitary-interrnal axis). Cortisol is responsible for organizing energy sources including glucose and lipids under stress to maintain homeostasis (Ozok et al., 2018). The current study revealed that bifenthrin exposure increased whole-body cortisol, which is corresponding to increased blood glucose level. The increased whole-body cortisol might be linked with increased energy demand or controlling abrupt change under stress. Moreover, the interaction, under stress, between the endocrine system and immune system is key to maintain life whereas the key product is cortisol secretion to regulate energy metabolism. An increase in cortisol levels in different species in response to synthetic exposure is reported by several authors such as Cypermethrin exposed Alburnus tarichi (Ozok et al., 2018) and deltamethrin intoxicated Hypophthalmichthys molitrix (Ullah et al., 2019b).
4.8 Hematological profiling
The exposure of grass carp to bifenthrin led to significant changes in their hematological profile. Hematology is one of the most widely employed biomarkers for evaluating the risk potential of pollutants and chemicals safety studies (Ullah et al., 2021). The current study revealed a disturbance in the hematological profile of the exposed grass carp. The exposure resulted in decreased RBCs count and hemoglobin, which might be associated with their elevated destruction or inhibited RBCs and hemoglobin synthesis. This decrease also indicated that bifenthrin exposure resulted in anemia in the experimental group. Similarly, the increased count of WBCs might be linked with the activation of leucopoiesis and lymphopoiesis to increase the production of WBCs under stress. The increase in platelets might be attributed to strengthening the phagocytic activity of exposed fish. There is well-documented research, on synthetic pyrethroids against different fish species, which coincide with the findings of the current research such as bifenthrin exposed Cyprinus carpio (Velisek et al., 2009), Cypermethrin exposed Alburnus tarichi (Ozok et al., 2018), and deltamethrin exposed Hypophthalmichthys molitrix (Ullah et al., 2019b).
5 Conclusion
The current study concluded that bifenthrin is highly toxic to fish. The LC50 of bifenthrin against grass carp was found to be 60 µg/L (EC10; corresponding to 6 µg/L) for 96 h. Exposure of grass carp to an acute concentration of bifenthrin mediated severe and drastic changes in their behavioral, biochemical, and hematological profile. The behavior was disrupted, subtle changes in serum biochemical profile were evident, and induction of oxidative stress in various tissues was followed by a series of other alterations such as increased lipid peroxidation coupled with increased activities of antioxidant enzymes, inhibited AChE activities, elevated whole-body cortisol, and damaged DNA.
The results of the current study classified bifenthrin as a highly toxic synthetic pyrethroid and demonstrate serious concerns regarding its use, keeping in view a continuous increase in its use and its prevalence in higher concentrations in the real world scenario. In the light of this study, we propose that the injudicious, unnecessary, extensive, and far-reaching use of bifenthrin should be monitored on a regular and strict basis. Its use should be under stern rules and regulations. The application of bifenthrin should be as per environmental laws and per environmental, public, and animal health safety.
Ethical statement
The current study was conducted following the ethical standard for animal handling and use. All applicable institutional, national, and international guidelines were followed.
Acknowledgements
This study was supported by the China Postdoctoral Science Foundation No. 2021M693373 and Investigation of Aquatic Biological Resources in the Aquatic Germplasm Resources Conservation Area of the Qinghai Section of the Yangtze River No. E039831D01. The authors express their sincere appreciation to the Researchers Supporting Project (RSP 2021/24), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Sublethal toxicity of commercial insecticide formulations and their active ingredients to larval fathead minnow (Pimephales promelas) Sci. Total Environ.. 2010;408(16):3169-3175.
- [Google Scholar]
- Changes in gene transcription and whole organism responses in larval fathead minnow (Pimephales promelas) following short-term exposure to the synthetic pyrethroid bifenthrin. Aquat. Toxicol.. 2011;105(1-2):180-188.
- [Google Scholar]
- Effects of bifenthrin exposure on the estrogenic and dopaminergic pathways in zebrafish embryos and juveniles. Environ. Toxicol. Chem.. 2017;37(1):236-246.
- [Google Scholar]
- Evaluation of acute toxicity of Karate and its sub-lethal effects on protein and Acetylcholinesterase activity in Cyprinus carpio. Int. J. Agric. Biol.. 2014;16:731-737.
- [Google Scholar]
- Pyrethroids pesticides as endocrine disruptors: molecular mechanisms in vertebrates with a focus on fishes. Environ. Sci. Technol.. 2016;50:8977-8992.
- [Google Scholar]
- Transcriptomic changes underlie altered egg protein production and reduced fecundity in an estuarine model fish exposed to bifenthrin. Aquat. Toxicol.. 2016;174:247-260.
- [Google Scholar]
- Antioxidant responses in Scytosiphon lomentaria (Phaeophyceae) inhabiting copper-enriched coastal environments. J. Phycol.. 2005;41:1184-1195.
- [Google Scholar]
- First report of pyrethroid bioaccumulation in wild river fish: a case study in Iberian river basins (Spain) Environ. Int.. 2015;75:110-116.
- [Google Scholar]
- Pyrethroids in human breast milk: occurrence and nursing daily intake estimation. Environ. Int.. 2012;47:17-22.
- [Google Scholar]
- Effects of deltamethrin on haematological indices of Indian major carp, Cirrhinus mrigala (Hamilton) Int. J. Pure Appl. Zool.. 2015;3:37-43.
- [Google Scholar]
- David, M., Sangeetha, J., Shrinivas, J., Harish, E., Naik, V., 2013. Alterations in the levels of ACh and associated AChE in the tissues of fresh water fish Cirrhinus mrigala exposed to deltamethrin. Int. J. Pharm. Biol. Arch. 4.
- A comparative study on the effects of a pesticide (cypermethrin) and two metals (copper, lead) to serum biochemistry of Nile tilapia, Oreochromis niloticus. Fish Physiol. Biochem.. 2011;37(3):657-666.
- [Google Scholar]
- The effects of the pyrethroid insecticide, bifenthrin, on steroid hormone levels and gonadal development of steelhead (Oncorhynchus mykiss) under hypersaline conditions. Gen. Comp. Endocrinol.. 2013;186:101-107.
- [Google Scholar]
- Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem.. 1974;249(22):7130-7139.
- [Google Scholar]
- Spatial analysis of bifenthrin sediment concentrations in California water bodies from 2001 to 2010: identification of toxic and non-toxic areas. Hum. Ecol. Risk Assess. Int. J.. 2014;20(2):497-509.
- [Google Scholar]
- Assessment of acute metals toxicity in Catla catla through hematological and biochemical blood markers. Pakistan J. Agric. Sci.. 2018;55:449-454.
- [Google Scholar]
- Pyrethroid insecticides in bed sediments from urban and agricultural streams across the United States. J. Environ. Monit.. 2012;14(7):1838-1845.
- [Google Scholar]
- Bifenthrin causes toxicity in urban stormwater wetlands: field and laboratory assessment using Austrochiltonia (Amphipoda) Environ. Sci. Technol.. 2017;51(12):7254-7262.
- [Google Scholar]
- Embryonic exposure to cis-bifenthrin enantioselectively induces the transcription of genes related to oxidative stress, apoptosis and immunotoxicity in zebrafish (Danio rerio) Fish Shellfish Immun.. 2013;34(2):717-723.
- [Google Scholar]
- Enantioselective disruption of the endocrine system by Cis-Bifenthrin in the male mice. Environ. Toxicol.. 2015;30(7):746-754.
- [Google Scholar]
- Exposure of maternal mice to cis-bifenthrin enantioselectively disrupts the transcription of genes related to testosterone synthesis in male offspring. Reprod. Toxicol.. 2013;42:156-163.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Ind. J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Enantioselective endocrine-disrupting effects of bifenthrin on hormone synthesis in rat ovarian cells. Toxicol.. 2011;290(1):42-49.
- [Google Scholar]
- Separation of bifenthrin enantiomers by chiral HPLC and determination of their toxicity to aquatic organism. J. Food Drug Anal.. 2005;12:357-360.
- [Google Scholar]
- Protein measurement with the Folin phenol reagent. J. Biol. Chem.. 1951;193(1):265-275.
- [Google Scholar]
- Enantioselective effect of bifenthrin on antioxidant enzyme gene expression and stress protein response in PC12 cells. J. Appl. Toxicol.. 2013;33(7):586-592.
- [Google Scholar]
- Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney. Possible implications in analgesic nephropathy. Biochem. Pharmacol.. 1984;33(11):1801-1807.
- [Google Scholar]
- Hemato-biochemical responses of Van fish (Alburnus tarichi Guldenstadt, 1814) during sublethal exposure to cypermethrin. Hum. Ecol. Risk Assess.. 2018;24(8):2240-2246.
- [Google Scholar]
- Pyrethroid and organophosphate pesticide-associated toxicity in two coastal watersheds (California, USA) Environ. Toxicol. Chem.. 2012;31(7):1595-1603.
- [Google Scholar]
- Effects of imidacloprid on the haematological and serum biochemical profile of Labeo rohita. Pakistan J. Zool.. 2014;46:1085-1090.
- [Google Scholar]
- Pesticides in house dust from urban and farmworker households in California: an observational measurement study. Environ. Health.. 2011;10:19-33.
- [Google Scholar]
- Correlation of tissue concentrations of the pyrethroid bifenthrin with neurotoxicity in the rat. Toxicology. 2011;290(1):1-6.
- [Google Scholar]
- Effects of dimethoate alone and in combination with Bacilar fertilizer on oxidative stress in common carp, Cyprinus carpio. Chemosphere. 2018;208:101-107.
- [Google Scholar]
- A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res.. 1988;175(1):184-191.
- [Google Scholar]
- Neurodevelopmental consequences of gestational and lactational exposure to pyrethroids in rats. Environ. Toxicol.. 2016;31(12):1761-1770.
- [Google Scholar]
- Urinary pyrethroid and chlorpyrifos metabolite concentrations in Northern California families and their relationship to indoor residential insecticide levels, part of the Study of Use of Products and Exposure Related Behavior (SUPERB) Environ. Sci. Technol.. 2014;48(3):1931-1939.
- [Google Scholar]
- Temperature changes alter the acute toxicity responses of cypermethrin in Zebrafish. Prog. Agric.. 2018;29(1):64-70.
- [Google Scholar]
- Urinary excretion of 3-phenoxybenzoic acid in middle-aged and elderly general population of Japan. Environ. Res.. 2009;109(2):175-180.
- [Google Scholar]
- Cypermethrin induced behavioral and biochemical changes in mahseer, Tor putitora. J. Toxicol. Sci.. 2014;39:829-836.
- [Google Scholar]
- A Multi-Scale Study on the Hostile Effects of Pollution on Humans and Fish: From Behavior, Physiology, Biochemistry, to Toxicology. P.R. China: submitted to Nanjing University; 2019. PhD Thesis
- Genotoxic effect of Endosulfan at sublethal concentrations in Mori (Cirrhinus mrigala) fish using single cell gel electrophoresis (comet) assay. Int. J. Pharmacol.. 2016;12(3):169-176.
- [Google Scholar]
- Multiple biomarkers based appraisal of deltamethrin induced toxicity in silver carp (Hypophthalmichthys molitrix) Chemosphere. 2019;214:519-533.
- [Google Scholar]
- Heavy metals bioaccumulation and subsequent multiple biomarkers based appraisal of toxicity in the critically endangered Tor putitora. Ecotoxicol. Environ. Safety. 2021;228:113032.
- [CrossRef] [Google Scholar]
- Cypermethrin induced toxicities in fish and adverse health outcomes: its prevention and control measure adaptation. J. Environ. Manag.. 2018;206:863-871.
- [Google Scholar]
- Global trends in the use of insecticides to control vector-borne diseases. Environ. Health Perspect.. 2012;120(4):577-582.
- [Google Scholar]
- Effects of acute exposure of bifenthrin on some haematological, biochemical and histopathological parameters of rainbow trout (Onchorhynchus mykiss) Veterin. Medic.. 2009;54(3):131-137.
- [Google Scholar]
- Wang, X., Gao, X., He, B., Jin, Y., F, Z., 2017. Cis-bifenthrin causes immunotoxicity in murine macropahges. Chemosphere 168, 1375-1382.
- Werner, I., Moran, K., 2008. Effects of pyrethroid insecticides on aquatic organisms. In Synthetic Pyrethroids: Occurrence and Behavior in Aquatic Environments, Gan, J., Spurlock, F., Hendley, P., Weston, D.P., Eds., American Chemical Society: Washington D.C., ACS Symposium Series 991, pp 310-335.
- Urban and agricultural pesticide inputs to a critical habitat for the threatened delta smelt (Hypomesus transpacificus) Environ. Toxicol. Chem.. 2014;33(4):920-929.
- [Google Scholar]
- Biomonitoring of pyrethroid exposure among rural and urban populations in northern Poland. Chemosphere. 2013;93(10):2547-2553.
- [Google Scholar]
- Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Arch. Biochem. Biophys.. 1981;206(2):296-304.
- [Google Scholar]
- Toxicity of the pyrethroid bifenthrin insecticide. Environ. Chem. Lett.. 2018;16(4):1377-1391.
- [Google Scholar]
- Integrative assessment of enantioselectivity in endocrine disruption and immunotoxicity of synthetic pyrethroids. Environ. Pollut.. 2010;158(5):1968-1973.
- [Google Scholar]
- Effect of confinement on water-borne and whole body cortisol in wild and captive-eared rainbowfish (Melanoteania duboulayi) Int. J. Agric. Biol.. 2014;16:183-188.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101752.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







