Translate this page into:
Azadirachta indica as a bio-material: Rapid synthesis of Cr5O12 shell nanoparticles to study its photocatalytic and antimicrobial properties
⁎Corresponding authors. rajarajanchem1962@gmail.com (Rajarajan Muthuramalangam), suganthitcarts@gmail.com (Suganthi Ayyadurai)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
A novel Cr5O12 nanoparticle were prepared by reducing K2Cr2O7 using Azadirachta indica plant extracts as a reducing agent. The synthesized nanoparticles shows orthorhombic phases with a band gap for 1.27 eV and it is further conformed by SEM. The average dimension of the nanoshell was about 56.99 nm. The FTIR spectrum explores the presence of the functional group of plant extract and Cr5O12. GC–MS of the aqueous extract shows the presence of many antioxidants in the leaf of Azadirachta indica. The photocatalytic performance was analyzed based on the degradation of Methyl Orange (MO) dye. The rate constant k of AzI-Cr5O12 is found to be 3.93 × 10−2 s−1 and follows pseudo first-order kinetic at a catalyst dosage of 0.050 g/L with concentration of 20 µM of dye. Further, the antimicrobial activity of the nanoparticles was tested against Staphylococcus aureus, Candida albicans and Enterobacter.
Keywords
Cr5O12
Azadirachta indica
Photocatalysis
Methyl orange
Antimicrobial activity
1 Introduction
Nanoparticles having one or more dimensions in the order of 100 nm or less has noticeable attention and charming properties like optical, electronic, magnetic, etc., over their bulk counterparts (Daniel and Astruc, 2004; Kato, 2011). Size and shape of synthesized nanoparticles by chemical technique can be controlled and their defined morphologies are restored and improved in many catalytic applications (Li et al., 2004). Currently, sustainability drive that use of green chemistry to develop and/or protect our environment is becoming principal issues in many fields of research. Plants or plant extract is a new simple and environmentally friendly biosynthesis method (Kaviyarasu et al., 2017; Kaviyarasu et al., 2017). It is more beneficial over chemical and physical method beyond using pressure, temperature and toxic chemicals (Stern and Grasselli, 1997; Sundaram and Nagaraja, 2004; Pandey et al., 2006; Asif, 2012).
Chromic Oxide is a transition metal oxide with a variety of applications in many fields like the heterogeneous catalyst, coating material, wear resistance, advanced colorant, pigment and solar energy collector (Cui et al., 2015; Kim et al., 2004; Bobet et al., 2003; Zhang et al., 2013). Cr2O3 is a p – type semiconductor with a wide gap of 3.4 eV synthesized by simple (Khamlich et al., 2012; Jankovsky et al., 2015; Pei and Zhang, 2008; Puerari et al., 2016) functinalized by WO3 nanorods (Choi et al., 2018), thermal decomposition (Gunnewiek et al., 2014) microwave refluxing and plasma (Su et al., 2014; Grzybowska et al., 1998) doped PVDF thin flims (Al-Hazmi et al., 2017) and coated with γ-Fe2O3 (Nadeema et al., 2018) have been employed. Green synthesis of Cr2O3 using plant such as Mukia Maderaspatna (Ananda and Gowda, 2013), Arachis Hypogaea (Ramesh et al., 2012) and synthesis of Cr5O12 has not reported earlier.
The treatment of textile dye wastewaters in order to reduce visual color and dissolved organic contaminants to meet the increasing environmental demands have continued to attract the interest of research (Ramesh et al., 2012; Gupta et al., 2015; Gupta et al., 2013; Ahmaruzzaman and Gupta, 2011) Adsorption is widely used techniques for the separation and removal of pollutants from wastewaters (Mohammadi et al., 2011; Robati et al., 2016; Gupta et al., 2016; Asfaram et al., 2015). Photocatalytic oxidation using semiconductor is one of the advanced cost effective and green technology for the eradication of toxic organic and inorganic pollutants from textile dye wastewater (Reddy et al., 2018; Karthikeyan et al., 2012; Rajendran et al., 2016; Saravanan et al., 2013; Saravanan et al., 2013; Saravanan et al., 2013; Saravanan et al., 2013; Saravanan et al., 2015; Magdalane et al., 2017; Magdalane et al., 2017; Biswas et al., 2002; Subapriya and Nagini, 2005).
A different section of the Azadirachta indica (Meliaceae family) has hold in universal medicine in the peculiar area around the nature (Uko and Kamalu, 2001; Angel Ezhilarasi et al., 2016). In particular, the leaf of neem is a “Storehouse” of organic compounds. There are ethnophormacological reports supporting their use against bacteria and worm infections by the oral use of their leave extracts. This effective pathogenic action is due to the existence of the alkaloids, flavonoids, glucoside, steroid, soluble carbohydrate, tannin, hydrogen cyanide and Azadirachtin (Matinise et al., 2017). In surface modification with plant extract the transition metal nanoparticles have various proven results that show cytotoxicity against microbes as is evident from the distortion of the morphology of the cells.
The phenolic compounds like flavonoids in the plant extracts are soluble in water, non toxic, bio-degradable and can function as both reducing agents for the synthesis of metal oxide (Raja et al., 2018; Angel Ezhilarasi et al., 2018; Valdez and Gomez, 2016; Karimiana and Pirib, 2013). On the surface of nanoparticles and this interaction could agglomerate more nanoparticles for making a bigger nanoparticle (Harborne et al., 1973). In addition, no reports on the synthesis of Cr5O12 using aqueous neem extract have been published. The chemical constituent present in the aqueous extracts of neem leaves was analyzed by photochemical testing and Gas-chromatography-Mass spectrum (GC–MS) techniques. The photocatalytic performance of the Cr5O12 nanoshell was measured related to the degradation of methyl orange. Further, the synthesized nanoshell was analyzed for cytotoxicity against antimicrobial like Candida albicans, Staphylococcus aureus, and Enterobacter as experimental pathogens which can be used for biomedical application.
2 Experiment
2.1 Materials and method
Analytical grade chemicals were purchased from ‘MERCK chemicals’ India. The young Azadirachta indica leaves were collected from C.P.A College, Bodinayakanur. Throughout the synthesis double distilled water was used. (i) Azadirachta indica plant leaves were used to make the aqueous extracts. Taxonomically authenticated healthy leaves were collected and about 25 g of leaves was thoroughly washed with distilled water, air desiccated, cut into fine pieces and boiled with 100 ml of distilled water in an Erlenmeyer flask for 10 min at 60 °C. The broth was filtered and stored at a temperature of 4 °C (Siddiqui and Ali, 1997). (ii) Typically 14.5 g of potassium dichromate was dissolved in 10 ml of plant extract and adjusted to 50 ml with distilled water. The plant extract was added to adjust the pH 8 and stirring is continued for 2 h. The synthesized AzI-Cr5O12 nanoshells was washed and dried at room temperature and calcination is continued for 1 h in a muffle furnace at 500 °C (Buvaneswari et al., 2015).
2.2 Identification tests for active compounds
The aqueous extract of Azadirachta indica leaves monitored for various test for the phytochemical constituents by the following test (Iyengar, 1995).
S.No
PHYTOCHEMICALS
EXPERIMENT
INFERENCE
1
Alkaloids
Extract residue + 2% hydrochloric acid heated in a boiling-water bath.
Yellow precipitation
2
Carbohydrate
2 ml extract + 2 drops of alcoholic α- naphthol + 1mlconcentrated sulphuric acid
Violetring junction at the
3
Reducing sugar
0.5 ml extract + 1 ml water + 5 drops Fehling’s solution
Brick red precipitate
4
Flavonoid
Extract + 1.5 ml of 50% methanol + metal magnesium and warmed, then 6 drops concentrated hydrochloric acid
Red colour solution
5
Glycoside
Glacial acetic acid + extract + ferric chloride + concentrated sulphuric acid
Reddish-brown colour at the junction
6
Tannins
1 ml of water + 0.5 ml of extract + 1 – drops of ferric chloride.
Formation of green or violet colour
7
Saponin
The extract was shaken for 15 min in a graduated cylinder with distilled water.
Formation of a layer of foam
8
Terpenoid steroid and
Extract + 0.5 ml of acetic anhydride + 0.5 ml of chloroform + concentrated sulphuric acid
Redvioletcolor (Trepenoid), bluish colour (steroids).
9
Protein
1 ml of 10% sodium hydroxide + heating + 0.7%coppersulphate solution
Violet or pink colour
2.3 Characterization
The phase composition and the crystallite size of Cr5O12 were determined using an X- ray diffractometer (XRD; XPERT PRO X-RAY) with Cu Ka radiation at 25 °C and the structural assignments were made with reference to the JCPDS files. JSM 6701F—6701 microscope is used to analyze the surface morphology in both secondary and backscattered electron modes, in addition to the elemental analysis. JASCO V-550 double beam spectrophotometer was used to identify the optical properties of nanoshell with PMT detector. JASCO-FT-IR-460 plus was used for surface structure analysis of the nanoshell. EUTECH instrument was used for pH monitoring. Perkin-Elmer GC Clarus 500 system comprising an AOC-20 i auto-sampler was used for phytochemical analysis of the leaves.
2.4 Photocatalytic activity
300 ml aqueous solution of MO with a certain amount of photocatalyst was taken in the cylindrical glass vessel, which was surrounded by a circulating water jacket to cool the lamp. The air was bubbled continuously into the aliquot by an air pump in order to provide a constant source of dissolved oxygen. Before switching on irradiation, the suspension was stirred in the dark for 30 min to ensure that the adsorption – desorption equilibrium (Valdez and Gomez, 2016). 300 W Xe arc lamp with an ultraviolet (λ < 400 nm) cutoff filter was used as the visible-light irradiation source. During light irradiation, 5 ml aliquots were withdrawn at a regular time interval of 30 min. Then the samples were centrifuged and filtered through a millipore filter to remove the photocatalyst. The filtrate was analyzed by UV–Vis spectrophotometer at λ max = 465 nm and the photodegradation percentage were calculated by the expression given below:
2.5 Assay for antimicrobial activity of Cr5O12 against microorganisms
The Cr5O12 nanoparticles in sterilized distilled water were tested for their antibacterial activity by the agar diffusion method. Fungal strains (Candida albicans) and bacterial strains (Staphylococcus aureus, Enterobacter) were used to study the antimicrobial property (Saravanan et al., 2013). These bacillus were matured in nutrient agar media for 24 h anterior to the trial, were shown in agar plates by the pour plate technique. The five test organisms were swapped over the nutrient agar medium and the disks containing Cr5O12 nanoparticles were kept over the medium using sterile forceps. The plates were nurtured at 37 °C for 24 h. The zone of restraint is deliberate in milimeter.
3 Result and discussion
3.1 Characterization
3.1.1 UV–Vis and UV–vis-DRS of Cr5O12
The UV–Visible spectroscopy is used to conform the reaction between metal ions and the leaf extracts for the formation of Cr5O12 nanoshell (Fig. 1(a)). The peak at 325 nm is due to inter band transition of core electrons of chromium and chromium oxide. The light absorption ability of Cr5O12 was studied by UV–Vis-DRS was shown Fig. 1(b). As seen in Fig. 1(c) the absorption edge of AzI-Cr5O12 was highly shifted to the visible region. The band gap of semiconductors is correlated to its range of absorption wavelength, and the band gap decreases with increasing of absorption edge. The evaluation of band gaps can be done using Tauc approach (Karthiga et al., 2015).

(a). UV–Vis absorption spectrum of AzI-Cr5O12 nanoparticles in aqueous solution, (b). UV–vis- DRS of AzI-Cr5O12, (c). Tauc plot of AzI-Cr5O12.
3.1.2 FTIR
The FTIR spectrum of the plant extract and plant extract (Fig. 2.) modified Cr5O12 nanoparticles exhibited peaks at 3386 cm−1, 1626 cm−1, 1389 cm−1, 1276 cm−1, 1078 cm−1, 760 cm−1 and 563 cm−1. The band at 3386 cm−1 is assigned to the hydroxyl group of polyphenols. The characteristic peaks at 1626 cm−1 represented the carbonyl group (–C⚌O) of amide in proteins. The peaks at 1389 cm−1 and 1276 cm−1 may be assigned to the –N⚌O bending and inplane (–C–H) bending, respectively (Ikram and Inamul, 1984; Scarano et al., 1993). The absorption band at 1078 cm−1 is attributed to the –C–N stretching vibration of aliphatic amines. The characteristic peak at 760 cm−1 is designated to the –C–C–C– deformations of the phenyl ring. The peak at 947 cm−1 and 564 cm−1 indicates that Cr⚌O and Cr–O vibration of Cr2O3 nanoparticles in AzI- Cr5O12 spectrum (Saleh and Gupta, 2011). From the examination terpenoids, flavonoids containing carboxyl group (capping agent) were found adsorbed on the surface of the nanoparticles. This also throws some light on the dual role of biological molecule in reducing metal ions and capping. Capping of nanoparticles by protein stabilizes Cr5O12 nanoparticles and prevents agglomeration in the medium (Saleh and Gupta, 2012).
FTIR spectra of AzI-Cr5O12.
3.1.3 XRD
Fig. 3. shows the XRD pattern of AzI-Cr5O12 nanoparticles. All the peaks can be indexed to the known orthorhombic structure of the Cr5O12 (JCPDS No 73–1787). A series of characteristic peaks at 24.06° (0 2 1), 26.39° (3 1 1) and 39.00° (4 2 1) was observed. The high crystallinity AzI-Cr5O12 was identified via strong intensity and narrow width diffraction peaks. In general, use of plant extract influence the polarity and coordinating ability can influence the morphology and crystallization behavior of the nanoshell and the reduction in microstrain are improved. The size of nanoparticles using XRD pattern has been estimated from scherrer equation and the average crystallite size for AzI-Cr5O12 was determined as 56.99 nm (Vinoth et al., 2012; Ramadass and Subramanian, 2018).

XRD for AzI-Cr5O12.
3.1.4 SEM and EDX
The morphology of AzI-Cr5O12 is shown in the Fig. 4(a). The image reveals that the nanoparticles have shells and plate like structure. An EDX analysis was conducted on AzI- Cr5O12 to determine the weight percentage of the AzI-Cr5O12 and is displayed in inset figure and Table 1. It is found that the major elements of nanoshell are Cr and O clearly observed at their corresponding keV values without any impurity (Ramadass and Subramanian, 2018). AzI-Cr5O12 was small in size, nearly spherical in shape and are agglomerated, due to the phytochemical present in the aqueous extract of Azadirachta indica and weak interparticle forces between the nanopartcles and secondary metabolites.
(a). SEM image of AzI-Cr5O12, (Inset figure EDX spectrum of Cr5O12).
S. No
Element
Weight (%)
Atomic %
keV
1
Cr
57.61
73.31
5.131
2
O
42.39
30.94
0.510
3.2 Qualitative determination of phytochemical in aqueous leaf extract
A various phytochemical that are present in the leaves of Azadirachta indica are responsible for the reduction and capping agent for the synthesis of nanoparticles. Qualitative test of phytochemical was shown in the Table 2. Aqueous extract of Azadirachta indica shows the presence of Alkaloids, Carbohydrate, Protein, Tannins, Phenolic compounds, Flavanoids and Triterpenoids and this were further conformed by GCMS. Table 2. Phytochemical study of aqueous Azadirachta indica leaves extract. Each test in triploids, “+” presence, “−” Absence.
S. No
Phytochemical constituent
Result
1
Alkaloids
+
2
Carbohydrate
+
3
Reducing Sugar
+
4
Flavanoids
+
5
Glycosides
–
6
Tannins and phenolic compounds
+
7
Triterpenoids
+
8
Protein
+
3.2.1 GC–MS
The aqueous extract of Azadirachta indica showed five major peaks in GC–MS chromatogram (Fig. 5) and the NIST library has been used for comparison of the mass spectra (Table 3) demonstrate the presence of 12 phytocomponents. From the results, it was observed that the presence of Phenol, 2-methoxy, 3-(3-propenyl)-, Hexadecane, Dibuthyl phthalate, 1-iodo-2-methyldecane, Eicosane, Docosane, Nonacosane, Phytol, Gamma- Sitosterol, Icosapentaenoic acid, Heptasiloxane, hexadecamethyl, Hexahydrofarnesyl acetone were the major components in the extract. Table 3 lists the phytochemicals that afford to the medicinal property of the Azadirachta indica leaves. Gamma-Sitosterol and Phytol (Vignesh et al., 2012) were known for its potential antioxidant, antibacterial, antifungal, anti arthritic and prophylactic activities. Hexadecane, Dibuthyl phthalate, 1-iodo-2-methyldecane, Eicosane, Docosane and Nonacosane has gained importance due to its antifungal and anticancer action, and also used as an insecticide. Phenol,2-methoxy, 3-(3-propenyl)- is reported to possess high level antimicrobial activity against fish pathogens (Bansal et al., 2009). These secondary metabolites present in the aqueous solution obtained from Azardirachta indica have a potential application in reduction of metal ions and act as a capping agent. Plant extracts act as secondary metabolites on the surface of nanoshells to increase the activity against pathogens to destroy the cell wall effectively.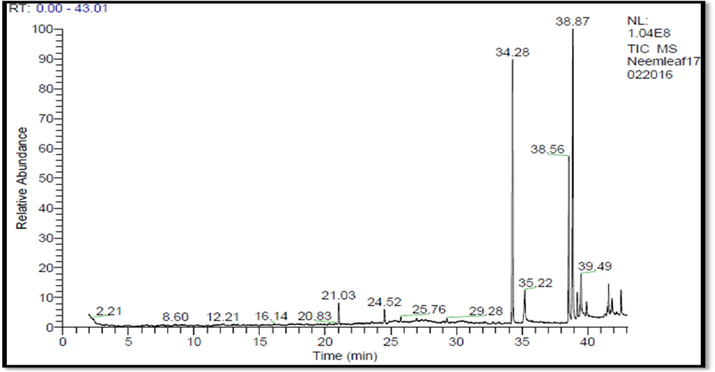
GC–MS of aqueous neem extract.
S. No
Retention Time
Name of the Compounds
Molecular Formula
Molecular Weight
1
12.21
Phenol, 2-methoxy, 3-(3-propenyl)-
C10H12O2
164
2
16.14
Hexadecane
C16H34
226
3
20.83
Dibuthyl phthalate
C16H22O4
278
4
21.03
1-iodo-2-methyldecane
C12H25I
296
5
21.03
Eicosane
C20H42
268
6
24.52
Docosane
C22H48
310
7
29.28
Nonacosane
C29H60
408
8
34.28
Phytol
C20H40O
296
9
34.28
Gamma-Sitosterol
C29H50O
414
10
38.56
Icosapentaenoic acid
C20H30O2
302
11
38.87
Heptasiloxane, hexadecamethyl
C16H48O6Si7
533
12
39.49
Hexahydrofarnesyl acetone
C18H36O
268
3.3 Photocatalytic activity
The photocatalytic activity of AzI-Cr5O12 was investigated in an aqueous solution of MO at 20 µM, at a catalyst concentration of 0.050 g/L and irradiation time of 90 min. The degradation of MO is negligible in the absence of photocatalyst due to chemical reaction rather than adsorption and the degradation follows pseudo first-order kinetic model (Maria Magdalane, 2017).

Kinetic plot of −ln(C/Co) versus irradiation time for the photodegradation of MO.
3.3.1 Mechanism
When the photocatalyst is irradiated by visible light, phytochemical present in the plant extract loaded on the surface of the Cr5O12 can easily excited and create mobile electron which transfer electrons into surface adsorbed O2 (Mobeen Amanulla et al., 2018; Reddy et al., 2018) resulting in generating more reactive oxygen species (O2•−). Therefore the photogenerated electrons of phytochemical will be easily transferred to the conduction band of Cr5O12 (wide band gap semiconductor) lead to the formation of new active sites, which enhance the electron-hole separation and facilitate the rapid transfer of electrons from the catalyst to molecular oxygen. This results in more charge carriers to form reactive species, which promote the degradation of MO (Jayaraj et al., 2018; Islam et al., 2018; Perillo, 2018) are shown in Fig. 7. The degradation efficiency of methyl orange solutions with other nanoparticles was compared at different reaction conditions were shown in Table 4. And the results show that Cr5O12 by plant extracts exhibit superior degradation efficiency when compared to chemically and green synthesized method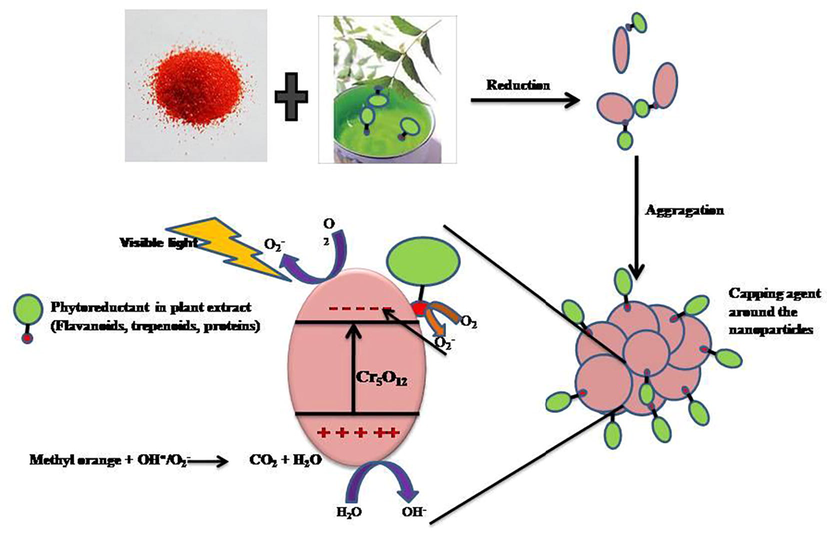
Mechanism of Photocatalytic activity of AzI-Cr5O12 under visible light irritation.
S. No
Nanoparticles
Light source
Dye Concentration
Percentage of degradation
References
1
V2O5 nanorods
Visible
5 µM
48(300 min)
(Liu et al., 2018)
2
AuNPs-TiO2
Visible
10 µM
95(180 min)
(Ahluwalia et al., 2016)
3
Cu-ZnO nanorods
Solar
10 µM
95(120 min)
(Rabea et al., 2003)
5
TiO2/HAP
UV
5 µM
92(150 min)
(Arunadevi et al., 2018)
6
Se-ZnS(green)
UV
20 µM
95(180 min)
(Angel Ezhilarasi et al., 2018)
7
AzI-Cr5O12
Visible
20 µM
95(90 min)
Present work
3.3.2 Effect of catalyst dosage
To evade the use of too much of a catalyst, it is comfortable to identify the optimum amount of catalyst for active degradation. A series of experiments was carried out by varying the dosage of catalyst from 0.030 to 0.060 g/L with a dye concentration of 20 µM and irradiation time of 90 min. The degradation efficiency of MO for various photocatalyst loadings is illustrated in Fig. 8. The results show that an increase in the catalyst loading from 0.030 to 0.050 g/L boost the dye degradation strongly from about 59% to 95%, due to the increases of mobile sites on the surface of the catalyst (Karthiga, 2015). Beyond 0.050 g/L of catalyst resulted in a decline in dye degradation. This abnormality is due to the interference and arrest of light penetration caused by the enormous amount of the catalyst. Particle aggregation is indicative at higher concentrations and reduces the active sites on the catalyst surface.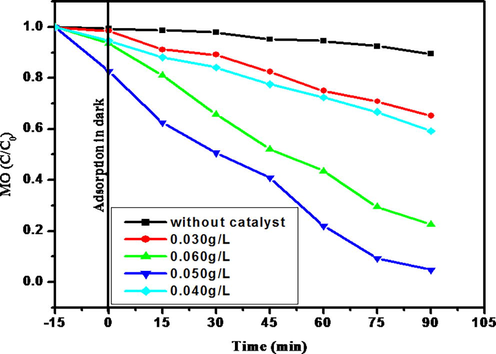
Effect of AzI-Cr5O12 dosage on the photodegradation of MO.
3.3.3 Effect of initial concentration of MO
MO varied from 10 to 30 µM to study the initial dye concentration (Fig. 9). The photodegradation of MO increases with increase of MO (10–20 µM) and then decreases with further increase of concentration (20–30 µM). The photodegradation performance was contrariwisely changed by the concentration of dye. The decrease in degradation with an increase in dye concentration was ascribed to the equilibrium adsorption of dye on the catalyst surface which results in the decrease of number of active sites. This phenomenon results in the formation of hydroxyl radicals, considered as a primary oxidizing agent of the dye (Saravanan et al., 2015).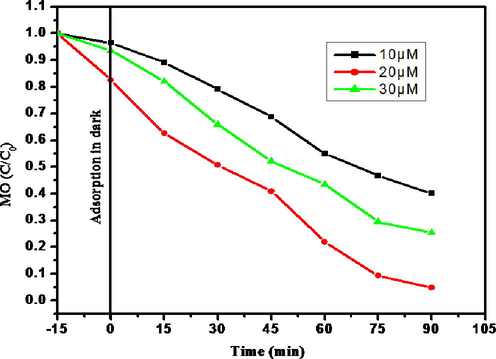
Effect of initial MO concentration.
3.4 Antimicrobial activity of Cr5O12
Fungal strains (Candida albicans) and bacterial strains (Staphylococcus aureus and Enterobacter) was analyzed using AzI-Cr5O12 are shown in Fig. 10 and Table 5. represent the antimicrobial activity of AzI-Cr5O12 for various microbes in a well diffusion assay. Amikacin was used as a reference drug and Ketokonazole as a control. The result revealed that AzI- Cr5O12 shows excellent antimicrobial activity against a range of bacteria and fungi. A larger zone of inhibition was observed to AzI-Cr5O12, whereas smaller zone of inhibition for resistant strains. According to the zone of inhibition Staphylococcus aureus and Enterobacter (bacterial strain) exhibited the highest sensitivity toward Cr5O12. While Candida albicans (fungal strains) show the least sensitivity among the tested microbes. The microbial performance of AzI-Cr5O12 depends on its degree of polymerization, molecular weight, nutrient composition, host, natural nutrient constituency, solvent, microorganism, physicochemical effect, and pH (Ezhilarasi et al., 2016; Mobeen Amanulla et al., 2018) was shown in Fig. 11.
Photograph of antimicrobial activity of AzI-Cr5O12.
Type of Pathogens
Name of organism
Zone of inhibition in mm
Control (c) (Ketokonazole)
Standard (s) (Amikacin)
AzI- Cr5O12(1)
Gram +ve Bacteria
Staphylococcus aureus
18
R
40
Gram −ve Bacteria
Enterobacter
17
R
35
Fungal Species
Candida albicans
25
R
25

Mechanism of Antimicrobial activity of AzI-Cr5O12.
4 Conclusion
AzI-Cr5O12 nanoparticles have been successfully synthesized by simple co- precipitation method. Cr5O12 nanoparticles were characterized by FT-IR, XRD, SEM and EDX techniques. The photocatalyst AzI-Cr5O12 is successfully applied for the degradation of MO under visible light irradiation. The enhanced photocatalytic activity of AzI-Cr5O12 is due to the suppression of electron-hole recombination by leaf extract in Cr5O12. The reaction conditions are optimized and maximum photodegradation is achieved within 90 min with an AzI-Cr5O12 dosage of 0.050 g/L and MO concentration of 20 µM. A highest zone of inhibition was observed for Staphylococcus aureus and Enterobacter of about 40 and 35 mm respectively compared to other microorganisms.
References
- Improved degradation of methyl orange dye using bio-co-catalyst Se nanoparticles impregnated ZnS photocatalyst under UV irradiation. Chem. Eng. J.. 2016;306:1041-1048.
- [Google Scholar]
- Rice husk and its ash as low-cost adsorbents in water and wastewater treatment. Ind. Eng. Chem. Res.. 2011;50:13589-13613.
- [Google Scholar]
- Evaluation of the spectroscopic ellipsometry and dielectric properties of Cr2O3 nanoparticles doped PVDF thin films for future application of organic ferroelectric junctions. Optik. 2017;138:207-213.
- [Google Scholar]
- Synthesis of chromium(III) oxide nanoparticles by electrochemical method and mukia maderaspatana plant extract, characterization, KMnO4 decomposition and antibacterial study. Mod. Res. Catal.. 2013;2(4):127-135.
- [Google Scholar]
- Green synthesis of NiO nanoparticles using Moringa oleifera extract and their biomedical applications: cytotoxicity effect of nanoparticles against HT-29 cancer cells. J. Photochem. Photobiol. B: Biol.. 2016;164:352-360.
- [Google Scholar]
- Green synthesis of NiO nanoparticles using Aegle marmelos leaf extract for the evaluation of in-vitro cytotoxicity, antibacterial and photocatalytic properties. J. Photochem. Photobiol. B: Biol.. 2018;180:39-50.
- [Google Scholar]
- Green synthesis of NiO nanoparticles using Aegle marmelos leaf extract for the evaluation of in-vitro cytotoxicity, antibacterial and photocatalytic properties. J. Photochem. Photobiol. B: Biol.. 2018;180:39-50.
- [Google Scholar]
- Investigation of the drastic improvement of photocatalytic degradation of Congo red by monoclinic Cd, Ba-CuO nanoparticles and its antimicrobial activities. Surf. Interfaces. 2018;10:32-44.
- [Google Scholar]
- Removal of basic dye Auramine-O by ZnS: Cu nanoparticles loaded on activated carbon Optimization of parameters using response surface methodology with central composite design. RSC Adv.. 2015;5(24):18438-18450.
- [Google Scholar]
- Antimicrobial potential of Azadirachta indica against pathogenic bacteria and fungi. J. Pharm. Phytochem.. 2012;4:78.
- [Google Scholar]
- Removal of Cr (VI) from aqueous solutions using preconsumer processing agricultural waste: a case study of rice husk. J. Hazard. Mater.. 2009;162(2009):312-320.
- [Google Scholar]
- Biological activities and medicinal properties of neem (Azadirachta indica) Curr. Sci.. 2002;82:1336-1345.
- [Google Scholar]
- Addition of nanosized Cr2O3 to magnesium for improvement of the hydrogen sorption properties. J. Alloys Compd.. 2003;351:217-221.
- [Google Scholar]
- Effect of FeWO4 doping on the photocatalytic activity of ZnO under visible light irradiation. Appl. Surf. Sci.. 2015;356:333-340.
- [Google Scholar]
- Cr2O3 nanoparticle functionalized WO3 nanorods for ethanol gas sensors. Appl. Surf. Sci.. 2018;432:241-249.
- [Google Scholar]
- Novel morphologies and growth mechanism of Cr2O3 oxide formed on stainless steel surface via Nd: YAG pulsed laser oxidation. J. Alloys Compd.. 2015;635:101-106.
- [Google Scholar]
- Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev.. 2004;104:293-346.
- [Google Scholar]
- Green synthesis of NiO nanoparticles using Moringa oleifera extract and their biomedical applications: cytotoxicity effect of nanoparticles against HT-29 cancer cells. J. Photochem. Photobiol. B: Biol.. 2016;164:352-360.
- [Google Scholar]
- Chromium oxide/alumina catalysts in oxidative dehydrogenation of isobutane. J. Catal.. 1998;178(2):687-700.
- [Google Scholar]
- Synthesis of Cr2O3 nanoparticles via thermal decomposition of polyacrylate/chromium complex. Mater. Lett.. 2014;129:54-56.
- [Google Scholar]
- Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Adv. Colloid Interface Sci.. 2013;193–194:24-34.
- [Google Scholar]
- Bioadsorbents for remediation of heavy metals: current status and their future prospects. Environ. Eng. Res.. 2015;20(1):1-18.
- [Google Scholar]
- Potential of activated carbon from Waste Rubber Tire for the adsorption of phenolics: effect of pre-treatment conditions. J. Colloid Interface Sci.. 2016;417:420-430.
- [Google Scholar]
- Phytochemical Methods (2nd ed.). London: Chapman and Hall Ltd; 1973. p. :49-188.
- Screening of medicinal plants for antimicrobial activity. Part I, Fitoterapia. 1984;55:231-235.
- [Google Scholar]
- Photocatalytic degradation effect of malachite green and catalytic hydrogenation by UV–illuminated CeO2/CdO multilayered nanoplatelet arrays: investigation of antifungal and antimicrobial activities. J. Photochem. Photobiol. B: Biol.. 2017;169:110-123.
- [Google Scholar]
- Fullerene stabilized gold nanoparticle supported on titanium dioxide for enhanced photocatalytic degradation of methyl orange and catalytic reduction of 4-nitrophenol. J. Environ. Chem. Eng.. 2018;6(4):3827-3836.
- [Google Scholar]
- Study of Crude Drugs (8th ed.). Manipal, India: Manipal Power Press; 1995. p. :2.
- Simple synthesis of Cr2O3 nanoparticles with a tunable particle size. Ceram. Int.. 2015;41(3):4644-4650.
- [Google Scholar]
- Enhanced photocatalytic activity of V2O5 nanorods for the photodegradation of organic dyes: a detailed understanding of the mechanism and their antibacterial activity. Mater. Sci. Semicond. Process.. 2018;85:122-133.
- [Google Scholar]
- Synthesis and investigation the catalytic behavior of Cr2O3. J. Nanostruct.. 2013;3:87-92.
- [Google Scholar]
- Photocatalytic and antimicrobial activity of NiWO4 nanoparticles stabilized by the plant extract. Mater. Sci. Semicond. Process.. 2015;40:123-129.
- [Google Scholar]
- Synthesis of MoO3 microrods via phytoconsituents of Azadirachta indica leaf to study the cationic dye degradation and antimicrobial properties. J. Alloys Compound.. 2018;753:300-307.
- [Google Scholar]
- Green synthesis of ZnO nanoparticles using sollanam santhocarbom to study its solar photocatalytic activity. IJSR. 2015;6(6):2370-2376.
- [Google Scholar]
- A new approach for the degradation of high concentration of aromatic amine by heterocatalytic Fenton oxidation: kinetic and spectroscopic studies. J. Mol. Liq.. 2012;173:153-163.
- [Google Scholar]
- In vitro assays: tracking nanoparticles inside cells. Nat. Nanotechnol.. 2011;6:139-140.
- [Google Scholar]
- In vitro cytotoxicity effect and antibacterial performance of human lung epithelial cells A549 activity of Zinc oxide doped TiO2 nanocrystals: investigation of bio-medical application by chemical method. Mater. Sci. Eng. C. 2017;74:325-333.
- [Google Scholar]
- Antiproliferative effects on human lung cell lines A549 activity of cadmium selenide nanoparticles extracted from cytotoxic effects: investigation of bio-electronic application. Mater. Sci. Eng. C. 2017;76:1012-1025.
- [Google Scholar]
- Black Cr/α-Cr2O3 nanoparticles based solar absorbers. Phys. B: Condens. Mater.. 2012;407(10):1509-1512.
- [Google Scholar]
- Preparation of chromia nanoparticles by precipitation–gelation reaction. Mater. Lett.. 2004;58:1894-1898.
- [Google Scholar]
- Low-temperature synthesis and microstructural control of titania nano-particles. J. Solid State Chem.. 2004;177:1372-1381.
- [Google Scholar]
- The growth mechanism of titania/hydroxyapatite and its application in the photodegradation of methyl orange dye under UV irradiation. Results Phys.. 2018;11:112-117.
- [Google Scholar]
- Facile synthesis of heterostructured cerium oxide/yttrium oxide nanocomposite in UV light induced photocatalytic degradation and catalytic reduction: synergistic effect of antimicrobial studies. J. Photochem. Photobiol. B: Biol.. 2017;173:23-34.
- [Google Scholar]
- Evaluation on the heterostructured CeO2/Y2O3 binary metal oxide nanocomposites for UV/Vis light induced photocatalytic degradation of Rhodamine – B dye for textile engineering application. J. Alloys Compound.. 2017;727:1324-1337.
- [Google Scholar]
- Evaluation on the heterostructured CeO2/Y2O3 binary metal oxide nanocomposites for UV/Vis light induced photocatalytic degradation of Rhodamine – B dye for textile engineering application. J. Alloys Compound.. 2017;727:1324-1337.
- [Google Scholar]
- ZnO nanoparticles via Moringa oleifera green synthesis: physical properties & mechanism of formation. Appl. Surf. Sci.. 2017;406:339-347.
- [Google Scholar]
- Antibacterial, magnetic, optical and humidity sensor studies of β-CoMoO4 - Co3O4 nanocomposites and its synthesis and characterization. J. Photochem. Photobiol. B: Biol.. 2018;180:39-50.
- [Google Scholar]
- Antibacterial, magnetic optical and humidity sensor studies of β-CoMoO4 - Co3O4 nanocomposites and its synthesis and characterization. J. Photochem. Photobiol. B: Biol.. 2018;183:233-241.
- [Google Scholar]
- Adsorption process of methyl orange dye onto mesoporous carbon material–kinetic and thermodynamic studies. J. Colloid Interface Sci.. 2011;362:457-462.
- [Google Scholar]
- Xiaoke Mu, Role of surface spins on magnetization of Cr2O3 coated γ-Fe2O3 nanoparticles. Solid State Sci.. 2018;83:43-48.
- [Google Scholar]
- Structural, optical, electrical and photovoltaic electrochemical characterization of spray deposited NiWO4 thin films. Electrochim. Acta. 2006;51:4659-4664.
- [Google Scholar]
- Solar-assisted photodegradation of Methyl Orange using Cu-doped ZnO nanorods. Mater. Today Commun. 2018
- [CrossRef] [Google Scholar]
- Synthesis characterization and toxicological evaluation of Cr2O3 nanoparticles using Daphnia magna and Aliivibrio fischeri. Ecotoxicol. Environ. Safety. 2016;128:36-43.
- [Google Scholar]
- Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4(6):1457-1465.
- [Google Scholar]
- Eco-friendly preparation of zinc oxide nanoparticles using Tabernaemontana divaricata and its photocatalytic and antimicrobial activity. J. Photochem. Photobiol. B: Biol.. 2018;181:53-58.
- [Google Scholar]
- Ce3+-ion-induced visible-light photocatalytic degradation and electrochemical activity of ZnO/CeO2 nanocomposite. Sci. Rep.. 2016;6:31641-31652.
- [Google Scholar]
- Study of phytochemical screening of neem (Azadirachta indica) Int. J. Zool. Stud.. 2018;3(1):209-212.
- [Google Scholar]
- Antibacterial activity of Cr2O3 nanoparticles against E. coli; reduction of chromate ions by Arachis hypogaea leaves. Achiev. Appl. Sci. Res.. 2012;4(4):1894-1900.
- [Google Scholar]
- Equilibrium and kinetic studies of the adsorption of acid blue 9 and Safranin O fromaqueous solutions by MgO decked FLG coated Fuller's earth. J. Phys. Chem. Solids. 2018;123:43-51.
- [Google Scholar]
- Equilibrium and kinetic studies of the adsorption of acid blue 9 and Safranin O from aqueous solutions by MgO decked FLG coated Fuller's earth. J. Phys. Chem. Solids. 2018;123:43-51.
- [Google Scholar]
- Removal of hazardous Dyes-BR 12 and methyl orange using graphene oxide as an adsorbent from aqueous phase. Chem. Eng J.. 2016;284:687-697.
- [Google Scholar]
- Functionalization of tungsten oxide into MWCNT and its application for sunlight-induced degradation of rhodamine B. J. Colloid Interface Sci.. 2011;362:337-344.
- [Google Scholar]
- Synthesis and characterization of alumina nano-particles polyamide membrane with enhanced flux rejection performance. Sep. Purif. Technol.. 2012;89:245-251.
- [Google Scholar]
- Synthesis, characterization and photocatalytic activity of novel Hg doped ZnO nanorods prepared by thermal decomposition method. J. Mol. Liq.. 2013;178:88-93.
- [Google Scholar]
- ZnO/Ag nanocomposite: an efficient catalyst for degradation studies of textile effluents under visible light. Mater. Sci. Eng. C. 2013;33:2235-2244.
- [Google Scholar]
- Visible light induced degradation of methylene blue using CeO2/V2O5 and CeO2/CuO catalysts. Mater. Sci. Eng.C. 2013;33:4725-4731.
- [Google Scholar]
- The photocatalytic activity of ZnO prepared by simple thermal decomposition method at various temperatures. J. Mol. Liq.. 2013;177:394-401.
- [Google Scholar]
- Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater. Sci. Eng. C. 2013;33:91-98.
- [Google Scholar]
- ZnO/Ag/Mn2O3 nanocomposite for visible light-induced industrial textile effluent degradation, uric acid and ascorbic acid sensing and antimicrobial activities. RSC Adv.. 2015;5:34645-34651.
- [Google Scholar]
- ZnO/Ag/CdO nanocomposite for visible light-induced photocatalytic degradation of industrial textile effluents. J. Colloid Interface Sci.. 2015;452:126-133.
- [Google Scholar]
- Interaction of CO with α-Cr2O3 surface: a FTIR and HRTEM study. Chem. Phys.. 1993;177(2):547-560.
- [Google Scholar]
- Practical Pharmaceutical Chemistry (1st ed.). New Delhi: CBS Publishers and Distributors; 1997. p. :126-131.
- Synthesis of spherical Cr2O3 nanoparticles by a microwave refluxing method and their photocatalytic properties. Ceram. Int.. 2014;40:15051-15055.
- [Google Scholar]
- Medicinal properties of neem leaves: a review. Curr. Med. Chem. AntiCancer Agents. 2005;5:146-156.
- [Google Scholar]
- Electrical and humidity sensing properties of lead(II) tungstate–tungsten(VI) oxide and zinc(II) tungstate–tungsten(VI) oxide composites. Mater. Res. Bull.. 2004;39:581-590.
- [Google Scholar]
- One-step green synthesis of metallic nanoparticles using sodium alginate. J. Nanomater.. 2016;1:9790345-9790411.
- [Google Scholar]
- Photocatalytic activity of AgI sensitized ZnO nanoparticles under visible light irradiation. Powder Technol.. 2012;224:331-337.
- [Google Scholar]
- Phytochemical analysis and antibacterial activity of Azadirachta indica A. Juss. Int. J. Res. Plant Sci.. 2012;2:50-55.
- [Google Scholar]
- NASICON-based potentiometric Cl2 sensor combining NASICON with Cr2O3 sensing electrode. Sens. Actuat. B Chem.. 2013;180:66-70.
- [Google Scholar]







