Translate this page into:
Association between H1N1 infection and pro-inflammatory Th-1 and Th-17 cytokines production
⁎Corresponding author. syalomar@ksu.edu.sa (Suliman Y. Alomar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
The pandemic H1N1 influenza A virus is considered one of the main causes of outbreaks of respiratory infection among humankind. Resident immune cells in the mucosal sites of the respiratory tract maintain an appropriate balance between protection and disease. Our research aims in the current study to investigate a profile of cytokines production, including TNF-α, IL-4, IFN-γ, IL-17A, IL-10, and IL-2, following stimulation of tonsillar MNCs using pH1N1 (A/California/04/2009 strain).
Methods
Patients who underwent tonsillectomy because of snoring and obstructive sleep apnoea (n = 31) were recruited in the current pilot study. Tonsillar monocular cells (MNCs) were isolated and then stimulated using the H1N1 viral antigen of the A/California/04/2009 strain. A BD™ Cytometric Bead Array Experiment (CBA) method was utilized to analyze different target pro-inflammatory cytokine markers in the cell-culture supernatants of H1N1-stimulated MNCs. The cytokine profiles comprise TNF-α, IL-4, IFN-γ, IL-17A, IL-10, and IL-2.
Results
A noticeable pro-inflammatory Th-1 and Th-17 response was seen in H1N1-stimulated MNCs, with significantly increased concentrations of IFN-γ, IL-10, and IL-17A compared to un-stimulated controls (p-value less than 0.001, and 0.01, respectively). TNF-α, IL-4, and IL-2 levels presented no significant differences between H1N1-stimulated MNCs compared to un-stimulated controls (P > 0.05). The results demonstrate a marked Th-1 and Th-17 cytokine response following stimulation of MNCs with the H1N1 viral antigen.
Conclusions
Stimulation of MNCs using pH1N1 viral antigen presented the vital role of T-cells in mucosal immunity against pH1N1 and its importance when considering designing and developing intranasal vaccine candidates that can trigger cellular in addition to humoral immune responses.
Keywords
pH1N1 viral antigen
IL-17A
IFN-γ
IL-10
MNCs
1 Introduction
Influenza is known as a human-transmittable infectious respiratory illness that is caused by either influenza A or B viruses. According to the WHO (2023), influenza viruses are responsible for causing seasonal epidemics and sporadic pandemic illnesses that have affected the social lives of many individuals across the globe. The incidence of novel variants of the influenza virus systematically poses significant challenges and a health threat to both scientific communities and public health systems across the world. In 2009, the initial influenza virus pandemic was caused by a new H1N1 influenza A virus (2009 H1N1) reassortant that was formerly flowing in pigs (Smith et al., 2009). The human seasonal and pandemic H1N1 influenza A virus was responsible for causing infectious disease in different age groups and was able to spread the infection and cause a severe outbreak, particularly among younger age groups. Several research reports found that in elderly persons (aged 75 years and over), there was noticeably lower influenza virus-associated respiratory cases percentage compared with people of younger age (Simonsen et al., 1998). Additionally, evidence of the detection of pre-existing immune responses to investigated influenza viruses has been obtained from the age-adjusted mortality rates. Individuals who examined influenza viruses among elderly people (>75 years) presented an influenza/pneumonia-related lower respiratory tract infection rate in 1918 than the pre-pandemic period between 1911 and 1917 (Taubenberger & Morens, 2006). Seasonal epidemics of influenza A and influenza B viruses cause the main load of disease in humans. Besides seasonal epidemics, the development of influenza A viruses from whichever avian or swine inhabitants has produced four pandemics since 1918, with those viruses next developing seasonal epidemic strains in the following years (Mahallawi et al., 2020) (Jiao et al., 2023; Wan et al., 2023).
Several infectious agents, such as respiratory viral, bacterial, and other pathogens, target cells that coat the airways and can cause direct damage to barrier sites and generate inflammation-associated tissue damage. Certainly, respiratory pathogens remain a noticeable source of worldwide mortality (Mettelman et al., 2022).
Every cell type, either tissue-resident or recruited, yields a sensibly coordinated equilibrium of inflammatory and chemotactic cytokines such as TNF-ɑ, and IL-6, and immune-regulatory factors such as IL-10 and TGF-β in a determination to fight and eradicate pathogens devoid of severe inflammation (Ho et al., 2011). Due to the risk of infection from inhaled air particles that are prone to carrying pathogens during the process of inspiration, the mucosal immune system is critical to protection against respiratory pathogens (Amersfoort et al., 2022; Waleed H. Mahallawi & Talal M. Aljeraisi, 2021). Resident immune cells in the mucosal sites of the respiratory tract maintain an appropriate balance between protection and disease, as they should continuously clear and remove inhaled contaminated air particles and quickly counter microbes via the much-synchronized enrolment of definite innate in addition to adaptive leukocytes (Holt et al., 2008).
We aimed in the current study to investigate a profile of cytokine production, including TNF-α, IL-4, IFN-γ, IL-17A, IL-10, and IL-2, following stimulation of tonsillar MNCs using pH1N1 (A/California/04/2009 strain).
2 Materials and methods
2.1 Influenza antigens
Influenza antigen (pH1N1) used for tonsillar MNCs stimulation was β-propiolactone inactivated (β-PLI), whole virus antigens of pH1N1 (partially purified) and were commercially ordered from the (NIBSC) and isolated from strain of influenza A virus-A/California/04/2009.
2.2 Tonsillar samples
Thirty-one human tonsils were collected after elective tonsillectomy from patients who had been diagnosed with tonsillitis and snoring. Those tonsils were obtained from the ENT department at Madinah Saudi German Hospital in the Kingdom of Saudi Arabia and recruited for this study. Participants with recurrent tonsillitis or with any history of immune suppression were excluded.
2.3 Isolation of tonsillar MNCs
Cell suspensions were prepared using the prior method with minor modifications. (W. H. Mahallawi & T. M. Aljeraisi, 2021) In brief, tonsils were processed within one hour of surgery. After adding 5 mL of cell-culture media (RPMI-1640) (Sigma-Aldrich), the tissues were minced. A 70-μm, sterile cell strainer mesh was used to rinse the cell suspension after adding it using a 5 mL sterile graduated dropper. MNCs were then isolated using Ficoll-Paque. Sterile phosphate-buffered saline (PBS) was used to wash MNCs and the washed cells were immediately re-suspended in a centrifugal tube containing 5 mL of RPMI-1640 (Mahallawi & Aljeraisi, 2021a, 2021b).
2.4 Cell culture and stimulation
Following the isolation of MNCs, we adjusted the cell concentration to 4 × 106 cells/mL. With minor modifications, our previous method used (Mahallawi & Khabour, 2024). Briefly, MNCs were then co-cultured in RPMI-1640 complete medium after adding the optimal vaccine concentration at 10 μL/mL, and un-stimulated MNCs were used as a control. 250 μL of stimulated and unstimulated MNCs were added to selected wells of the cell-culture plate (Costar). The cell-culture-treated plate was then placed in the incubator (5 % CO2 atmosphere at 37 °C). The stimulated and unstimulated supernatants were gathered on the third day and directly kept in the freezer at −20 °C, for further analysis.
2.5 Cytokines beads Array
The BDTM CBA was performed to measure multi-target cytokines. (https://www.bdbiosciences.com/en-us/products/reagents/immunoassay-reagents/cba/cba-kits/human-th1-th2-th17-cba-kit.560484). The BD™ CBA (San Jose, CA), CBA Human Th-1/Th-2/Th-17 Pro-inflammatory Cytokine Kit was utilized to quantify several cytokine profiles such as TNF-α, IL-4, IL-17A, IFN-γ, IL-10, and IL-2. Kit performance was manually optimized for the measurement of generating cytokine marker concentrations (pg/mL) in stimulated and un-stimulated cell-culture supernatants. Tonsillar MNCs were stimulated with the A/California/04/2009 strain, and then the armed effector T-cells were examined in the cell supernatants for detecting the existence of Th-1, Th-2, and Th-17 and the expression of their target pro-inflammatory cytokine markers. The capture bead-based immunoassays in the CBA kit have been performed and labelled with conjugated specific antibodies. A mixture of phycoerythrin (PE)-conjugated antibodies (the detection reagent) was provided in the kit. It uses fluorescent dyes for fluorescent staining and was used to produce a fluorescent signal to determine the amount of each analyte in the cell-culture supernatants.
2.6 Statistical data analysis
Calculations and statistical data analyses were achieved by using GraphPad Prism Statistical 5.0. software (GraphPad Software, USA). The analyzed data were stated as the mean ± SD. Comparisons between two age groups were conducted, and a Student’s t-test was used. A p-value less than 0.05 was judged as significant.
3 Results
3.1 Infection with pH1N1 virus elicits a type-II interferon response
A BDTM CBA assay was performed to measure the secretion of cytokine profiles (e.g., IFN-γ) in cell-culture supernatants following the stimulation of tonsillar MNCs with pH1N1 influenza virus antigen. Stimulation of MNCs with pH1N1 virus antigen-induced significant mucosal IFN-γ production in tonsillar MNCs compared to un-stimulated control (Fig. 1, p < 0.001, n = 31). IFN-γ is an effector cytokine that belongs to type II interferon, and it is formed by numerous immune cell types, such as CD4 Th-1 cells, natural killer cells (NKs), and CD8+ cytotoxic T-cells. IFN-γ plays a crucial role by interfering with viral replication and shows imperative roles in acquired as well as innate immunity (Castro et al., 2018).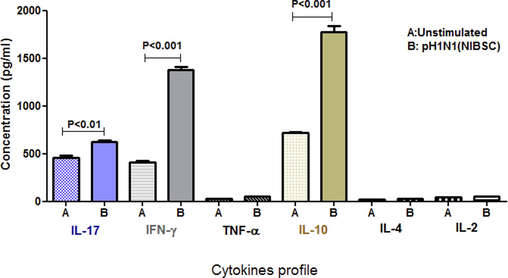
Cytokines markers expression in tonsillar MNCs following the stimulation with 2009 pH1N1 virus antigen. Tonsillar MNCs were prepared, and cell-culture supernatants were stimulated with the 2009 pH1N1 influenza virus antigen. The production of numerous types of cytokines markers such as TNF-α, IL-4, IFN-γ, IL-17A, IL-10, and IL-2, were analyzed utilizing CBA immune assay. The high production of IL-10, IFN-γ, and IL-17A was identified and results were compared with un-stimulated control (n = 31). Mean ± SD are presented as (p-value was lower than 0.001 and 0.01, respectively).
3.2 Th-17 cytokine is associated with infection with pH1N1 virus antigen
Cell-culture supernatants were analysed using a BDTM CBA assay to measure the expression of cytokine markers following tonsillar MNCs stimulation with the pH1N1 influenza virus. Significant mucosal IL-17A concentrations were observed following the stimulation of MNCs with the pH1N1 virus compared with the unstimulated control (see Fig. 1, p < 0.01, n = 31). IL-17 is a cytokine made by T-helper cells, and it is known as a hallmark molecule of Th-17 cells. It is secreted in response to STAT3 as well as NF-κB signaling pathways (Ge et al., 2020; Manni et al., 2014). This result suggests that the pH1N1 virus in humans triggers a Th-17 pro-inflammatory cytokine. Also, there were no significant concentrations of IL-2, TNF-α, and IL-4 in stimulated cell-culture supernatants compared with the unstimulated control (p < 0.05).
3.3 pH1N1 virus induces the expression of IL-10
To investigate the capability of pH1N1 virus antigen to induce IL-10, a CBA assay was conducted to quantify the IL-10 secretion in stimulated and un-stimulated cell-culture supernatants of tonsillar MNCs with 2009 pH1N1 virus protein. Interestingly, the use of the 2009 pH1N1 virus protein to stimulate cells was able to induce and increase significant mucosal secretions of IL-10 in tonsillar MNCs compared to the unstimulated control (Fig. 1, p < 0.001, n = 31). IL-10 is a vital cytokine that is essential in regulating pro-inflammatory responses. IL-10 is one of the class II cytokines and has a key anti-inflammatory consequence mediated through the JAK-STAT pathway (Carey et al., 2012; Renauld, 2003).
4 Discussion
Human adenotonsillar organs form the foremost part of nasopharyngeal-associated lymphoid tissue (NALT) or Waldeyer's ring and are known to be functionally connected with the nasopharyngeal-associated lymphoreticular tissues of rodents and other kinds, and they are accounted for as secondary immune organs (Aljeraisi et al., 2023; Brombacher et al., 2003; Komorowska et al., 2005; Mahallawi & Khabour, 2024).
In the adenotonsillar-derived lymphoid tissue, B-lymphocytes share about seventy percent of the entire lymphocyte population (Mahallawi & Zhang, 2023), whereas the residual lymphocytes involve both T lymphocytes and plasma cells (Brandtzaeg, 2011). Amongst T lymphocytes, Th cells show important roles in immune defense upon their capability to activate B cells to yield antibodies in addition to employing different leukocytes to locations of infection plus inflammation (Morris et al., 2016). It has been shown that Th cells are able to segregate into parallel sorts of effector CD4+ T cells (Th-17, Th-2, and Th-1) subgroups to defend against diverse types of pathogens by stimulating the creation of diverse cytokines that can work as immune effectors to abolish infected cells (Mahallawi et al., 2018; Zhang et al., 2011).
The current study shows that significant levels of IL-17A were identified in the cell-culture supernatants following stimulation with pH1N1. It has been observed that children with higher IL-l7 levels in response to the carriage of pneumococcal could display a raised capability to clear mucosal carriage (Huang et al., 2018). Therefore, induction of IL-17A at the mucosal sites is critical to defending against respiratory pathogens such as influenza viruses, in addition to helping in the eradication of pathogenic bacterial colonization (Gray et al., 2014). Consequently, describing the role of IL-17A and its related immune pathway to different pathogens is vital to investigating, designing, executing, and modifying management strategies for efficaciously treating and preventing these infectious diseases (Khan & Pichichero, 2014).
Th-17 cell stimulation results in the expression of chemokine receptors (i.e., CCR4, CCR6) that enable their movement to inflammatory sites at mucosal sites (Weaver et al., 2013). Along with IL-17A, IL-17F, and even IFN-γ, the Th-17 pathway is able to help cells that express IL-22, which targets the mucosal site of the epithelium for advanced barrier character (Sonnenberg et al., 2011). Therefore, the result proves that MNCs stimulation with pH1N1 virus antigen would be of great advantage in considering the important role of mucosal immunity in combating influenza viruses. Consequently, focusing on developing influenza vaccines that are administered via mucosal sites, such as intranasal routes, will be beneficial.
The activation of CD4+ T helper cells (the central-memory cells) in the human tonsils by either flu vaccines or natural infection results in the formation of CD4+ T-cells (the effector-memory cells) that are considered the initial source of releasing IFN-γ (Sallusto et al., 1999). It was proposed that the secreted effector-memory CD4+ T-cells be moved toward the mucosal tissues to fight infection. These migrations lead to the secretion of IFN-γ, which is vigorously involved in the immune response to influenza virus infection. They have been proven that tonsillar MNCs contain huge numbers of cell-secreting IFN-γ, which are crucial at the mucosal surfaces of the aerodigestive tract and are important for both innate and adaptive immune responses (Bhaskaran et al., 2021; Kang et al., 2004). However, the cellular immune response in the tonsillar tissues is stronger than the humoral immune response (Komorowska et al., 2005; Seethaler et al., 2020). Our study found that the 2009 pH1N1 virus protein was able to stimulate tonsillar MNCs and express a significant amount of IFN-γ. This may be a result of the activation of central-memory CD4+ T-cells in the human tonsils, which diverts to effector-memory CD4+ T-cells to secrete a high amount of IFN-γ. Therefore, the existence of effector-memory CD4+ T-cells at the site of infection may be vigorously involved in the immune system response against influenza virus infection.
Following the activation of T-cells, CD4+ naïve T cells develop into two functional helper T-cells, Th-1 and Th-2, based on their release of cytokine markers, mainly IL-4 and IFN-γ. Th-1 cells play a key function by protecting against a diverse range of intracellular pathogens, whereas Th-2 cells are responsible for protecting against extracellular microbes (Seder & Ahmed, 2003). Th-1 cells are categorized by producing large amounts (IFN-γ) markers and small volumes (IL-4) molecules. CD4+ T cells play a critical function in the enhancement of antigen-specific T-cells and regulation of effector-memory T-cells (TEM cells) immunity.
IFN-γ is a pro-inflammatory factor, that plays a vital role in defense against influenza virus infection, in particular at the nasal mucosa surface (McKinstry et al., 2010). A study by Guthrie has reported that IFN-γ was highly produced in tonsil tissues following stimulation with influenza virus proteins, which were almost three times higher than IFN-γ molecules expression in PBMCs. These findings can indicate the essential functional role of activation of IFN-γ markers in human nasal mucosal tissues at the upper respiratory tract in protection against influenza virus infection. They also observed that there was high expression of IFN-γ by Th-1 cells, which dominated the production of IFN-γ by Th-2 in the nasal mucosal tissues when stimulated cells with influenza virus antigens (Guthrie et al., 2004). While several investigations support Th-1 cells and IFN-γ as inhibitors of Th-2-type responses, further research demonstrates that Th-1 cells enrich inflammation and do not enhance disease. The pro-inflammatory properties of Th-1 cells are maintained by the linkage of viral respiratory infections, which is likely to persuade Th-1 responses in addition to the worsening of symptoms in patients with lung diseases such as asthma (Ibitokou et al., 2023; Yuan et al., 2023; Zhou et al., 2023).
The current study found that there are no significant differences in the production of IL-4 by Th-2 cells in human tonsillar MNCs following stimulation with the 2009 pH1N1 virus protein. This result is different from the substantial secretion of high IFN-γ levels. These results propose that the 2009 pH1N1 protein was able to enhance a predominant Th-1 cytokine immune response.
This study also found that pH1N1 virus antigen induced a massive amount of mucosally derived IL-10 and was the highest among all significant cytokines. It has to be highlighted that IL-10, a “cytokine synthesis inhibiting factor” (CSIF), is a powerful cytokine molecule with specific anti-inflammatory activity, commonly formed by subgroups of T-cells such as Treg and Th-2 (Bant et al., 2022). It is also vital to stress that a different T cell population generates huge quantities of IL-10 and powerfully inhibits the immune response in human nasopharyngeal-associated lymphoreticular tissues (Sumitomo et al., 2017). It has been recently designated the presence of IL-10, one in which the cells act in the course of chronic viral infection in mice (Xin et al., 2018) and in which they exist in human tonsils (Canete et al., 2019). IL-10 is known as a major immunomodulatory cytokine, and it plays a crucial role in the termination of infectious agents as well as regulating adaptive immune responses. The regulation of cellular immunity is critical for the protection of host cells against lethal influenza virus infection and, simultaneously, preventing the excessive inflammation of human tissues (Kingsley et al., 2002).
5 Conclusions
Overall, the results demonstrate a marked Th-1 and Th-17 cytokine response following stimulation of MNCs with the H1N1 viral antigen. Stimulation of MNCs using pH1N1 viral antigen presented the vital role of T-cells in mucosal immunity against influenza virus. Its importance increases when considering designing and developing intranasal vaccine candidates that are able to trigger cellular and humoral immune responses.
CRediT authorship contribution statement
All the authors have contributed equally to the current article.
Acknowledgments
The authors would like to thank the Researchers Supporting Project Number (RSP2024R35), King Saud University, Riyadh, Saudi Arabia.
References
- BCG vaccine-induced mucosal humoral immunity in human nasal associated lymphoid tissue. Journal of King Saud University - Science. 2023;35(6):102773
- [CrossRef] [Google Scholar]
- Immunomodulation by endothelial cells — partnering up with the immune system? Nat. Rev. Immunol.. 2022;22(9):576-588.
- [CrossRef] [Google Scholar]
- Occurrence of IL-1, IL-10, CD25, CD40, and CD69 in the tissue of palatine tonsils. Postepy Dermatol Alergol. 2022;39(1):182-188.
- [CrossRef] [Google Scholar]
- Oral immune dysfunction is associated with the expansion of FOXP3+PD-1+Amphiregulin+ T cells during HIV infection. Nat. Commun.. 2021;12(1):5143.
- [CrossRef] [Google Scholar]
- Potential of nasopharynx-associated lymphoid tissue for vaccine responses in the airways. Am J Respir Crit Care Med. 2011;183(12):1595-1604.
- [CrossRef] [Google Scholar]
- Novel IL-12 family members shed light on the orchestration of Th1 responses. Trends Immunol.. 2003;24(4):207-212.
- [CrossRef] [Google Scholar]
- Regulatory roles of IL-10-producing human follicular T cells. J Exp Med. 2019;216(8):1843-1856.
- [CrossRef] [Google Scholar]
- Interferon-gamma at the crossroads of tumor immune surveillance or evasion [review] Front. Immunol.. 2018;9
- [CrossRef] [Google Scholar]
- Biology of Interleukin-17 and its pathophysiological significance in sepsis [review] Front. Immunol.. 2020;11
- [CrossRef] [Google Scholar]
- Activation of memory Th17 cells by domain 4 pneumolysin in human nasopharynx-associated lymphoid tissue and its association with pneumococcal carriage. Mucosal Immunol. 2014;7(3):705-717.
- [CrossRef] [Google Scholar]
- Parenteral influenza vaccination influences mucosal and systemic T cell-mediated immunity in healthy adults. J Infect Dis. 2004;190(11):1927-1935.
- [CrossRef] [Google Scholar]
- Lung CD103+ dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC class I for presentation to CD8 T cells. J. Immunol.. 2011;187(11):6011-6021.
- [CrossRef] [Google Scholar]
- Regulation of immunological homeostasis in the respiratory tract. Nat. Rev. Immunol.. 2008;8(2):142-152.
- [CrossRef] [Google Scholar]
- IL-17A expression in the adenoid tissue from children with sleep disordered breathing and its association with pneumococcal carriage. Sci Rep. 2018;8(1):16770.
- [CrossRef] [Google Scholar]
- Effects of low-level persistent infection on maintenance of immunity by CD4 T cell subsets and Th1 cytokines. Infect Immun. 2023;91(3):e0053122.
- [Google Scholar]
- Analysis of the conserved protective epitopes of hemagglutinin on influenza a viruses. Front Immunol. 2023;14:1086297.
- [CrossRef] [Google Scholar]
- Immunomodulatory effect of astragali radix extract on murine TH1/TH2 cell lineage development. Biol Pharm Bull. 2004;27(12):1946-1950.
- [CrossRef] [Google Scholar]
- The host immune dynamics of pneumococcal colonization: implications for novel vaccine development. Hum Vaccin Immunother. 2014;10(12):3688-3699.
- [CrossRef] [Google Scholar]
- CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses [Research Support, Non-U.S. Gov't] J Immunol. 2002;168(3):1080-1086. http://www.ncbi.nlm.nih.gov/pubmed/11801641
- [Google Scholar]
- Cytokines locally produced by lymphocytes removed from the hypertrophic nasopharyngeal and palatine tonsils. Int. J. Pediatr. Otorhinolaryngol.. 2005;69(7):937-941.
- [CrossRef] [Google Scholar]
- In vitro cell culture model of human nasal-associated lymphoid tissue (NALT) to evaluate the humoral immune response to SARS-CoV-2 spike proteins. Saudi J Biol Sci. 2021;28(8):4516-4521.
- [CrossRef] [Google Scholar]
- Infection with SARS-CoV-2 primes immunological memory in human nasal-associated lymphoid tissue. Clin. Immunol.. 2021;231:108850
- [CrossRef] [Google Scholar]
- Natural immunity to influenza a and B among saudi blood donors in Al Madinah al Munawarah, Saudi Arabia. Saudi Medical Journal. 2020;41(12):1301-1307.
- [CrossRef] [Google Scholar]
- Pandemic H1N1 influenza virus triggers a strong T helper cell response in human nasopharynx-associated lymphoid tissues. Saudi J Biol Sci. 2024;31(3):103941
- [CrossRef] [Google Scholar]
- MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8-13.
- [CrossRef] [Google Scholar]
- Live attenuated influenza vaccine induces broadly cross-reactive mucosal antibody responses to different influenza strains in tonsils. Saudi Journal of Biological Sciences. 2023;30(10):103809
- [CrossRef] [Google Scholar]
- A tale of two cytokines: IL-17 and IL-22 in asthma and infection. Expert Rev. Respir. Med.. 2014;8(1):25-42.
- [CrossRef] [Google Scholar]
- Mucosal immune responses to infection and vaccination in the respiratory tract. Immunity. 2022;55(5):749-780.
- [CrossRef] [Google Scholar]
- Adenoidal follicular T helper cells provide stronger B-cell help than those from tonsils. Laryngoscope. 2016;126(2):E80-E85.
- [CrossRef] [Google Scholar]
- Class II cytokine receptors and their ligands: key antiviral and inflammatory modulators. Nat. Rev. Immunol.. 2003;3(8):667-676.
- [CrossRef] [Google Scholar]
- Two subsets of memory T lymphocytes with distinct homing potentials and effector functions [Research Support, Non-U.S. Gov't] Nature. 1999;401(6754):708-712.
- [CrossRef] [Google Scholar]
- Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4(9):835-842.
- [CrossRef] [Google Scholar]
- IL-8 and IFN-γ as preoperative predictors of the outcome of tonsillectomy. Ear Nose Throat J.. 2020;100(5_suppl):822S-S827.
- [CrossRef] [Google Scholar]
- Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis. 1998;178(1):53-60.
- [Google Scholar]
- Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza a epidemic. Nature. 2009;459(7250):1122-1125.
- [CrossRef] [Google Scholar]
- Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12(5):383-390.
- [CrossRef] [Google Scholar]
- Identification of tonsillar CD4+CD25−LAG3+ T cells as naturally occurring IL-10-producing regulatory T cells in human lymphoid tissue. J. Autoimmun.. 2017;76:75-84.
- [CrossRef] [Google Scholar]
- 1918 influenza: the mother of all pandemics [historical article] Emerg Infect Dis. 2006;12(1):15-22.
- [CrossRef] [Google Scholar]
- Investigating epidemiologic and Molecular links between patients with community- and hospital-acquired influenza a: 2017–2018 and 2019–2020, Michigan. Open Forum Infect Dis. 2023;10(2):ofad061.
- [CrossRef] [Google Scholar]
- The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol. 2013;8:477-512.
- [CrossRef] [Google Scholar]
- Single-cell RNA sequencing unveils an IL-10-producing helper subset that sustains humoral immunity during persistent infection. Nat Commun. 2018;9(1):5037.
- [CrossRef] [Google Scholar]
- Th1-involved immune infiltrates improve neoadjuvant chemoradiotherapy response of esophageal squamous cell carcinoma. Cancer Lett. 2023;553:215959
- [CrossRef] [Google Scholar]
- Characterisation of regulatory T cells in nasal associated lymphoid tissue in children: relationships with pneumococcal colonization. PLoS Pathog. 2011;7(8):e1002175
- [CrossRef] [Google Scholar]
- Prednisone acetate modulates Th1/Th2 and Th17/Treg cell homeostasis in experimental autoimmune uveitis via orchestrating the notch signaling pathway. Int Immunopharmacol. 2023;116:109809
- [CrossRef] [Google Scholar]







