Translate this page into:
Assessment of different heavy metals in cigarette filler and ash from multiple brands retailed in Saudi Arabia
⁎Corresponding author. nauman.khalid@umt.edu.pk (Nauman Khalid)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Tobacco smoking is considered one of the major global health concerns due to its known carcinogenic effects on the human body. In this study, 36 samples of tobacco products were evaluated for the approximation of the heavy metal content, including chromium (Cr), cadmium (Cd), copper (Cu), iron (Fe), lead (Pb), manganese (Mn) and zinc (Zn) using the inductively coupled plasma - optical emission spectrometry (ICP-OES). The heavy metal content was evaluated in the tobacco filler, ash, and tobacco products (with and without filters). The average concentrations in filler tobacco products were 0.66 mg/kg, 0.09 mg/kg, 2.61 mg/kg, 245.55 mg/kg, 0.38 mg/kg, 3.985 mg/kg, and 1.64 mg/kg, for Cr, Cd, Cu, Fe, Pb, Mn, and Zn, respectively. Ash obtained from the combustion of different tobacco products had various toxic metals in different concentrations. Furthermore, the concentration of heavy metals in tobacco products (with and without filters) was also analyzed to evaluate the effect of filters. The results of the study showed that the number of heavy metals present, and their concentrations vary in different brands of tobacco products. Moreover, the concentrations for both Pb and Cd, the potent human carcinogens, were greater than the recommended threshold set forth by WHO and FAO.
Keywords
Heavy metals
Tobacco
Cigarette brands
Waterpipe smoking
Risk assessment
1 Introduction
Various metals are significant for their functional roles in a host of physiological and biochemical processes in the human body. Their presence in high concentrations, however, could be a cause of considerable concern. In particular, heavy metals are regarded as toxic for both human health, as well as the environment even at low concentrations (Engida and Chandravanshi, 2017). Tobacco (Nicotiana tabacum L.) smoking usually refers to the practice of inhaling and exhaling the smoke that is liberated as a result of the combustion of the plant material. A diverse range of tobacco products is available in the market worldwide. More popular choices include cigarettes, cigarillos, cigars, pipes, bidis (tobacco rolled in dry leaf and smoked without a filter), kreteks, and waterpipes (or hookah). Smokeless tobacco products have also been traditionally consumed in various regions of the world in the form of either chewing tobacco preparations, by placing a wad in the mouth between gums and cheeks, or in the form of snuff, whereby tobacco is sniffed directly through the nose (West, 2017). However, both types of tobacco products have serious health concerns and have been linked to an increased incidence of morbidity and mortality over the years. A recent survey-based study has suggested that the new trend of waterpipe smoking is on the rise among the youth throughout the world, and is being regarded as a fun, social activity, based on the misplaced belief that it is comparatively less harmful than cigarette smoking. Reported in low, middle, as well as high-income countries alike, waterpipe smoking is becoming a global phenomenon (Jafari et al., 2020). Tobacco-associated diseases killed more than 7 million people throughout the world in 2016 (Drope and Schluger, 2018). Another research reported that the death rate due to excessive smoking of tobacco will be reduced by 9% by the end of 2030 in high-income countries. However, the trend has been projected to be opposite in the middle and low-income countries as the death rate will be doubled by the end of 2030, with the estimated number of deaths estimated between 3.4 and 8.3 million (Mathers and Loncar, 2006).
Tobacco smoking can decrease the overall life expectancy of smokers by 10 years when compared to non-smokers. This can be attributed to tobacco smoke containing various harmful constituents produced due to pyrosynthesis. According to some studies, more than 5000 chemical compounds including carbon dioxide (CO2), carbon monoxide (CO), nitrogen oxides (NOx), various hydrocarbons, heavy metals, and even radioactive compounds, such as polonium-210 and lead-210 are present in this highly complex, reactive, and dynamic mixture (Talaiekhozani and Amani, 2018; Talhout et al., 2011). The repeated inhalation of tobacco smoke results in the accumulation of significant amounts of toxic metals, specifically chromium (Cr), lead (Pb), cadmium (Cd), and nickel (Ni), which have low permissible daily exposure (PDE) limits (5 µg, 5 µg, 2 µg, and 3 µg, for Pb, Ni, Cd, and Cr, respectively) (Ting et al., 2020). The excessive accumulation of toxic heavy metals in the human body can significantly increase the risk of various chronic illnesses such as cancer, and other non-cancer diseases such as chronic obstructive pulmonary disease (COPD) (including emphysema and chronic bronchitis), and cardiovascular disorders. There is a need to enforce strict regulations and educate the people about a reduction in smoking keeping in view the harmful effects of toxic heavy metals (Khlifi and Hamza-Chaffai, 2010; Zazouli et al., 2020).
Many studies have also reported that prolonged exposure to tobacco smoking, and in turn, to heavy metals, can negatively affect the health of pregnant women as it interferes with the development of the fetus, manifesting in elevated risk of many fetal disorders such as intrauterine growth restriction (IUGR), ectopic pregnancy, low birth weight (LBW), preterm birth, as well as spontaneous abortions. Some of the major components of tobacco smoke implicated in this regard include CO, nicotine, and heavy metals including lead, arsenic, and cadmium. The pregnancy is particularly at high risk in women with repeated inhalation exposure to tobacco smoke, as these factors stimulate the changes in the levels of microelements, disruptions associated with oxidative stress by way of oxidative imbalances, and disturbances affecting the concentration and structure of various proteins, particularly elevated concentrations of α1-, α2-, and β-globulins, and decrease in the levels of albumin, and γ-globulins (Wrześniak et al., 2016).
Chronic obstructive pulmonary disease (COPD) is a major health concern associated with cigarette smoking, and as a consequence, with long-term exposure to tobacco smoke (and other particulate matter), although, there is significant evidence that passive smoking may also contribute to the development of respiratory symptoms and progression of the disease resulting from the abnormalities in lung functioning as in the active form of tobacco smoking. A direct consequence of COPD is progressive, and generally irreversible obstruction of the airflow from the lungs, owing to an inflammatory response triggered by noxious particles such as those present in cigarette smoke. There is also significant evidence indicating that a higher prevalence of respiratory problems and lung function anomalies was observed in cigarette smokers, and that cessation of smoking was shown to significantly reduce the decline in FEV1/FVC ratio (or the Tiffeneau-Pinelli index), a ratio used for categorization of the severity of obstructive lung disorders. Patients with preexisting COPD also face an increased risk of morbidity and mortality with markedly reduced life expectancy (Papadopoulos et al., 2011).
The tobacco plant is highly capable of accumulating toxic elements (TEs) from its growing environment, with the greatest concentrations detected in the leaves, and the smoke particulate. Other contributing factors to the uptake of TEs include the pH of the soil, and the sludge loaded with TEs, as well as the fertilizers applied to plants at the time of growth. For instance, in the few countries where arsenic-containing pesticides are still permissible, arsenic accumulation has been detected in the tobacco plant (Kazi et al., 2009b). Among the heavy metals that mostly get accumulated through chronic exposure to tobacco smoking i.e. nickel (Ni), cadmium (Cd), aluminum (Al), mercury (Hg), lead (Pb), arsenic (As), silver (Ag), barium (Ba), titanium (Ti), Cd and Pb have been reported as the most toxic, and are known human carcinogens. Moreover, the toxicity associated with these heavy metals has been linked to serious health problems such as damage to the central nervous system (CNS), reduced brain function, increased concentration in the bloodstream, and damage to vital organs such as the liver, lungs, and kidneys, as well as progressive degenerative disorders that mimic Alzheimer’s disease, multiple sclerosis (MS), Parkinson’s disease, and muscular dystrophy. Furthermore, Cd and Pb have also been associated with hypertension, and consequent cardiovascular problems (Alrobaian and Arida, 2019).
India ranks as a major market for tobacco products, and both chewing and smoking forms of tobacco are liberally used across the country, for various purposes, including pleasure, habit, ritualistic significance, addiction, and self-medication. Smokeless tobacco products are most popular (40%), alongside bidi (40%), followed by cigarettes (20%). The heavy metals that have been reported in most of the tobacco plants (as a result of uptake from the soil) grown in India include Cd, Cr, Pb, Ni, Zn, Fe, and Cu. Consequently, the presence of these metals was observed, in varying concentrations, in three different types of tobacco products i.e., cigars, cigarettes, and bidi. The results of the study indicated that bidi contained a lower concentration of these toxic heavy metals, as compared to cigars and cigarettes. Further analysis of the results suggested that the concentration of these heavy metals gets enriched during the chemical treatment and processing of cigars and cigarettes. The higher content of heavy metals in both smoking and smokeless tobacco products, therefore, could have long-term health implications, not only for active smokers but also for passive smokers (Verma et al., 2010).
The heavy metals easily get inhaled by both active and passive smokers and absorbed in the body through the lungs, ultimately reaching the bloodstream, and blood then transports them to other parts of the body. It has been reported that Pb gets easily absorbed in the lungs of the children as a result of passive smoking (Hagstad et al., 2014). Each year, between 25,000–30,000 tons of Cd is released into the environment as a result of many activities, both natural and man-made. However, the main sources through which Cd makes its way into the human body are agricultural produce (food), and smoke produced by the combustion of tobacco. It was observed in a study that one cigarette generally contains approximately 1–2 µg of Cd, and half of this amount enters the lungs by way of smoking and becomes part of the systemic circulation (Dip et al., 2017). In the past, the exposure of heavy metals through cigarette smoking has been studied by many researchers, as it was reported that smoking of tobacco products is the main source of Cd exposure, and the significant increase in Pb levels in the blood owing to its tendency of binding with the erythrocytes in the circulatory system (Järup, 2003). Moreover, it has been suggested that although the accumulation of Cd occurs in many tissues and organs of the human body, the highest degree of accumulation was observed in the kidney cortex, and it has been estimated that Cd-associated nephropathy is responsible for an increase in mortality risk by 40 to 100% (Satarug and Moore, 2004). Another research study found that the concentrations of both Pb and Cd were significantly higher in the blood, urine, hair, and nails samples in the case of smokers, as compared to non-smokers (Mortada et al., 2004).
In the Kingdom of Saudi Arabia (KSA), a high-income country, the prevalence of smoking habits has been reported to be notably higher than most of the regional countries. This increase in the practice of cigarette smoking witnessed a statistically significant spike between 1980 and 2012, resulting in the KSA importing tobacco products worth an estimated USD 3.4 billion from 2010 to 2014 alone. Furthermore, between the years 2004 and 2014, tobacco consumption cost KSA’s economy over USD 20.5 billion, also manifesting in a death toll of 280,000 premature deaths (from 2001 to 2010). In particular, the popularity and usage of cigarettes and their allied products were more predominant in the case of males, especially male college students (Alotaibi et al., 2019). Many studies have been conducted in the KSA to date in order to determine the concentration of toxic heavy metals in the blood of both active and passive smokers by using inductively coupled plasma (ICP), and optical emission spectrometry (OES) techniques. The reported data from these studies showed that the concentration of toxic elements was significantly higher in the blood of smokers than non-smokers (Al-Ramadi et al., 2016).
The current study was conducted to report the concentration of toxic elements or heavy metals present in tobacco products being sold in the markets of the KSA and to assess the burden of tobacco smoking on the public health system. Samples from 36 different brands of cigarettes, cigars, and waterpipes (Shisha, Narghile, and Hookah) were obtained from the country’s commercial enterprises. The heavy metal content in the filler of tobacco (dried tobacco leaves), the filter pad (after smoking), and the ash content of the cigarettes was analyzed, and their levels were compared with the recommended permissible limits of daily intake suggested by the World Health Organization (WHO), and Food and Agriculture Organization (FAO).
2 Materials and methods
2.1 Instrument
An Inductive Coupled Plasma-Optical Emission Spectrometer (ICP-OES), Thermo-Fisher iCAP 6000, Thermo Fisher Scientific, Waltham, MA, USA), was used to determine the concentration of metals with high sensitivity. The conditions followed in determining the heavy metals concentration by ICP-OES were as: nebulizer (V groove) for gas flow 0.53/min, with RF power 1150 W and stabilization time was 0 s, and meanwhile, sample injection pump flow rate was 50 rpm. Draeger Gas Detection Pump was used for the smoking of both cigar and cigarette to detect heavy metals.
2.2 Standard and reagents
All the chemicals and reagents used for the heavy metal analysis were of analytical grade. Nitric acid (70%, Sigma-Aldrich), hydrochloric acid (35–38%, Sigma-Aldrich), and hydrogen peroxide (30%, Sigma-Aldrich) were used to digest the organic matter in tobacco samples of both cigarette and shisha. The mono-element containing stock solutions of Cd, Cr, Cu, Fe, Zn, Pb, Mn with a concentration of (1000 μg mL−1) (Specpure®) were used for the preparation of reference standard solution required for the calibration curve and optimization of analytical conditions. The reference standards include 0, 1, 5 and 10 mg L-1. The standard curve of each element is presented in Fig. 1.
Standard curves of each heavy metal detected in the present study.
2.3 Collection of samples
A total of 32 samples including cigarette, cigar, and shisha (water pipe) were collected from different retail markets of Saudi Arabia, from the month of July to September 2020. Three packs of each cigarette brand were bought from three different places of Dammam, Khobar, and Saudi Arabia to ensure the random sampling. The collected samples were not more than 6 months old from the date of production. These cigarette samples were further classified as light (or low tar), or regular (full flavor) cigarettes depending upon nicotine concentration. 10 samples of cigarettes were classified as ‘light’, 11 as ‘regular’, while 3 samples were classified between light and regular. The brands of tobacco products including shisha, cigars, and cigarettes are presented in this study with alphabetic letters.
2.4 Preparation of the samples and analytical procedures
To prepare the samples, glass containers were soaked in a solution of 5% nitric acid (HNO3) for 24 h, and later these soaked containers were washed with deionized water to prevent any external heavy metal contamination. To prepare the homogenized sample for drying purposes, about 1.5 g of cigarette tobacco sample was taken from 3 cigarette sticks from each brand using three different cigarette packets. Tobacco samples (cigar, cigarette, and shisha) were placed and spread in covered clean containers until they were fully dried at 80 ± 5 °C for 6 h in a closed air-dry oven to prevent cross-contamination from the airborne heavy metal contamination (AOAC, 1997). The dried samples of tobacco ground in a mortar with the pestle to obtained fine particles and to facilitate the maximum organic matter digestion. Then the sample was weighed and the mean weight of each cigarette was obtained by weighing its contents in triplicates.
The samples were then placed in a microwave digestion system (One Touch-Mars 6, CEM, USA) for 60 mins using HNO3 and H2O2. The digestion was performed to reduce interference by organic matter and to ensure the elaboration of all bound elements from the tobacco leaves into a free form (by following the ISO 4387 standard protocol) that could later be determined by ICP-OES (ISO, 2000). The tobacco contents were cooled and filtered through Whatman® no.1 acid-washed filter paper. The resulting solution was preserved in a refrigerator at 4 °C and later used for spectrophotometric determination of the various metal analysis.
2.5 Inductively coupled plasma - Optical emission spectrometry (ICP–OES)
The digested samples were then detected for target heavy metals in Inductively coupled plasma - optical emission spectrometry (ICP–OES). Briefly, a 10 mL solution of each sample was prepared, and the solution was then filtered through a Whatman® grade filter paper 40 into a centrifuge tube. The quantification of metals was achieved by interpolating the relevant calibration five-point curves prepared from aqueous solutions of metal standards in the same acid concentration, to minimize matrix effects.
2.6 Statistical analysis
For every sample, three replicates were taken, and the average value was calculated. The results were statistically analyzed using the ANOVA (one-way analysis of variance) at the level of 95% probability. The mean significant differences were obtained using the LSD test.
3 Results and discussion
3.1 Calibration and detection limit
The analytical wavelength, linear range, and instrumental detection limit for the determination of heavy metals (Cd, Cr, Cu, Fe, Mn, Pb and Zn) by ICP-OES including the quantification limits and permissible limits of heavy metals used for the reference as per WHO /FAO standard mentioned in Table 1.
Metal
Wavelength (nm)
Detection limit (µg/L)
Quantification limits (mg/kg)
WHO/FAO Tolerable weekly intake (mg/kg per week)
WHO/FAO Tolerable daily intake (mg/kg/day)
Reference
Cd
214.43
0.02
0.21
3.5
0.2–1
(Sebiawu et al., 2014)
Cr
205.55
0.08
0.67
3–5
0.2–1
(Azeez et al., 2018)
Cu
324.75
0.14
7.46
500
100
(Sebiawu et al., 2014)
Fe
259.94
0.54
316.94
–
0.05
(Anhwange et al., 2009)
Mn
257.61
0.05
7.93
–
25
(Anhwange et al., 2009)
Pb
220.35
0.43
0.98
25
5
(Sebiawu et al., 2014)
Zn
257.61
0.04
2.91
500
50
(Onojah et al., 2015)
3.2 Heavy metals concentration in cigarettes, cigars and sheesha product
The results of target elemental analysis by ICP–OES for tobacco products being retailed in the markets of KSA were presented in Tables 2–5. The average concentration of tobacco reported in a cigarette is 0.62 g with a moisture content of 15%, as the moisture content in tobacco is considered an important quality characteristic. A similar study reported in Pakistan for local and imported cigarette brands, the average value of 8.9% of moisture content in tobacco of imported brands with the range of 4.5–13.1% and 10.3% for local brands with the range of 4.9–17.2% (Ajab et al., 2014). The results of moisture content in the present study relate to the concentrations of moisture reported in the past studies. * Mean Value ± Standard Deviation; ** Values present within each column followed by different alphabetic letters are significantly different from one another (p < 0.05).
Brands
Cd
Cr
Cu
Fe
Mn
Pb
Zn
A
0.15 ± 0.00*B
0.55 ± 0.01D
4.87 ± 0.00B
44.22 ± 0.56D
4.04 ± 0.01D
1.18 ± 0.04A
2.52 ± 0.02B
B
0.07 ± 0.00CD**
0.46 ± 0.00E
1.09 ± 0.00G
23.95 ± 0.00EF
7.89 ± 0.01A
0.11 ± 0.03G
1.51 ± 0.02C
C
0.04 ± 0.00EF
0.23 ± 0.00G
0.17 ± 0.00 J
3.138 ± 0.04H
1.44 ± 0.02I
0.29 ± 0.01EF
0.56 ± 0.02FG
D
0.07 ± 0.01CD
0.21 ± 0.04GH
1.41 ± 0.00D
20.75 ± 0.05FG
2.75 ± 0.01F
0.23 ± 0.04F
0.87 ± 0.03E
E
0.09 ± 0.01C
0.24 ± 0.00G
1.06 ± 0.00G
18.19 ± 0.33G
4.39 ± 0.02D
0.15 ± 0.01G
1.35 ± 0.06CD
F
0.09 ± 0.00C
0.18 ± 0.00HI
1.28 ± 0.01E
25.39 ± 0.37E
3.12 ± 0.06E
0.11 ± 0.00G
1.21 ± 0.02D
G
0.19 ± 0.00A
2.12 ± 0.00B
17.60 ± 0.04A
1075.50 ± 5.03B
6.53 ± 0.04B
0.37 ± 0.03D
2.58 ± 0.34B
H
0.06 ± 0.01DEF
2.22 ± 0.01A
1.19 ± 0.03F
1087.90 ± 2.39A
6.50 ± 0.56B
0.31 ± 0.02E
2.65 ± 0.19B
I
0.04 ± 0.00F
1.07 ± 0.00C
1.73 ± 0.00C
633.21 ± 1.45C
5.52 ± 0.01C
0.57 ± 0.01B
3.88 ± 0.02A
J
0.06 ± 0.00DE
0.32 ± 0.01F
0.23 ± 0.06I
3.08 ± 0.05H
1.88 ± 0.09GH
0.26 ± 0.01EF
0.39 ± 0.02G
K
0.19 ± 0.00A
0.22 ± 0.04G
0.46 ± 0.00H
6.58 ± 0.06H
2.13 ± 0.00G
0.58 ± 0.06B
0.68 ± 0.05EF
L
0.05 ± 0.04DEF
0.15 ± 0.00I
0.24 ± 0.00I
4.69 ± 0.07H
1.64 ± 0.00HI
0.44 ± 0.01C
1.48 ± 0.05C
Average
0.09 ± 0.01
0.66 ± 0.01
2.61 ± 0.01
245.55 ± 0.87
3.98 ± 0.07
0.38 ± 0.02
1.64 ± 0.07
Brands
Cd
Cr
Cu
Fe
Mn
Pb
Zn
A
0.29 ± 0.01MN
1.38 ± 0.03JK
12.59 ± 0.14I
243.67 ± 0.18H
83.11 ± 0.05H
17.59 ± 0.44D
21.91 ± 0.12D
B
0.37 ± 0.00LMN
1.69 ± 0.04EFG
17.21 ± 0.30E
312.98 ± 0.08D
101.56 ± 0.38E
4725.00 ± 1.15A
26.64 ± 0.07B
C
0.50 ± 0.01KLMN
1.75 ± 0.03EF
11.17 ± 0.02 J
267.19 ± 0.02F
72.74 ± 0.40 K
1.34 ± 0.08GHI
16.39 ± 0.52H
D
0.65 ± 0.02KL
1.35 ± 0.04 K
14.19 ± 0.04G
243.21 ± 0.11H
73.76 ± 0.19 J
1.14 ± 0.07HIJK
19.42 ± 0.36F
E
1.11 ± 0.06 J
2.31 ± 0.05C
9.80 ± 0.02L
162.40 ± 0.21 N
98.52 ± 0.41F
1.56 ± 0.07FGH
18.35 ± 0.16G
F
10.87 ± 0.02G
1.58 ± 0.06FGHI
9.03 ± 0.23 M
195.50 ± 0.27 J
62.80 ± 0.31L
29.71 ± 0.20C
14.59 ± 0.37I
G
0.54 ± 0.04KLM
1.40 ± 0.11IJK
13.55 ± 0.11H
193.70 ± 0.19 K
92.34 ± 0.48G
0.59 ± 0.07JKL
0.19 ± 0.04 N
H
4.38 ± 0.05I
2.87 ± 0.11B
19.20 ± 0.08D
327.63 ± 0.47C
115.28 ± 0.21B
2.04 ± 0.13EFG
89.06 ± 0.12A
I
0.24 ± 0.04MN
1.59 ± 0.05EFGH
7.29 ± 0.32 N
205.60 ± 0.02I
56.13 ± 0.13 M
1.08 ± 0.19HIJK
13.55 ± 0.07 J
J
13.36 ± 0.42F
2.73 ± 0.30B
12.44 ± 0.06I
291.61 ± 0.26E
75.47 ± 0.02I
2.71 ± 0.43E
21.87 ± 0.12DE
K
0.19 ± 0.02 N
1.55 ± 0.05GHIJ
40.70 ± 0.26A
244.72 ± 0.57G
18.69 ± 0.19R
1.02 ± 0.282HIJK
11.91 ± 0.18 K
L
9.19 ± 0.05H
2.04 ± 0.14D
7.01 ± 0.02 N
165.09 ± 0.01 M
104.90 ± 0.00D
1.29 ± 0.05HIJ
13.75 ± 0.07 J
M
147.18 ± 0.09B
1.77 ± 0.07E
16.63 ± 0.25F
818.49 ± 0.35A
113.52 ± 0.32C
2.14 ± 0.15EF
23.57 ± 0.43C
N
4.20 ± 0.023I
1.62 ± 0.05EFG
7.23 ± 0.00 N
128.77 ± 0.33O
51.35 ± 0.04 N
0.11 ± 0.05L
7.27 ± 0.07 M
O
0.28 ± 0.04MN
1.41 ± 0.02IJK
23.29 ± 0.13C
191.21 ± 0.31L
134.81 ± 0.18A
1.18 ± 0.05HIJK
21.58 ± 0.11DE
P
138.70 ± 0.36C
1.11 ± 0.06L
33.53 ± 0.15B
119.44 ± 0.32P
45.59 ± 0.38O
0.67 ± 0.08IJKL
12.27 ± 0.06 K
Q
0.710 ± 0.03 K
4.27 ± 0.02A
10.37 ± 0.10 K
483.07 ± 0.09B
72.76 ± 0.27 K
0.77 ± 0.08IJKL
14.57 ± 0.05I
R
30.53 ± 0.07E
1.41 ± 0.02HIJK
3.64 ± 0.17Q
93.59 ± 0.06Q
62.35 ± 0.11L
1464.90 ± 0.74B
26.19 ± 0.10B
S
78.36 ± 0.07D
0.92 ± 0.05L
4.02 ± 0.06P
66.33 ± 0.09R
34.15 ± 0.10P
0.48 ± 0.06KL
21.41 ± 0.38E
T
306.06 ± 0.38A
1.43 ± 0.02HIJK
5.55 ± 0.30O
39.77 ± 0.15S
30.29 ± 0.09Q
0.18 ± 0.03L
10.10 ± 0.22L
Average
37.39 ± 0.09
1.81 ± 0.07
13.92 ± 0.14
239.69 ± 0.20
75.01 ± 0.22
312.77 ± 0.22
20.23 ± 0.18
Brands
Cd
Cr
Cu
Fe
Mn
Pb
Zn
A
4.09 ± 0.03E
1.49 ± 0.04CD
0.62 ± 0.06DE
4.61 ± 0.04DEF
0.17 ± 0.07AB
2.23 ± 0.18C
0.52 ± 0.08BCD
B
0.17 ± 0.04I
0.91 ± 0.02F
0.34 ± 0.05F
3.13 ± 0.22H
0.19 ± 0.03AB
1.07 ± 0.02E
0.49 ± 0.07CDE
C
0.63 ± 0.05H
1.70 ± 0.01B
0.35 ± 0.05F
9.05 ± 0.09A
0.05 ± 0.02B
0.44 ± 0.11G
0.32 ± 0.03FG
D
0.44 ± 0.02HI
1.22 ± 0.01E
0.31 ± 0.02F
2.19 ± 0.24I
0.02 ± 0.01B
0.001 ± 0I
0.18 ± 0.02H
E
14.08 ± 0.35B
1.31 ± 0.24DE
1.14 ± 0.07B
7.07 ± 0.09B
0.37 ± 0.06AB
0.29 ± 0.03GH
0.38 ± 0.05EF
F
7.20 ± 0.06C
0.91 ± 0.11F
0.72 ± 0.06CD
5.43 ± 0.58C
0.24 ± 0.07AB
0.04 ± 0.01I
0.41 ± 0.06DEF
G
1.76 ± 0.36G
1.94 ± 0.07A
0.34 ± 0.05F
4.47 ± 0.11EF
0.59 ± 0.71A
0.23 ± 0.04H
0.25 ± 0.06GH
H
2040.10 ± 0.08A
1.57 ± 0.07BC
13.85 ± 0.11A
4.12 ± 0.78FG
0.13 ± 0.04B
0
0.52 ± 0.01BCD
I
2.49 ± 0.03F
1.29 ± 0.08E
0.62 ± 0.04DE
3.56 ± 0.12GH
0.19 ± 0.03AB
3.00 ± 0.12B
0.63 ± 0.07B
J
0.78 ± 0.05H
0.94 ± 0.04F
0.30 ± 0.02F
5.16 ± 0.24CDE
0.14 ± 0.03AB
0.66 ± 0.08F
0.63 ± 0.02B
K
6.32 ± 0.15D
0.50 ± 0.09G
0.82 ± 0.03C
5.26 ± 0.08CD
0.09 ± 0.01B
38.89 ± 0.14A
0.94 ± 0.07A
L
0.59 ± 0.17H
0.51 ± 0.06G
0.52 ± 0.04E
6.53 ± 0.31B
0.20 ± 0.01AB
1.28 ± 0.06D
0.54 ± 0.07BC
Average
173.22 ± 0.12
1.19 ± 0.07
1.66 ± 0.05
5.04 ± 0.24
0.20 ± 0.09
4.01 ± 0.07
0.48 ± 0.05
Brands
Cd
Cr
Cu
Fe
Mn
Pb
Zn
A
1.07 ± 0.03D
2.84 ± 0.20A
0.60 ± 0.06FG
4.48 ± 0.09H
0.64 ± 0.04DE
0.08 ± 0.01E
0.71 ± 0.02E
B
0.21 ± 0.03E
2.59 ± 0.01AB
0.24 ± 0.05IJ
3.67 ± 0.10I
0.36 ± 0.04GH
0.06 ± 0.01E
0.40 ± 0.01G
C
5.49 ± 0.57B
0.89 ± 0.15DE
0.11 ± 0.02 J
0.94 ± 0.06 K
0.17 ± 0.06I
0.01 ± 0.01E
0.23 ± 0.03H
D
0.56 ± 0.33E
1.18 ± 0.05CDE
1.25 ± 0.19C
5.65 ± 0.07G
0.50 ± 0.04EFG
0.25 ± 0.03D
0.68 ± 0.02E
E
0.23 ± 0.03E
0.97 ± 0.06DE
0.73 ± 0.08EF
3.29 ± 0.08 J
0.37 ± 0.04FGH
0.01 ± 0.01E
0.45 ± 0.04G
F
0.27 ± 0.05E
1.36 ± 0.07CD
0.40 ± 0.00HI
8.79 ± 0.15F
0.52 ± 0.02EF
1.91 ± 0.14B
0.46 ± 0.04G
G
0.30 ± 0.03E
0.73 ± 0.07E
0.39 ± 0.08HI
8.66 ± 0.08F
1.54 ± 0.07A
0.50 ± 0.01C
1.09 ± 0.01C
H
1.82 ± 0.15C
2.35 ± 0.24B
0.49 ± 0.03GH
10.25 ± 0.08E
0.33 ± 0.05H
0.55 ± 0.06C
0.59 ± 0.04F
I
130.03 ± 0.03A
1.49 ± 0.09C
1.71 ± 0.09B
122.10 ± 0.01B
0.86 ± 0.17C
0.59 ± 0.02C
0.69 ± 0.01E
J
0.59 ± 0.01E
2.49 ± 0.08AB
0.79 ± 0.13E
23.43 ± 0.06D
1.14 ± 0.07B
0.59 ± 0.01C
1.20 ± 0.02B
K
0.28 ± 0.04E
2.42 ± 0.60AB
8.67 ± 0.09A
52.97 ± 0.11C
0.70 ± 0.03D
0.52 ± 0.03C
1.83 ± 0.06A
L
0.51 ± 0.04E
2.63 ± 0.25AB
1.07 ± 0.04D
150.13 ± 0.05A
0.39 ± 0.06FGH
2.74 ± 0.14A
0.85 ± 0.09D
Average
11.78 ± 0.11
1.83 ± 0.15
1.37 ± 0.07
32.86 ± 0.08
0.63 ± 0.06
0.65 ± 0.04
0.77 ± 0.03
The average concentration of the target elements in cigarettes, cigars, and shisha (waterpipe), recorded in the present study was in the range of 0.09–3.98 mg/kg, with the maximum concentration reported for Fe (245.55 mg/kg) in shisha products. While a study from Turkey reported the Cd content in the range between 0.44 and 1.55 mg/kg, Cu contents between 10.36 and 30.47 mg/kg, Fe contents between 306.03 and 595.42 mg/kg, Pb contents between 0.16 and 7.37 mg/kg in Indian and some imported cigarette brands being used in Turkey (Özcan et al., 2019). The reported values in this study for Cd, Cu, and Fe on an average basis (sample A-L) are slightly lower (Table 2) than the concentrations reported in the study from Turkey, but Pb contents were comparable which indicated that there may be variations in the concentrations owing to the soil conditions, agronomic practices, area, and processing technology. The mean concentrations of the target elements per brand in tobacco filler are presented in Table 2, which showed that there was no significant difference in the concentrations of these heavy metals from one another.
The long-term Pb exposure in the human body has been linked to several nervous and peripheral system disorders, kidney malfunction, cardiovascular problems, and skeletal, and muscle system abnormalities (Mishra, 2017). The obtained mean value for Pb in tobacco filler of cigarettes in the present study was lower than the maximum tolerable certified value of Pb recommended by WHO/FAO of 5 mg/kg for daily use, and 25 mg/kg for the weekly intake through tobacco leaves (Onojah et al., 2015). Another comparable study conducted in the Nasarawa state of Nigeria reported the Pb concentrations in the range of 5.43–10.55 mg/kg (Yahaya et al., 2019), which was higher than the recommended value by WHO/FAO, as well as significantly higher than the concentration of Pb reported in the present study (0.389 mg/kg). Another study reported the Pb concentration in 5 different brands of cigarettes in the range of 2.76 mg/kg–3.20 mg/kg which was higher than the maximum permissible limit recommended by WHO (0.05 mg/kg) (Kaličanin and Velimirović, 2012). The results of the present study also exhibited high concentrations of Pb. The continuous accumulation of the Pb in the human body results in lead poisoning which ultimately can manifest in hypertension, deafness and hearing loss, kidney malfunction, and various neurological disorders. A further study reported the mean concentration of Pb at 0.0304 mg/L (ElMohr et al., 2020), which was less than the concentration in the present study. However, it is evident the concentration of Pb reported in the present analysis (0.389 mg/kg) was more than that is suggested by WHO (0.05 mg/kg) (Nathaniel et al., 2018).
Cd is one of the non-essential elements of the human body and is regarded as a strong human carcinogen. The WHO reported that about 10–20% of Cd enters into the human body by way of inhalation. Going by these estimates, the amount of Cd inhaled as a result of tobacco smoking (one pack of 20 cigarettes) ranges between 1.6 and 3.2 µg. The findings of the current study reported the mean concentration of Cd in the range of 0.093 mg/kg, which is comparable with the reported results of a study from the Zaria region of Nigeria for the Cd concentrations in various cigarette brands (0.06–0.40 mg/kg (Kaličanin and Velimirović, 2012). Another study reported the concentration of Cd in the range of 1.3–7.6 µg/g (Engida and Chandravanshi, 2017), while the reported concentrations of Cd in another investigation ranged between 0.53 and 0.59 mg/kg in four different brands of cigarettes, which in the former, is higher than the concentration of Cd reported in the present study. Furthermore, it can be seen that the Cd concentrations reported in all these studies are higher than the threshold values of Cd (0.05 mg/kg) recommended by WHO (Nathaniel et al., 2018).
Similarly, a study conducted in Pakistan reported the Cd concentrations in the range of 1.66–2.96 µg/g in the locally branded cigarettes, which is again, higher than the concentration reported in the present study, as well as the recommended threshold by WHO (0.05 mg/kg) (Kazi et al., 2009a). The literature showed a significant difference between the determined values in the current study and the concentrations reported in the literature on Cd. The possible factors that can be attributed to these differences are the variations in the tobacco species from one region to another, distribution of heavy metals in soils of different regions, adsorption ratio of Cd by plant, soil pH, and the dissolved organic matter in soils containing Cd traces. The long-term exposure of Cd in smokers has been shown to induce sperm function defects, lung diseases, bone resorption (in women), interference with bone metabolism, microproteinuria, nephrotoxicity, and renal tubular disorders with a progressive reduction in the glomerular filtration rate (GFR). The accumulation of Cd in the urinary tract and the consequent damage to kidneys has been reported to be more prevalent in women than men (Mortensen et al., 2011).
Many studies further showed that not only active but passive smoking also causes the accumulation of heavy metals, in particular, Cd, Pb, and, As, in the follicular fluid present around the oocytes which negatively affects the quality of the oocyte in females. A study on passive smoking reported that the concentration of Cd was 0.0202 mg/L in passive smokers which correlated with the concentration of Cd reported in the current study (0.093 mg/kg) (Mortensen et al., 2011). A study in this regard recorded the Cd accumulation in smokers at about 0.27 µg/L and in nonsmokers, at 0.17 µg/L. Similarly, for Pb, the reported concentration in the blood of smokers was 32.8 µg/L, while in nonsmokers, it was 17.6 µg/L. The study further suggested that the accumulation for both Cd and Pb was more predominant in the blood than other organs of the body. It was also suggested that the samples collected from the functional endometrium and endocervix (layer rich with blood vessels), as well as other reproductive parts of women, exhibited a significant amount of heavy metals accumulation, particularly, Cd and Pb. Also, in the case of women, frequent smoking caused a gradual increase in the concentration of both metals by way of accumulation through a hematogenous route, with consequent causation of endometrial cancer (Pinto et al., 2017).
Chromium (Cr) is also a known human carcinogen when it gets oxidized from the Cr (III) to Cr (VI), and its effects from the perspective of tobacco smoking have also been studied by many researchers. In nature, Cr exists in different oxidation states, however, the most prevalent forms are trivalent (Cr III), and hexavalent (Cr VI), which if accumulated in the body, tend to produce toxicity. The concentration usually recorded for chromium in the mainstream cigarette smoke is in the range of 0.0002–0.5 µg, and in the human body, Cr predominantly accumulates in tissues such as in the lungs. The Cr concentrations reported in smokers are significantly higher (4.3 mg/kg dry weight) than those in nonsmokers (1.3 mg/kg). The non-carcinogenic effects of Cr include ulceration of the respiratory tract, chronic pharyngitis, impaired lung function, and emphysema (Bernhard et al., 2005). Additionally, a study reported that the prolonged accumulation of heavy metals, such as, As, Cd, Ni, and Cr is a causative factor for cancer of the head and neck. In the case of the samples from patients with tumors of the neck and head for determination of the concentration of toxic metals (using the advanced atomic absorption spectrometry technique), the observed median levels of Cr, As, Ni, and Cd in tumor cells were 2.85, 7.42, 3.41, and 0.31 µg, respectively, which were significantly higher than the normal healthy tissues (1.89, 3.41, 2.19, and 0.13 µg for Cr, As, Ni, and Cd respectively) (Khlifi et al., 2013). The current study reported the Cr concentration of 0.663 mg/kg which was in the range of maximum permissible level (0.01–1.2 mg/kg or 0.5 mg/kg) suggested by WHO. A study reported the levels of Cr in five brands of cigarettes (12.30, 17.86, 13.44, 14.58, and 16.10 mg/kg), and that was significantly higher than the values reported in the current study, as well as over the WHO permissible limit. The toxicity of Cr has been linked to lung cancer, as well as genotoxicity and mutations in the DNA (Nathaniel et al., 2018).
Copper (Cu) enters the soil, in the case of agricultural practices, by way of phosphate fertilizer application (Sebiawu et al., 2014). The mean concentration of Cu reported in the present study was 2.61 mg/kg. Some of the previous studies have reported significantly higher concentrations (than the present study). For instance, in the commonly smoked cigarette brands and local snuff retailed in the Nigerian market, the concentrations ranged between 6.02 and 15.85 mg/kg (Vincent et al., 2011), 14.53–21.8 mg/kg in a study in Ghana (Sebiawu et al., 2014), 20 to 50 µg/g in a study on the Indian cigarette tobacco (Shaikh et al., 2002), 10.2 to 21.8 µg/g in another study in Ghana (Engida and Chandravanshi, 2017), and 2.80 to 25 µg/g in a study on tobacco products in Ethiopia (Engida and Chandravanshi, 2017). This significant difference between the reported concentrations of Cu indicates that the concentration of metals can vary across different regions and countries, and to a considerable extent, depends upon the soil condition. However, the levels of Cu in all the studies are below the WHO/FAO recommended daily and weekly permissible limits of 100 mg/kg, and 500 mg/kg respectively (Sebiawu et al., 2014). Cu, although, is involved in many biochemical processes of the plants, long-term exposure can result in the causation of Menkes disease, Wilson’s disease, and Indian childhood cirrhosis (Iwuoha et al., 2013).
Zinc is another essential element in terms of its contribution to the growth, development, and proper functioning of the human body. However, elevated concentrations have been linked to copper deficiency in the liver, serum, and heart, interference with the functioning of copper metalloenzymes, as well as the storage of iron, and the consequent causation of anemia (Kaličanin and Velimirović, 2012). The mean concentration reported for Zn in the current study was 1.641 mg/kg, which is significantly lower than the permissible levels for Zn in cigarettes (100 mg/kg) (Poorolajal et al., 2020). However, two separate studies have reported far higher Zn concentrations in the range of 8.5–23.18 mg/kg (Kaličanin and Velimirović, 2012), and 27.75–39.50 mg/kg (Engida and Chandravanshi, 2017). The possible reasons for such variations could be the condition of the soil, the area under tobacco cultivation, the presence of heavy metals in soils, as well as a host of biotic, and abiotic factors (Rai et al., 2019). The concentration of Mn determined in this study was 75.006 mg/kg which is lower than the values reported in a comparable study (87.42–106.22 mg/kg) from Port Harcourt, Rivers State, Nigeria) (Poorolajal et al., 2020). However, both of these concentrations exceed the permissible limit for Mn (6.61 mg/kg) recommended by WHO/FAO. The reported levels for Fe in this study were 245.55 mg/kg and are comparable to the levels reported in an investigation conducted in Turkey involving various cigarette brands (306.03–595.42 mg/kg) (Onojah, Daluba and Odin, 2015).
3.3 Heavy metals concentration in the ash of tobacco products
The present study also reported the concentration of the various heavy metals detected in the ash of different tobacco products available in the KSA. The values reported were, 37.39, 1.81, 13.92, 239.69, 75.01, 312.78, and 20.23 mg/kg, for Cd, Cr, Cu, Fe, Mn, Pb, and Zn, respectively which, in the case of Fe, Mn, and Pb, are comparable to the reported values in a study involving the Marlboro cigarettes (filter) sourced from nine different countries, and the Russian Belomorkanal ‘papierosy’ (filter-less), where the ash still retained 71–86% of the initial elemental component (Lisboa et al., 2020; Zinicovscaia et al., 2018). A similar study conducted in China also reported the high metal concentration in ash with concentrations for various heavy metals ranging between 55.60 and 125.99 µg/g (Ren et al., 2017), which are fairly comparable to the levels of toxic metal contents reported in the ash of different tobacco products for the present study (1.81–312.78 mg/kg) as shown in Table 6.
Heavy Metals
Cd
Cr
Cu
Fe
Mn
Pb
Zn
Tobacco filler
0.09 ± 0.01
0.66 ± 0.01
2.61 ± 0.01
245.55 ± 0.87
3.98 ± 0.07
0.38 ± 0.02
1.64 ± 0.07
Ash
37.39 ± 0.09
1.81 ± 0.07
13.92 ± 0.14
239.69 ± 0.20
75.01 ± 0.22
312.78 ± 0.22
20.23 ± 0.18
With filter
173.22 ± 0.12
1.19 ± 0.07
1.66 ± 0.05
5.05 ± 0.24
0.20 ± 0.09
4.01 ± 0.07
0.48 ± 0.05
Without filter
11.78 ± 0.11
1.83 ± 0.15
1.37 ± 0.07
32.86 ± 0.08
0.63 ± 0.06
0.65 ± 0.04
0.77 ± 0.03
3.4 Heavy metals concentration in tobacco product with or without filters
The presence of cigarette filters has been shown to significantly reduce the toxic metals in cigarette smoke as evidenced by the reported values of a 38% reduction for As, 49% for Cd, and 57% for Cr. It has been reported that on average, every 1 mm increase in the length of the cigarette filter can significantly increase the absorption of heavy metal content, such as a 1.3% increase in absorption of As, 1.9% for Cd, 2% for Cr. Moreover, the study also provided a comparison of the extent of chemical components in cigarette smoke, when used with and without a filter. The concentrations for As, Cd, and Cr without a filter were recorded at 20.38 µg/cig, 11.41 µg/cig, and 11. 24 µg/g respectively, while with a filter, the concentrations were 12.73 µg/cig, 5.80 µg/g, and 4.83 µg/g (Poorolajal et al., 2020). The concentrations of toxic metals in tobacco products (with or without filters), detected during the current study are mentioned in Tables 4 and 5. The results from Tables 4 and 5 indicate that the average absorption of Cd (173.22 mg/kg) was reduced (11.78 mg/kg) after removing the filter. Similar results were observed for Pb, whereby the absorption reduced from 4.01 mg/kg to 0.65 mg/kg after the removal of the filter, and for Cu, the concentration reduced from 1.66 mg/kg to 1.37 mg/kg. However, the trend was opposite for other metals (Cr, Fe, Mn, and Zn), owing to the reason that filter lengths could vary from metal to metal. A similar study suggested that the absorption of heavy metals by the filters varies for different brands as well, and this plays a significant role in the release of toxic metals by way of cigarette smoke into the environment (Ziarati et al., 2017). These filters normally entrap heavy metals, as well as about 70% of the carcinogens, and the simple dumping of these filters contributes to the toxic metals getting accumulated in the soil, as well as increasing the future risk of uptake by different plant species (Qamar et al., 2020).
Smoking of tobacco products is considered as one of the main routes for exposure (both actively and passively) to toxic heavy metals in the human body, and the second major cause of health concerns such as lung cancer and COPD worldwide (Mishra, 2017). A study reported that close to 21.4% of the total population of Saudi Arabia were smokers, with males (aged 25 to 44 years) predominating (32.5%) over females (3.9%) (Algabbani et al., 2018). The present study of the 7 analyzed metals (Cd, Cr, Cu, Fe, Mn, Pb, and Zn) in 36 different brands of tobacco products including cigarettes, cigars, and waterpipe revealed that heavy metal concentrations vary significantly, reaching the maximum mean value of 245.55 mg/kg for Fe to a minimum value of 0.09 mg/kg for Cd. A comparison between the concentrations of heavy metals in different sources recorded during the present study is presented in Table 6 and Fig. 2. The comparison showed that among all the heavy metals the maximum concentration observed was for Cd, Pb, and Fe in tobacco products. The Fe content present in filler tobacco and ash of tobacco products is representative of the absorption capability of the tobacco plant in terms of taking up maximum Fe content. However, as this accumulated Fe can be utilized in different biochemical processes, the toxicity of Fe is rarely reported. Cd mostly present in tobacco ash and the filter of a cigarette is a result of uptake from the soil during the growth and further enrichment during the processing of tobacco products. The filters of tobacco products are considered a rich source of Cd which contributes to environmental pollution. The maximum Pb content has been found in the ash of the tobacco products, which indicates that Pb is enriched in tobacco products during the growth and processing of tobacco products. Other heavy metals (Cr, Cu, Mn, and Zn) though found in lower concentrations; their repeated intake can cause toxicity in the human body.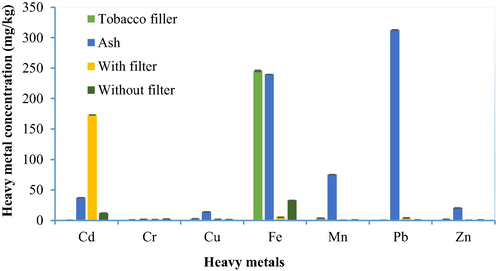
Heavy metals comparison in different brands of shisha, cigarettes, and cigars, before and after removing filters.
Other than this, there could be many reasons for the variations in the heavy metal content depending on factors such as contamination of tobacco cultivated land with toxic metals, physical and chemical treatment of the tobacco during the tobacco products processing, and the size of the filters used in tobacco products. It is recommended that the Saudi Food and Drug Authority (SFDA) promulgate legislation for regulating the maximum concentrations of toxicologically relevant heavy metals in all commercially available tobacco products. Tobacco smoke influences the protein profile of the serum in non-pregnant women; however, the additive effects of pregnancy and tobacco smoking on proteins have not been fully examined. The present study also suggested that the concentration of heavy metals absorbed by the cigarette filter of different brands in varying amounts contributes to the distribution of toxic metals in soil and produces environmental pollution. Tobacco plants absorb these heavy metals from the soils and subsequently transfer them to different plant parts, with major accumulation occurring in the leaves of the tobacco plant.
4 Conclusion
The concentrations of the heavy metals (Cd, Cr, Cu, Fe, Mn, Pb, and Zn) in the current study were approximated in 36 popular brands of cigarettes, cigars, and water pipes available in the markets of KSA. The results of the study showed that the number and quantities of heavy metals vary in different brands of tobacco products. The concentration of the most carcinogenic metals, Cd and Pb, was higher in all tobacco samples when compared to the threshold suggested by the WHO. The major risk associated with these metals is that they can easily accumulate in the body of the smoker's liver, lungs, brain, and blood, manifesting in chronic health effects, and thereby posing a serious threat to the life of the individuals smoking the tobacco products. The studies also reported that the levels of heavy metals, in particular, Pb and Cd, were low in samples from the higher-priced cigarettes. However, the concentrations were still greater than the threshold value recommended by the WHO. Therefore, no brand can be regarded as ‘safe’ for human health. The present study provided new data that can be utilized by many health and regulatory authorities in the KSA, as well as other countries. Moreover, this study can be extended to determine the concentration of heavy metals in different parts of the tobacco plant, and their mobility rates under different growth conditions. Furthermore, the ash content of tobacco leaves and burned cigarettes can be compared to determine the ratio of metal content added during the processing of the cigarettes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ajab, H., Yaqub, A., Malik, S.A., Junaid, M., Yasmeen, S., Abdullah, M.A., 2014. Characterization of toxic metals in tobacco, tobacco smoke, and cigarette ash from selected imported and local brands in Pakistan. Sci. World J., 2014.
- Evaluation of some toxic metals in blood samples of smokers in Saudi Arabia by inductive coupled plasma mass spectrometry. Trop. J. Pharm. Res.. 2016;15:2669-2673.
- [Google Scholar]
- The prevalence of cigarette smoking in Saudi Arabia in 2018. Food Drug Regul. Sci. J.. 2018;1:1.
- [Google Scholar]
- Smoking tobacco prevalence among college students in the Kingdom of Saudi Arabia: systematic review and meta-analysis. Tobacco Induced Dis.. 2019;17
- [Google Scholar]
- Alrobaian, M., Arida, H., 2019. Assessment of heavy and toxic metals in the blood and hair of saudi arabia smokers using modern analytical techniques. Int. J. Anal. Chem., 2019.
- Trace metal contents of some common vegetables grown on irrigated farms along the banks of river Benue within Makurdi metropolis. Electron. J. Environ. Agric. Food Chem.. 2009;8(12)
- [Google Scholar]
- Official Method of Analysis (14th ed.). Washington, D.C.: Association of Analytical Chemistry; 1997. p. :16.
- Assessment of Cr, Cd and Pb levels in tobacco leaves and selected cigarette samples from Ilorin Metropolis Kwara State, Nigeria. J. Appl. Sci. Environ. Manag.. 2018;22(12):1937-1939.
- [Google Scholar]
- Effects of age, gender, BMI, settlement and smoking on lead and cadmium accumulation in heart tissue. Medicine. 2017;6(3):531-536.
- [Google Scholar]
- The tobacco atlas. American Cancer Society; 2018.
- Effect of passive smoking on heavy metals concentration in blood and follicular fluid of patients on going ICSI. Ind. J. Sci. Technol.. 2020;13:2035-2040.
- [Google Scholar]
- Assessment of heavy metals in tobacco of cigarettes commonly sold in Ethiopia. Chem. Int.. 2017;3(3):212-218.
- [Google Scholar]
- Passive smoking exposure is associated with increased risk of COPD in never smokers. Chest. 2014;145(6):1298-1304.
- [Google Scholar]
- Levels of selected heavy metals in some brands of Cigarettes marketed in. Rivers State: University of Port Harcourt; 2013.
- Determination of metals and BTEX in different components of waterpipe: charcoal, tobacco, smoke and water. J. Environ. Health Sci. Eng. 2020:1-9.
- [Google Scholar]
- Potentiometric stripping analysis of zinc, cadmium and lead in tobacco leaves (Nicotiana Tabacum L.) and soil samples. Int. J. Electrochem. Sci.. 2012;7:313-323.
- [Google Scholar]
- Toxic metals distribution in different components of Pakistani and imported cigarettes by electrothermal atomic absorption spectrometer. J. Hazard. Mater.. 2009;163(1):302-307.
- [Google Scholar]
- Determination of toxic elements in different brands of cigarette by atomic absorption spectrometry using ultrasonic assisted acid digestion. Environ. Monit. Assess.. 2009;154:155.
- [Google Scholar]
- Head and neck cancer due to heavy metal exposure via tobacco smoking and professional exposure: a review. Toxicol. Appl. Pharmacol.. 2010;248(2):71-88.
- [Google Scholar]
- Arsenic, cadmium, chromium and nickel in cancerous and healthy tissues from patients with head and neck cancer. Sci. Total Environ.. 2013;452:58-67.
- [Google Scholar]
- ISO, I., 2000. Cigarettes-determination of total and nicotine-free dry particulate matter using a routine analytical smoking machine ISO 4387 [Online]. Geneva, Switzerland. In.
- Chromium levels in tobacco, filter and ash of illicit brands cigarettes marketed in Brazil. J. Anal. Toxicol.. 2020;44(5):515-520.
- [Google Scholar]
- Mathers, C.D., Loncar, D., 2006. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine 3e, 442.
- Are e-cigarettes beneficial for public health: Hume's guillotine–The debate continues?. Elsevier; 2017.
- Mortada, W.I., Sobh, M.A., El-Defrawy, M.M., 2004. The exposure to cadmium, lead and mercury from smoking and its impact on renal integrity. Medical Sci. Monitor 10(30), CR112–CR116.
- Smoking status and urine cadmium above levels associated with subclinical renal effects in US adults without chronic kidney disease. Int. J. Hyg. Environ. Health. 2011;214:305-310.
- [Google Scholar]
- Analysis of heavy metals content of tobacco cigarette brand sold in Samaru Area of Zaria, Nigeria. Industr. Eng.. 2018;2(2):52-55.
- [Google Scholar]
- Investigation of heavy metals in selected samples of cigarette randomly purchased from local markets in Anyigba and its environment and tobacco leaves grown in Kogi State, Nigeria. Int. J. Innov. Res. Technol. Sci.. 2015;3:1-7.
- [Google Scholar]
- Distribution of heavy metal and macroelements of Indian and imported cigarette brands in Turkey. Environ. Sci. Pollut. Res.. 2019;26(27):28210-28215.
- [Google Scholar]
- Smoking cessation can improve quality of life among COPD patients: validation of the clinical COPD questionnaire into Greek. BMC Pulmonary Med.. 2011;11:13.
- [Google Scholar]
- Metals transfer from tobacco to cigarette smoke: evidences in smokers’ lung tissue. J. Hazard. Mater.. 2017;325:31-35.
- [Google Scholar]
- Impact of cigarettes’ filter length and diameter on cigarette smoke emissions. Clin. Epidemiol. Global Health. 2020;8(2):377-382.
- [Google Scholar]
- Cigarette waste: assessment of hazard to the environment and health in Riyadh city. Saudi J. Biol. Sci.. 2020;27(5):1380-1383.
- [Google Scholar]
- Heavy metals in food crops: health risks, fate, mechanisms, and management. Environ. Int.. 2019;125:365-385.
- [Google Scholar]
- Determination of heavy metals in cigarettes using high-resolution continuum source graphite furnace atomic absorption spectrometry. Anal. Methods. 2017;9:4033-4043.
- [Google Scholar]
- Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ. Health Perspect.. 2004;112:1099-1103.
- [Google Scholar]
- Analysis of heavy metals content of tobacco and cigarettes sold in Wa Municipality of Upper West Region, Ghana. Chem. Process Eng. Res.. 2014;25:24-33.
- [Google Scholar]
- Characterization of Indian cigarette tobacco and its smoke aerosol by nuclear and allied techniques. J. Radioanal. Nucl. Chem.. 2002;253:231-234.
- [Google Scholar]
- Preparing emission factors of carbon dioxide, carbon monoxide, hydro-carbons and nitrogen oxides for cigarette. J. Air Pollut. Health 2018
- [Google Scholar]
- Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health. 2011;8:613-628.
- [Google Scholar]
- Heavy metals (Cr, Pb, Cd, Ni) in aerosols emitted from electronic cigarettes sold in Malaysia. J. Environ. Sci. Health. 2020;55:55-62.
- [Google Scholar]
- Trace metal concentration in different Indian tobacco products and related health implications. Food Chem. Toxicol.. 2010;48:2291-2297.
- [Google Scholar]
- A comparative evaluation and toxicity assessment of heavy metals in commonly smoked cigarette brands and local tobacco snuff purchased and consumed in Nigeria. Res. J. Environ. Toxicol.. 2011;5(6):359.
- [Google Scholar]
- Tobacco smoking: health impact, prevalence, correlates and interventions. Psychol. Health. 2017;32:1018-1036.
- [Google Scholar]
- The Influence of tobacco smoke on protein and metal levels in the serum of women during pregnancy. PLoS ONE. 2016;11(8):e0161342.
- [Google Scholar]
- Evaluation of concentration of copper, lead, and zinc in different brands of cigarette sold in Keffi Main Market of Nasarawa State, Nigeria. Asian J. Adv. Res. Rep. 2019:1-7.
- [Google Scholar]
- Heavy metal content in cigarette and Hookah Tobacco in Iran. J. Mazandaran Univ. Med. Sci.. 2020;30:95-106.
- [Google Scholar]
- Major-and trace-element distribution in cigarette tobacco, ash and filters. J. Radioanal. Nucl. Chem.. 2018;316(2):629-634.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101521.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







