Translate this page into:
Assessing the growth-promoting traits of actinobacteria spp. isolated from Cleome africana: Implications on growth and root enhancement of Medicago sativa
⁎Corresponding author at: Microbiology, Health and Environment Team, Faculty of Sciences, Chouaïb Doukkali University, El Jadida 24000, Morocco. ahmed.nafis@edu.uca.ac.ma (Ahmed Nafis)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Endophytes actinobacteria isolated from the Cleome africana shoot and root tissues were compared for their growth promotion (PGP) traits and their beneficial effects on shoot and root enhancement evaluated using Medicago sativa as test crop.
Methods
Healthy plants of C. africana evolved for long periods on heavy metal mining sites were sampled and the resident endophytes actinobacteria communities in root and leaves tissues isolated and characterized based on their 16 s rRNA regions and a culture dependent approach. The growth-promoting traits in terms of phosphate solubilization activity, siderophore production, indole acetic acid synthesis and ACC deaminase activity, resistance to drought and salt stresses were studied for the selected endophytes. The beneficial effect of the two selected actinobacteria and their consortium in promoting growth of M. sativa plants from an inoculation assay that comprised four treatments: [a control (Con), inoculation with Streptomyces sp. (A3), inoculation with Amycolatopsis sp. (A4), and a consortium of A3+A4]. Sixty days after sowing, plants were harvested, and the biomass production was measured.
Results
The six isolated actinobacteria strains were identified as members of the Streptomyces, Amycolatopsis and Nocardia genus. Isolates of A2 and A4 presented increased identity to A. endophytica (99.10%) and A. nivea (99.44%) respectively, isolates A1 were in the Nocardia genus, with high affinity to the N.m exicana (98.7% 16S rRNA similarity). Isolates A3 was a non-cultured and not yet identified, while the isolates A5 and A6 all from the Streptomyces genus. Isolates A6 showed high affinity S. xishensis, strain YIM M 10378, and the isolates A5 were closely related to S. nogalater (100%). Cultures of isolates A3 and A4 and their consortium was used to inoculate M. sativa and the shoot, root dry weight and total biomass (shoot+root) was higher in the inoculated plants than uninoculated ones.
Conclusion
The significant increase in plant growth implies that these actinobacteria can be used as inoculants to improve crop plant growth in semi-arid regions.
Keywords
Biochemical properties
Indole acetic acid contents
Inorganic phosphate (Pi) solubilization
- ACC
-
1-aminocyclopropane-1-carboxylate
- IAA
-
indole acetic acid
- PGP
-
plant growth promoting
- RP
-
rock phosphate
- TCP
-
Tricalcium phosphate
Abbreviations
1 Introduction
Plant internal tissues (endosphere) are a dynamic niche colonized by numerous group of microorganisms, bacteria and fungi, and actinobacteria which are defined as endophytes (Hardoim et al., 2015; Passari et al., 2016). Actinobacteria are among endophytes groups that have attracted special interest for their diverse plant growth-promoting effects (Nafis et al., 2019; Qin et al., 2015), strong plant colonization activities, increased stress resistance (through pigment and spore production) (Hamedi and Mohammadipanah, 2015), as well as their ability to produce an enormous array of secondary metabolites (Nafis et al., 2018; Ibrahimi et al., 2023). Actinobacteria improve plant growth by various mechanisms (Sathya et al., 2017), including, nitrogen fixation (Qin et al., 2015), phosphate solubilization (Passari et al., 2016), siderophores biosynthesis (El-Tarabily et al., 2019), phytohormone production [e.g. auxins (IAA) (Qin et al., 2015), gibberellins (Etminani and Harighi, 2018) and cytokinin’s (El-Tarabily et al., 2019)], biosynthesis of volatile organic compounds (Passari et al., 2020) and polyamines (El-Tarabily et al., 2019), and 1-aminocyclopropane −1-carboxylate (ACC) deaminase activity (Qin et al., 2015). Moreover, endophytic actinobacteria are considered efficient biocontrol agents (Oubaha et al., 2019; Yadav et al., 2020). These characteristics are considered vital and of crucial importance in sustainable agriculture, since they are based on biological processes to stimulate plant growth and productivity and permit to maintain soil fertility (Raklami et al., 2019).
Endophytic actinobacteria have evolved the potential to adapt to a variety of environmental stress condition, and the evolved traits from environment genetical, physiological and biochemical are now showing a great interest for plant growth promotion, especially under abiotic stresses such as drought and salinity, two important adverse constraints for crop yield in the North Africa (Ma et al., 2016; Sharma and Kumar, 2021). These endophytic microbes provide a consistent and effective enhancement in the crops productivity (White et al., 2019). The use of endophytic actinobacteria is considered as economical, safer source of nutrition, eco-friendly, viable alternatives to replace or reduce fertilizers and pesticides for increasing agricultural production and improving soil fertility, especially in the arid and semiarid regions (Sathya et al., 2017). Yet, research regarding endophytic actinobacteria diversity and their beneficial effects in plant growth promotions is scarce. In that vein, it is essential to understand the ecological function of the endophytic actinobacteria and pinpoint their precise functional role in the host plant system.
There are currently several abandoned mines in the Marrakech area, which is situated at the base of two massif (the Hight Atlas Mountain to the south and the Jebilets to the north). Particular attention has been paid to the Kettara mine (Raklami et al. 2021). Pyrrhotite (FeS) extraction was carried out in the mine up to 1982, mostly to produce sulfuric acid. From 1964 to 1981, the mine was also used to recover other metals, including Cu, Zn, Fe, and Cd (Hakkou et al., 2008; Raklami et al., 2021). Around three million tons of wase were heaped up during the operation period over an area of about 37 ha in a dyke and ponds, where they have produced acid mine drainage for more than 24 years (Toughzaoui et al., 2015). The Kettara mine is the main source of pollution and an environmental problem in the Marrakech region since Zn, Cu, Pb, Co, As, and Cd are the most prevalent heavy metals found in the mine waste. Upon vegetative recolonization, the pioneer plant was the Cleome africana that belong to the family of Cleomaceae, and exhibit many biological activities such as antidiabetic, antiviral, anticancer, antidiarrhea, analgesic, anti-inflammatory, and hepatoprotective (Abdullah et al., 2021). The plants are indigenous to North Africa regions, accumulate heavy metals in their tissues with higher content, and host several soil microbiomes that have evolved in heavy metals and saline and drought conditions (El Alaoui et al., 2021).
The overall objective of the characterize the growth-promoting endophytes actinobacteria isolated from C. africana leaves and roots exposed to long terms soil contaminated with heavy metal, compared the growth beneficial traits, and benefit in promoting growth of Medicago sativa. Specially, we aimed to:
-
Characterize the diversity of resident’s endophyte actinobacteria inside the roots and leaves of C. africana.
-
Compare the traits such as phosphate solubilization, IAA and siderophores production, ACC deaminase activity.
-
Examine their potential to promote M. sativa growth and development. Ultimately, selected endophytic actinobacteria may be used as future inoculants to boost plant growth in semi-arid regions.
2 Materials and methods
2.1 Mining sites description and plant sampling
The plant material was collected in the Kettara mine (31° 52′00 “N and 8° 9′00 ”W) located approximately 23 km Northwest of Marrakech, Morocco. C. africana was chosen based on its abundance in the polymetallic contaminated site and its known adaptation to the semi-arid climatic conditions (El Alaoui et al, 2021). Furthermore, earlier research suggested that microbes associated with plants might be crucial in plant adaptation to stresses. Five healthy adult C. africana plants distanced 100 m from each other were sampled and the rhizosphere soil collected for chemical analysis (Supplement Table S1). To remove any associated soil, the plants were thoroughly cleaned with tap water.
2.2 Isolation of the endophytes actinobacteria
Plant tissues were surface sterilized following the five-step method described by Qin et al. (2009). Briefly, plant tissues were washed in 5% NaOCl for 10 min, promptly washed in 2.5% Na2S2O3 and 75% ethanol for 10 min and 5 min, respectively. A final wash was done in 10% NaHCO3 for 10 min. Tissues were aseptically crushed into smaller pieces, after being heated at 80 °C for 30 min. The crushed tissues were then spread in Bennett’s agar or chitin-vitamin agar (Nafis et al., 2019). Nalidixic acid (100 µg/mL) and cycloheximide (50 µg/mL) were added to the media to prevent the growth of endophytic Gram-negative bacteria and fungi, respectively. Actinobacterial colonies were regularly checked, and those emerged were purified using repeated streaking on the same isolation medium. Purified isolates were stored at −20 °C in glycerol (25%). Aliquots of the sterile distilled water used in the final rinse were poured into the mediums to make sure the surface sterilization was successful. The disinfection process was considered successful when no microorganism growth occurred after 7 days incubation at 28 °C.
2.3 Growth media and morphological characteristics of endophytic actinobacteria
On Bennett agar media, growth traits such as color of aerial mycelium and color of substrate mycelium of the selected actinomycetes isolates were recorded. Scanning electron microscopy (SEM) of the endophytic actinobacteria was performed using the sterile cover slips methods without any chemical fixation. Briefly, the sterile cover slips were inserted in the Bennett agar medium at an angle of around 45° until about half. After 24 h, an inoculum of the endophytic actinobacteria was then spread along the line where the surface if the cover slips meet the medium. The plates were incubated for 3 days at 28 °C.
2.4 Identification of cultivable endophytic actinobacteria
Following manufacturer’s instructions, genomic DNA was extracted using a Bacterial DNA kit (MPure™, Ottawa, ON, Canada. Using the universal primers FD1 (5′ AGAGTTTGATCCTGGCTCAG 3′) and S17 (50CGGTCACGTTCGTTGC30), the 16S rRNA gene was amplified by PCR (Weisburg et al., 1991; Pawlowski and Holzmann, 2002;). An initial denaturation of the PCR was performed at 95 °C for 5 min, followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 53 °C for 30 s, extension at 72 °C for 1.5 min, and a final extension at 72 °C for 5 min. BLASTN was used to compare the obtained 16S rDNA sequences (GenBank and accession numbers, OK263090-OK263095) with the publicly accessible nucleotide sequences in GenBank databases (https://www.ncbi.nlm.nih.gov/nucleotide/BLASTN). The sequences were aligned in the Molecular Evolution Genetics Analysis (MEGA) software (v5.0) using ClustalW (Larkin et al., 2007; Hall, 2013). Phylogenetic tree was constructed based on the Tamura-Nei model using neighbor-joining analyses (bootstrap of 1500).
2.5 Characterizing the isolated endophytic actinobacteria
2.5.1 Salt and drought tolerance
Salt and drought tolerance were assessed in microtiter plates containing serial dilution of NaCl and of polyethylene glycol P6000 (Sigma-Aldrich, France), respectively. Briefly, Bennett medium was supplemented with an increasing range of NaCl (0.092–4 mM) or polyethylene glycol P6000 (1.25–60%), then, the plates were incubated for 96 h at 28 °C. Using a microtiter plate reader (800 TS Absorbance Reader, BioTek, Winooski, USA), the optical density was measured at 600 nm.
2.5.2 Phosphate solubilization activity
Actinobacterial isolates were inoculated into NBRIY broth supplemented with tricalcium (Ca3(PO3)2 or the Moroccan rock phosphate as a source of inorganic phosphate Nafis et al. (2019). For 196, the medium was incubated at 28 °C and 180 rpm on rotary shaker. The concentration of soluble P was determined using the method described by Olsen et al. (1982), while the pH was estimated using a digital pH (pH21, Hanna Instruments, Romania).
2.5.3 Siderophores production
Siderophores production was determined using the Chrome-Azurol-S (Sigma Aldrich, France) medium. The appearance of an orange halo around the colony was considered as a positive result and the halo diameter was measured (Patel et al., 2018).
2.5.4 Biosynthesis of indole acetic acid (IAA)
IAA strains production was evaluated in 100 mL of Luria Bertani broth containing 1.02 g/L of L-tryptophan (Sigma Aldrich, France) as a precursor of IAA. After centrifugation, 1 mL of the bacterial supernatant was mixed with 2 mL of Salkowski’s reagent (10 mM FeCl3, 35% perchloric acid) and 2 drops of phosphoric acid (Sigma Aldrich, France). After 30 min incubation in the darkness, the optical density was measured at 530 nm (VR-2000 Spectrophotometer, Selecta, Spain). Following the linear regression analysis, the amount of IAA amount was calculated using a calibration curve of pure IAA (Sigma-Aldrich) (Bano and Musarrat, 2003).
2.5.5 1-aminocyclopropane-1-carboxylic acid deaminase activity
The ACC deaminase activity was evaluated as described in Nascimento et al., (2019). Briefly, actinobacteria isolates were picked and grown in 50 mL Falcon tubes containing 5 mL of Bennet’s broth, at 25 °C, 200 rpm, over 7 days. After centrifugation at 7200 rpm for 10 min, cells were washed two times with 5 mL DF minimal media, followed by cell pellet resuspension in 5 mL of the DF minimal media supplemented with ACC to a final concentration of 3 mM. These cultures were incubated for 48 h at 25 °C, 200 rpm. Bacterial pellets were then collected by centrifugation and carefully washed twice with 10 mL of 0.1 M Tris HCl buffer (pH 8). The pellets were finally resuspended in 400 µL of 0.1 M Tris HCl buffer (pH 8), the cells disrupted with toluene, and the ACC deaminase activity quantified.
2.5.6 Rapid biochemical characterization of endophytic actinobacteria
The isolates were biochemically characterized. Biochemical tests generally used were nitrate reduction to nitrite and to dinitrogen, glucose fermentation, arginine dihydrolase, urease, β-glucosidase, protease hydrolysis, β-galactosidase, glucose, arabinose, mannose, mannitol, N-Acetyl-glucosamine, maltose, potassium gluconate, capric acid, adipic acid, malate, trisodium citrate, and phenylacetic acid assimilation. The biochemical tests were investigated and read using the API 20NE according to the manufacturer instructions (API systems, Biomerieux).
2.6 Bioassay
2.6.1 Biological materials
Medicago sativa seeds (Demnate variety) were disinfected for 5 min with sodium hypochlorite diluted 1/5 (v/v) and placed on wet filter paper for germination in the dark at 25 °C for 24 h. Sprouted seeds were sown in 2.2 L plastic pots filled with previously sterilized peat and perlite at 1:1 ratio (w/w).
The bacterial inocula was prepared by culturing the selected strains in Bennett’s agar for 5–6 days at 28 °C) were harvested by centrifugation (6000 rpm, 10 min), washed once with sterile distilled water, and then re-suspended in sterile distilled water to the final concentration (OD600 = 1). Each pot was inoculated with 10 mL of the appropriate strains (the mixed inoculation was obtained by mixing equal volume of each strain).
2.6.2 Experimental design
The design of the experiment was a randomized complete block (RCBD) with four treatments (Trt) and 10 replicates. The first Trt was the control (Con) uninoculated, Trt 2 was inoculation with Streptomyces sp. (A3); Trt 3 was inoculation with Amycolatopsis sp. (A4), and Trt 4 was the consortium of A3 × A4. Pots filled with previously sterilized peat and perlite at 1:1 ratio (w/w) and one week old seedling of M. sativa transplanted. Pots were placed controlled greenhouse at Cadi Ayyad University under natural daylight (250 – 1000 μmol/m2/sec). The temperature was maintained at 25/21 °C day/night and 40%-60% relative humidity. Plants were irrigated with distilled water (250 mL) twice a week to maintain the water holding capacity at about 75%. The shoot and root length were measured at 60 days after sowing. Plants were harvested at 60 days after sowing, their roots separated from the shoots. Roots were carefully rinsed, and excess water was removed using a paper towel. Dry weights of the shoots and roots were measured after oven drying at 70 °C for 72 h after which their shoot dry weight and root dry weight were measured.
2.7 Statistical analysis
The statistical analyses of the data were conducted using Statistical Analysis System (SAS) (JMP, 2019). One-way analyses of variance (ANOVA) were carried out to assess the significant difference of the strains. The TUKEY’s test method was used to separate means that were different at p ≤ 0.05. Levels of significance are given by ‘ns’ (not significant, p > 0.05), *p < 0.05, **p < 0.01, and ***p < 0.001. Values followed by the same letter are not significantly different at p < 0.05 (TUKEY’s test).
3 Results
3.1 Identification and characterization of cultivable endophytic actinobacteria
Six different actinobacteria isolates (named A1 to A6) were culture- dependent identified based on the morphological, aerial hyphae and substrate mycelia development (Fig. 1). The isolates A4 and A5 were screened from root tissues, while other isolated strains (A1, A2, A3, A6) were obtained from shoots of C. africana. The A1 (Fig. 1 a) and A6 (Fig. 1f) shows a white substrate mycelium and white aerial mycelium on Bennet medium. A2 and A4 show yellow substrate mycelium and white aerial mycelium (Fig. 1b and d). While A3 and A5 shows a brown substrate mycelium and orange aerial mycelium (Fig. 1c and e). A1 strain exhibits a fragmentation of the mycelium and extensively branched like the roots (Fig. 1g and m). The hyphae of the A2 and A4 is characterized by a long branching hypha and generally fragmented into coccoid to red-shaped (Fig. 1h and n). While A3, A5, and A6 had a dense-long hyphae under SEM. According to 16S rRNA sequencing, the strains were found to belong to three genera: Streptomyces, Nocardia and Amycolatopsis (Fig. 2). Isolates of A2 and A4 were identified as Amycolatopsis endophytica and Amycolatopsis nivea, respectively, The A1 isolates were in the Nocardia genus, with high affinity to the N. Mexicana (Fig. 2). The Streptomyces (A3) isolates was non-cultured and not yet identified, while the isolates A5 and A6 all from the Streptomyces genus. Isolates A6 showed high affinity S. xishensis, strain YIM M 10378, and the isolates A5 were closely related to Streptomyces nogalater (100%).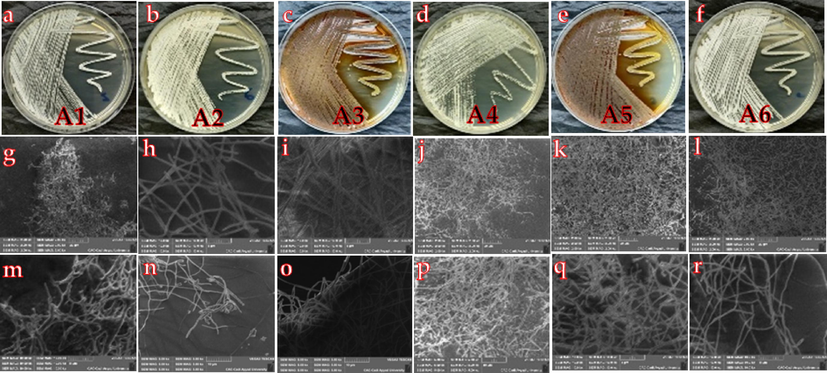
Morphological characteristics (A-E) and scanning electron microscopy photos (F-R) of the isolated endophytic strains (A1-A6) from Cleome Africana organs.

Maximum-likelihood tree based on 16S rRNA gene sequence showing the relations between the isolated endophytic Actinobacteria strains. The numbers at the nodes indicate the levels of bootstrap support based on maximum-likelihood analyses of 1500 resampled data sets (only values > 50% are shown). Bar, 0.01 nt substitution per nt position.
3.2 Improved traits under stress among the isolates
We further compared the six actinobacteria isolates for the salt and drought tolerance, TCP, and RP solubilization, IAA production, ACC activity and siderophore production (Table 1). The actinobacteria isolates showed different resistance to salt stress, ranging from 0.75 to 21.25 ML-1 NaCl. For instance, Amycolatopsis sp. A4 tolerated up to 0.75 ML-1 NaCl in the growth media, isolate A3 salt tolerance was 1.25 M NaCl; strains A1, A2, and A5 grew in the presence of up to 1.5 M L-1 NaCl and Streptomyces sp. A6 resisted to 2 M NaCl. Regarding drought tolerance, strains A2, A3, A4, A5 tolerated 60% of P6000, while the strains A1 and A6 tolerated up to 40% P6000. Overall, Streptomyces sp. A6 presented the highest resistance to both salt and drought stress (Table 1). +: presence of PGP trait, −: absence of PGP trait. Means (±SD) within the same colony followed by different letters are significantly different at p < 0.05.
Strain
16 rRNA Gene
Accession NumberSalt tolerance (M)
Drought tolerance (%P6000)
Tricalcium phosphate solubilization
Rock phosphate solubilization
IAA production (µg/ml)
ACC deaminase activity
(µmol/mg protein/hour)Siderophores production (halo diameter)
pH
P content (mg/l)
pH
P content (mg/l)
Nocardia sp. A1
OK263095
1.5
40
6.24
93.69 ± (0.9)c
6.76
33.17 ± (8.76)c
7.21 ± (0.5)f
−
−
Amycolatopsis sp. A2
OK263094
1.5
60
5.35
171.39 ± (5.99)b
5.91
11.64 ± (2.88)d
16.57 ± (1.68)d
0.051
1.2
Streptomyces sp. A3
OK263093
1.25
60
5.16
181.64 ± (9.34)ab
4.62
49.62 ± (2.56)b
102.92 ± (0.5)a
−
−
Amycolatopsis sp. A4
OK263092
0.75
60
5.45
198.03 ± (5.9)a
4.65
102.46 ± (2.9)a
21.51 ± (0.68)c
0.012
1.2
Streptomyces sp. A5
OK263091
1.5
60
5.09
191.89 ± (12.38)a
4.48
51.09 ± (0.76)b
63.75 ± (0.77)b
−
−
Streptomyces sp. A6
OK263090
2
40
6.04
165.08 ± (6.58)b
5.48
59.34 ± (4.95)b
12.95 ± (0.09)e
−
2
The isolated actinobacteria strains displayed multiple PGP activities, particularly, the TCP and RP solubilization, IAA production, ACC activity and siderophore production (Table 1). All isolates solubilized the inorganic phosphate (Pi) from the TCP and RP sources after 196 h of incubation. The Pi released in the media ranged from 93.9 to 198.03 mg L-1 in case of tricalcium phosphate, and from 11.64 to 102.46 mg/L in case of rock phosphate, Amycolatopsis sp. A4 solubilized the highest Pi content than other isolates (Table 1). Similarly, all strains produced the IAA, Streptomyces sp. A3 produced the highest (102.92 µg/ml) followed by Streptomyces sp. A5 (63.75 µg/ml), while Nocardia sp. A1 produced the least (7.21 µg/ml) (Table 1). The ACC activity was observed in only two (Amycolatopsis sp. A2 and Amycolatopsis sp. A4) out of six the isolates of actinobacteria (Table 1). Siderophore production was higher for Streptomyces sp. A6 compared to Amycolatopsis sp. A2, Amycolatopsis sp. A4 (Table 1).
3.3 Biochemical traits
Several rapid biochemical test of functional traits of the isolated actinobacteria were measured and the results are shown in Table 2. The six isolates qualitatively expressed the β-glucosidase, β-galactosidase, and arabinose assimilation, glucose reductases, protease hydrolysis (except A1) functions (Table 2). The isolates A1, A3 and A6 expressed the nitrate and dinitrogen reductase functions that were not observed in the A2, A4, and A5 isolates (Table 2). However, in case of maltose assimilation, only A1 and A2 showed a positive reaction. Similarly, A1 was the only strain that showed a positive reaction in case of potassium gluconate assimilation test (Table 2). NO2: nitrate reduction to nitrite; N2: nitrate reduction to dinitrogen; GLU: glucose fermentation; ADH: arginine dihydrolase; URE: urease; ESC: β-glucosidase; GEL: protease hydrolysis; PNPG: β-galactosidase; GLU*: glucose assimilation; ARA: arabinose assimilation; MNE: mannose assimilation; MAN: mannitol assimilation; NAG: N-Acetyl-glucosamine assimilation; MAL: maltose assimilation, GNT: potassium gluconate assimilation; CAP: capric acid assimilation; ADI: adipic acid assimilation; MLT: malate assimilation; CIT: trisodium citrate assimilation; PAC: phenylacetic acid assimilation; +: presence; −: absence.
NO2
N2
GLU
ADH
URE
ESC
GEL
PNPG
GLU*
ARA
MNE
MAN
NAG
MAL
GNT
GNT
ADI
MLT
CIT
PAC
A1
+
+
−
−
−
+
−
+
+
+
−
+
−
+
−
+
+
+
+
A2
−
−
−
−
−
+
+
+
+
+
+
−
+
+
−
−
−
+
+
+
A3
+
+
−
−
−
+
+
+
+
+
−
−
+
−
+
−
+
−
−
−
A4
−
−
−
+
−
+
+
+
−
+
+
+
+
−
−
−
−
−
−
−
A5
−
−
−
−
−
+
+
+
+
+
−
−
+
−
+
−
−
−
−
−
A6
+
+
−
−
−
+
+
+
+
+
−
−
+
−
+
−
−
−
−
+
3.4 Growth-promoting effect of isolate on Medicago sativa growth
We evaluated the ability of two selected endophytic actinobacterial isolates (Streptomyces sp. A3 and Amycolatopsis sp. A4) and the consortium of A3 + A4 to promote shoot growth and root development of M. sativa (Fig. 3). The ANOVA revealed highly significant effects of the tested treatments on the shoot, root, and total biomass dry weight (Fig. 3a). Inoculated plants showed higher dry matter biomass of shoot and roots than the uninoculated ones (Fig. 3a). There was not specific difference due to the consortium effect compared to A3 and A4 inoculation alone for the shoot, root, and total biomass, except in for the A4 for the root dry matter (Fig. 3a). The shoot dry and root dry weight increased by 72% for the Streptomyces sp. A3 and inoculated and 80% for the Amycolatopsis sp. A4 treated plants (Fig. 3a). The A3 + A4 treatment showed shoot and root dry matter increase by 67% and 88%, respectively (Fig. 3a). The inoculation with the mixture of Streptomyces sp. A3 + Amycolatopsis sp. A4 increased the shoot length by 31% compared to the non-inoculated plants (Fig. 3b). The root length was higher for the A3 strain and the consortium (A3 + A4) than in the control plants (Fig. 3b). The inoculation with the mixture of both endophytic actinobacteria increased the number of leaves of M. sativa plants, resulting in 91% relative increase (Fig. 3b).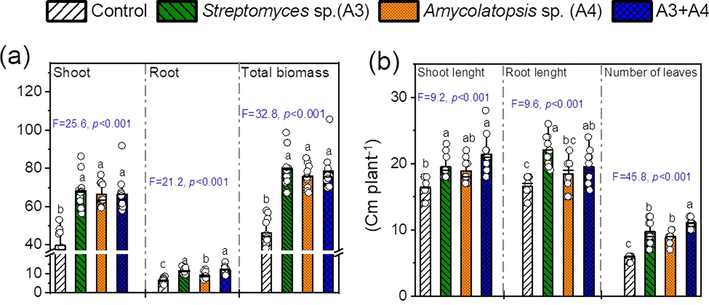
Shoot and root, and total biomass dry weight (a) and lengths of shoot and root, and numbers of leaves of Medicago sativa submitted to different treatments [control without inoculation Streptomyces sp. A3, and Amycolatopsis sp. A4; and the consortia of A3 + A4. Means (±SD) within the same parameter followed by different letters are significantly different at P < 0.05. Symbols are the number of replicate (12). The TUKEY’s test method was used to separate means that were different at p ≤ 0.05. Levels of significance are given by ‘ns’ (not significant, p > 0.05), *p < 0.05, **p < 0.01, and ***p < 0.001. Values in columns followed by the same letter are not significantly different at p < 0.05 (TUKEY’s test).
4 Discussion
We hypothesized that in polluted environments (e.g., Kettara mine in this study) have attracted individual-special endophytic actinobacteria with distinctive PGP traits to support their growth as a response to the harsh environment. The diversity and functional capabilities of the endophytic actinobacteria that are associated with plants in particular ecosystems are still poorly understood.
In this study, the growth-promoting characteristics of numerous endophytic actinobacteria connected to C. africana and their influence on the growth of M. sativa were examined. Six actinobacteria strains were isolated from C. africana tissues, and their molecular identification revealed that these strains belonged to the Streptomyces (isolates A3, A5, A6), Amycolatopsis (isolates A2, A4) and Nocardia (isolate A1) genera. Zamoum et al. (2015) reported the isolation of Streptomyces and non-Streptomyces strains from the root tissues of C. Africana plants native to Algerian Sahara. Our findings support earlier research that showed the wide prevalence of Streptomyces in the interior tissues of various plant species including Solanum lycopersicum (Passari et al., 2016), Jatropha curcas (Qin et al., 2015), Thymus roseus (Mohamad et al., 2022), Artemisia princeps, Capsella bursa, Iris rossii, Lamium purpureum, Rudbeckia bicolor, and Setaria viridis (Kim et al., 2012). Many plants' have been found to harbors members of the Nocardia genus (e.g. Capsella bursa) (Passari et al., 2016). Notably, Amycolatopsis have been isolated less frequently and studies reporting this genus as endophytic are scarce. Currently, several studies report novel species belonging to Amycolatopsis genus, such as Amycolatopsis anabasis (Wang et al., 2020), Amycolatopsis pittospori (Kaewkla and Franco, 2021), Amycolatopsis dendrobii (Tedsree et al., 2021).
The results of our investigation show that culturable endophytes may be involved in a wide range of biological activities, with potential uses in the plant growth promotion. Most of the obtained endophytic actinobacterial isolates presented stress resistance and active PGP traits, including i) inorganic phosphate solubilization (best PO4 solubilization for Amycolatopsis sp. A4); ii) IAA production (best production was observed in the case of Streptomyces sp. A3); iii) ACC deaminase activity (positive activity was detected in the case Amycolatopsis sp. A2 and Amycolatopsis sp. A4), iv) siderophores production (Streptomyces sp. A6 showed higher production). In agreement with these findings, it has been reported that Amycolatopsis isolated from the Tenfit mine, an abandoned mine in the Marrakech region, can produce hydroxamate siderophores (El baz et al., 2015). In a recent study, Streptomyces sp. NEAU-S7GS2, which was isolated from rhizosphere soil and Glycine max’s root, exhibit several plants growth-promoting traits (Liu et al., 2019). The production of 1-aminocyclopropane-1-carboxylate (ACC) deaminase, IAA, and the solubilization of inorganic phosphate were all factors in NEAU-ability S7GS2′s to promote plant development. The majority of endophytic actinobacteria isolated from Thymus roseus were shown by Mohamed et al. (2022) to be capable of having direct PGP traits, such as auxin, ammonia, siderophore production, and phosphate solubilization, in addition to cell-wall degrading enzymes like protease, cellulase, lipase, and chitinase. The obtained values for phosphate solubilization, IAA and siderophore biosynthesis and ACC deaminase activity were within the range of those reported from other plant associated actinobacteria (Borah and Thakur, 2020; Jog et al., 2014; Passari et al., 2016; Qin et al., 2015).
Endophytic actinobacterial strains with multiple PGP traits improve plant growth through various direct and indirect mechanisms (Nafis et al., 2019). The actinobacterial isolates, Streptomyces sp. A3 and Amycolatopsis sp. A4 presented multiple and potent PGP functional traits such as inorganic phosphate solubilization, IAA production (Streptomyces sp. A3), ACC deaminase activity (Amycolatopsis sp. A4) and were therefore selected to be used as inoculants to promote M. sativa plant growth. The greenhouse experiment showed that the inoculation of M. sativa with the isolated endophytic actinobacteria (single or a mixture) yielded a significant improvement in terms of different plant growth parameters such as shoot and root dry weight, shoot and root length and leaves number, when compared to non-inoculated control plants. These results are consistent with previous reports demonstrating the beneficial impact of endophytic actinobacteria in plants such as Triticum aestivum (Jog et al., 2014), Jatropha curcas (Qin et al., 2015), and Camellia sinensis (Borah and Thakur, 2020).
Improvement in M. sativa growth by Streptomyces sp. A3 and Amycolatopsis sp. A4 could be correlated with the strains capacity to modulate plant hormones. For instance, Streptomyces sp. A3 produced increased levels of IAA, an essential phytohormone that induces plant growth by stimulating root elongation (Raklami et al., 2019). In addition, Amycolatopsis sp. A4 presented ACC deaminase activity which may decrease plant ethylene levels by converting the ethylene precursor to ammonia and α-ketobutyrate (Sathya et al., 2017).
5 Conclusions
For the decades to come, actinobacteria has a great opportunity to become an essential component as biofertilizers and biocontrol agents for sustainable agriculture. Actinobacteria, particularly Streptomyces, are considered as one of the most promising microorganisms for enhancing plant growth, agricultural productivity, and overall soil health. The Overall obtained results displayed the valuable role of endophytic Streptomyces sp. A3 and Amycolatopsis sp. A4 (single or in consortia) as biofertilizers agent. The isolated actinobacteria strains are considered PGP bacteria and could be used for the development of commercial inoculants for agricultural applications, especially for use in semi-arid regions.
Funding
The authors extend their appreciation to the researchers supporting project number (RSP2023R15), King Saud University, Riyadh, Saudi Arabia, and by CNRST/TUBITAK cooperation project (Morocco-Turkey).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Biological activity and investigation of some active constituents with molecular docking from two Cleome species. Egy. Pharm. J.. 2021;20(2):115.
- [Google Scholar]
- Characterization of a New Pseudomonas aeruginosa strain NJ-15 as a potential biocontrol agent. Curr. Microbiol.. 2003;46:324-328.
- [CrossRef] [Google Scholar]
- Phylogenetic and functional characterization of culturable endophytic actinobacteria associated with Camellia spp. for growth promotion in commercial tea cultivars. Front. Microbiol.. 2020;11:1-23.
- [CrossRef] [Google Scholar]
- Use of native plants and their associated bacteria rhizobiomes to remediate-restore Draa Sfar and Kettara mining sites. Morocco. Environ. Monit. Assess. 2021;193:1-14.
- [CrossRef] [Google Scholar]
- Resistance to and accumulation of heavy metals by actinobacteria isolated from abandoned mining areas. Sci. World J.. 2015;2015
- [CrossRef] [Google Scholar]
- Growth promotion of Salicornia bigelovii by Micromonospora chalcea UAE1, an endophytic 1-aminocyclopropane-1-carboxylic acid deaminase-producing actinobacterial isolate. Front. Microbiol.. 2019;10:1-17.
- [CrossRef] [Google Scholar]
- Isolation and identification of endophytic bacteria with plant growth promoting activity and biocontrol potential from wild pistachio trees. Plant Pathol. J.. 2018;34:208-217.
- [CrossRef] [Google Scholar]
- Acid mine drainage at the abandoned kettara mine (Morocco): 1. environmental characterization. Mine Water Environ.. 2008;27:145-159.
- [CrossRef] [Google Scholar]
- Building phylogenetic trees from molecular data with mega. Mol. Biol. Evol.. 2013;30:1229-1235.
- [CrossRef] [Google Scholar]
- Biotechnological application and taxonomical distribution of plant growth promoting actinobacteria. J. Ind. Microbiol. Biotechnol.. 2015;42:157-171.
- [CrossRef] [Google Scholar]
- The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev.. 2015;79:293-320.
- [CrossRef] [Google Scholar]
- The potential of facultative predatory Actinomycetota spp. and prospects in agricultural sustainability. Front. Microbiol.. 2023;13:1081815.
- [CrossRef] [Google Scholar]
- Mechanism of phosphate solubilization and antifungal activity of Streptomyces spp. isolated from wheat roots and rhizosphere and their application in improving plant growth. Microbiol. (United Kingdom). 2014;160:778-788.
- [CrossRef] [Google Scholar]
- Amycolatopsis pittospori sp. nov., an endophytic actinobacterium isolated from native apricot tree and genome mining revealed the biosynthesis potential as antibiotic producer and plant growth promoter. Antonie Van Leeuwenhoek. 2021;114:365-377.
- [CrossRef] [Google Scholar]
- Diversity and physiological properties of root endophytic actinobacteria in native herbaceous plants of Korea. J. Microbiol.. 2012;50:50-57.
- [CrossRef] [Google Scholar]
- Antifungal, plant growth-promoting, and genomic properties of an endophytic actinobacterium Streptomyces sp. NEAU-S7GS2. Frontiers in microbiology. 2019;10:2077.
- [Google Scholar]
- Beneficial role of bacterial endophytes in heavy metal phytoremediation. J. Environ. Manage.. 2016;174:14-25.
- [CrossRef] [Google Scholar]
- The metabolic potential of endophytic actinobacteria associated with medicinal plant Thymus roseus as a plant-growth stimulator. Microorganisms. 2022;10(9):1802.
- [CrossRef] [Google Scholar]
- Screening for non-polyenic antifungal produced by actinobacteria from moroccan habitats: assessment of antimycin a19 production by Streptomyces albidoflavus AS25. Int. J. Mol. Cell. Med.. 2018;7:133-145.
- [CrossRef] [Google Scholar]
- Actinobacteria from extreme niches in morocco and their plant growth-promoting potentials. Diversity. 2019;11:139.
- [CrossRef] [Google Scholar]
- Taxonomical over splitting in the Rhodnius prolixus clade, Are R. taquarussuensis and R. neglectus the same species. PLoS One. 2019;14
- [CrossRef] [Google Scholar]
- Determination of available phosphorus. In: Methods of Soil Analysis. Madison, WI: American Society of Agronomy; 1982. p. :403-430.
- [Google Scholar]
- The potential of antagonistic moroccan Streptomyces isolates for the biological control of damping-off disease of pea (Pisum sativum L.) caused by Aphanomyces euteiches. J. Phytopathol.. 2019;167
- [CrossRef] [Google Scholar]
- Detection of biosynthetic gene and phytohormone production by endophytic actinobacteria associated with Solanum lycopersicum and their plant-growth-promoting effect. Res. Microbiol.. 2016;167:692-705.
- [CrossRef] [Google Scholar]
- In Vivo studies of inoculated plants and in vitro studies utilizing methanolic extracts of endophytic Streptomyces sp. Strain DBT34 Obtained from Mirabilis jalapa L. exhibit ROS-scavenging and other bioactive properties. Int. J. Mol. Sci.. 2020;21:7364.
- [CrossRef] [Google Scholar]
- Modified chrome azurol S method for detection and estimation of siderophores having affinity for metal ions other than iron. Environ. Sustain.. 2018;1:81-87.
- [CrossRef] [Google Scholar]
- Isolation, diversity, and antimicrobial activity of rare actinobacteria from medicinal plants of tropical rain forests in Xishuangbanna China. Appl. Environ. Microbiol.. 2009;75:6176-6186.
- [CrossRef] [Google Scholar]
- Biodiversity and plant growth promoting traits of culturable endophytic actinobacteria associated with Jatropha curcas L. growing in Panxi dry-hot valley soil. Appl. Soil Ecol.. 2015;93:47-55.
- [CrossRef] [Google Scholar]
- Use of rhizobacteria and mycorrhizae consortium in the open field as a strategy for improving crop nutrition, productivity and soil fertility. Front. Microbiol.. 2019;10:1-11.
- [CrossRef] [Google Scholar]
- Restoring the plant productivity of heavy metal contaminated soil using phosphate sludge, marble waste, and beneficial microorganisms. J. Environ. Sci.. 2021;99:210-221.
- [CrossRef] [Google Scholar]
- Plant growth-promoting actinobacteria: a new strategy for enhancing sustainable production and protection of grain legumes. 3 Biotech. 2017;7:102.
- [CrossRef] [Google Scholar]
- Bioremediation of heavy metals from industrial effluents by endophytes and their metabolic activity: Recent advances. Bioresour. Technol.. 2021;339:125589
- [CrossRef] [Google Scholar]
- Amycolatopsis dendrobii sp. nov., an endophytic actinomycete isolated from Dendrobium heterocarpum Lindl. Int. J. Syst. Evol. Microbiol.. 2021;71(7):004902
- [CrossRef] [Google Scholar]
- Hydrogeochemicaland isotopic studies of the Kettara mine watershed. Morocco. Water Environ.. 2015;34:308-319.
- [CrossRef] [Google Scholar]
- Amycolatopsis anabasis sp. nov., a novel endophytic actinobacterium isolated from roots of Anabasis elatior. Int. J. Syst. Evol. Microbiol.. 2020;70(5):3391-3398.
- [CrossRef] [Google Scholar]
- 16S ribosomal DNA amplification for phylogenetic studyitle. J. Bacteriol.. 1991;173:697-703.
- [CrossRef] [Google Scholar]
- Review: Endophytic microbes and their potential applications in crop management. Pest Manag. Sci.. 2019;75:2558-2565.
- [CrossRef] [Google Scholar]
- Microbial biotechnology for sustainable biomedicine systems: Current research and future challenges. In: New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; 2020. p. :281-292.
- [CrossRef] [Google Scholar]
- Biocontrol capacities and plant growth-promoting traits of endophytic actinobacteria isolated from native plants of Algerian Sahara. J. Plant. Dis. Prot.. 2015;122:215-223.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102722.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







