Translate this page into:
Ascorbic acid alleviates oxidative stress and improves major salivary glands’ structure and function in diabetic rats: A histological and immunohistochemical study
⁎Corresponding author. rabab.rasheed@ksiu.edu.eg (Rabab Ahmed Rasheed),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Diabetes Mellitus and the associated hyperglycemia trigger oxidative damage to tissues and organs. DM is obviously related to the salivary glands’ dysfunction. Ascorbic acid is a powerful dietary antioxidant. In this study, we investigated the ascorbic acid’s putative potential to conserve the structure and function of the major salivary glands in diabetic patients. Adult male albino rats (n = 24) were equally divided into four groups: Group I (control, only distilled water), Group II (ascorbic acid 200 mg/Kg/day), Group III (experimentally-induced diabetes by single intraperitoneal injection of streptozotocin 60 mg/kg), and Group IV (ascorbic acid after one week of diabetes induction). Blood glucose level was assessed. After eight weeks, samples from the salivary glands were processed for histopathological and immunohistochemical examination. Group III showed degenerative changes in the salivary glands and increased collagen deposition, whereas the glycoprotein content and antiapoptotic activity decreased. Group IV showed marked histologic and functional improvement. Although further research is required to fully understand its mode of action, ascorbic acid is advised as an adjuvant medication for diabetes to keep the integrity of major salivary glands.
Keywords
DM
Salivary glands
Ascorbic acid
Antioxidant
Histopathology
Immunohistochemical
1 Introduction
The production of adequate saliva is necessary for maintaining the mouth cavity's homeostasis and overall health since saliva is a special, complicated bodily fluid (Wang et al., 2017).
While the parasympathetic nerve controls the secretion of the fluid portion of saliva, which contains water, electrolytes, and minute amounts of protein, sympathetic innervation controls the secretion of saliva's proteins. Salivary composition is affected by a person's metabolic, neurological, immunological, hormonal, and nutritional conditions (Chen et al., 2020). Both oral, dental protective and digestive functions of saliva depend mainly on the precise composition of organic substances in the secretory product. Accordingly, any alteration in the composition of the saliva may result in significant deterioration in salivary functions and triggers long-term complications both in oral and dental environments (Dodds et al., 2015).
Latest studies have revealed a substantial link between disorders of the salivary glands and Diabetes Mellitus (DM). DM is characterized by hyperglycemia, which causes oxidative and cellular damage that disrupts the structure of organs and tissues (Rohani, 2019). Persistent hyperglycemia results in chronic oxidative stress, which triggers the overproduction of reactive oxygen species (ROS). The development of DM problems and their onset are brought on by ROS (Kaludercic and Di Lisa, 2020).
Many disorders related to impaired salivary secretion are more predominant in diabetic patients, for example, periodontitis, delayed wound healing, and xerostomia (Fouani et al., 2021). Ascorbic acid (AA), a powerful dietary antioxidant, combats free radicals by neutralizing hydroxyl and superoxide radicals (Santosh and David, 2017).
Studies have demonstrated that AA supplements can boost insulin synthesis, sensitivity, and secretion besides lowering blood glucose levels and the occurrence of DM complications (Shi et al., 2020). This research sought to histologically explore the potential protective role of AA supplementation against DM-induced alterations in the main salivary glands.
2 Materials and methods
2.1 Animal housing and ethical declaration
This experiment was approved by the Institutional Animal Care and Use Committee of Beni-Suef University, per the NIH guidelines for the Care and Use of Laboratory Animals (approval no. 022–245 on 20th Mars 2022). In the Animal House of the Faculty of Medicine, Beni-Suef University, Twenty-four adult male albino rats five to six months old, weighing 180–200 gm, were accommodated in polycarbonate cages (six rats/cage) in a controlled laboratory setting at a steady temperature (22–24 °C) and light–dark cycle (12:12 h) for one week before the onset for acclimation. A standard pellet diet, as shown in S1 Table (Atta et al., 2017), and access to drinking tap water were freely allowed for rats.
2.2 Medications
From Sigma-Aldrich (St. Louis, MO, USA), we purchased l-Ascorbic acid (water-soluble powder, Pack size 100 gm, product number: A92902) and streptozotocin (powder form, 500 mg in glass bottle stored at − 20 °C, product number: S0130).
2.3 Experimental design
For a total duration of eight weeks, the rats were divided into four groups in random fashion (n = 6): Group I (control): distilled water, Group II (AA): AA powder liquified in distilled water (200 mg/kg/day) via oral gavage (Afifi and Embabi, 2016), Group III (STZ): After 12-h fast, experimental induction of DM by intraperitoneal (i.p) injection (once, 60 mg/kg) of STZ freshly dissolved in 0.1 ml citrate buffer, pH 4.5 within ten minutes after preparation. Four to six hours later, depending on the high level of insulin secretion from the pancreas, 5 ml glucose solution (20 %) was injected i.p to eliminate the fatal hypoglycemia. 5 % glucose solution was added to the drinking water for 24 h, and free intake of food was allowed (Gilani et al., 2022). Group IV (STZ + AA): injected with the same dose of STZ as in Group III, and after a one-week interval, AA powder dissolved in distilled water and administered through oral intubation (200 mg/kgm per day) for a further seven weeks. Fig. 1 demonstrates the experimental design.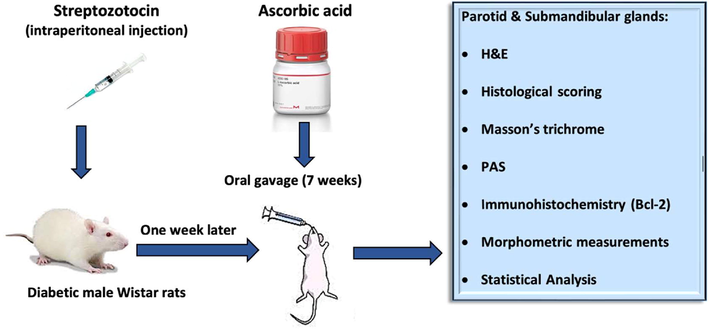
The experimental design.
2.4 Assessment of blood glucose levels
Before STZ injection, collection of blood samples from tail veins of all rats was done, set as (Time 0; T0) to assess fasting blood glucose levels and on days 1, 4, 7, and just before sacrifaction (Ali et al., 2021). Blood glucose level was measured using Glucocheck (Biotest Medical Corp., Tortola, VI, USA).
2.5 Euthanasia and tissue sampling
At the end of the eighth week, no deaths were observed among rats. All rats were euthanized by ether inhalation followed by cervical dislocation.
2.6 Histopathology
Samples from parotid and submandibular glands from rats were fixed in NBF 10 %, dehydrated in graded alcohol, xylene cleared, and further processed for hematoxylin and eosin (H&E), Masson's trichrome, Periodic acid Schiff’s (PAS) stains as per (Bancroft and Layton, 2013).
2.7 The rationale for histopathological scoring
A semiquantitative histopathological scoring in H&E-stained sections was done. An experienced pathologist assessed four parameters in a blinded way: irregular acini, vacuolated cells, leukocytic infiltration, and vascular congestion. The pathological changes were measured in 24 indiscriminately-chosen non-intersecting microscopic fields for each group (two fields/section & two sections/rat of each of the six rats per group). Score scale: 0 = no lesions; 1 = less than 25 %; 2 = 26–50 %; 3 = 51–75 %; and 4 = 76–100 % (Gibson-Corley et al., 2013).
2.8 Immunohistochemistry
Following the avidin–biotin-peroxidase system, Rabbit polyclonal anti-Bcl2 antibodies (Catalogue number SC-492, Santa Cruz Biotechnology, CA, USA, dilution 1:300, antigen retrieval at 90 °C for 20 min) were used as primary antibodies, then incubated with secondary anti-rabbit antibodies. Finally, counterstained with Mayer’s hematoxylin (Noreldin et al., 2018).
2.9 Morphometric study
The image analysis system Leica Qwin DW3000 (Cambridge, England) was used to snap the most representative five random non-intersecting fields in each section to measure the mean area% using light microscopy. All measurements were performed in the Department of Medical Histology, Faculty of Medicine, King Salman International University, South Sinai.
2.10 Statistical analysis
The histopathological scoring data were presented as median and interquartile range (IQR) in S2 Table. Other outcomes were abridged as mean ± standard deviation (SD). With the Kolmogorov-Smirnov test, the normality of the results was evaluated. The findings indicated that the majority of the data were parametric. To conduct the statistical analysis, we used SPSS 19.0 (Statistical Package for Scientific Studies, SPSS, Inc., Chicago, IL, USA) for Windows. One-way ANOVA and Tukey's post hoc tests were then run. p ≤ 0.05 was set as the statistical significance threshold.
3 Results
3.1 Effect of STZ injection on blood glucose levels
S3 Table and Histogram 1 shows the mean ± SD of blood glucose values of the STZ and the STZ + AA groups. The assumption of diabetes was based upon the presence of hyperglycemia and polyuria 48 h after STZ injection. Rats exceeding 220 mg/dl after 24 h of STZ injection were set as diabetics (Guo et al., 2018) and were nominated for tissue sampling.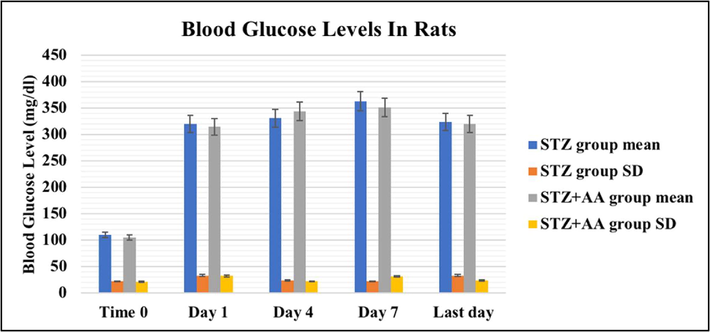
Blood glucose levels in STZ injected rats as mean ± SD (mg/dl).
3.2 Effect of STZ-induced diabetes and/or AA on the histopathological picture of salivary glands
Control and AA groups showed normal histologic features of both glands as ill-defined lobules of serous acini with pyramidal basophilic cell lining with rounded nuclei separated by thin septa with occasional blood vessels. The striated ducts were lined with cuboidal to columnar basally striated cells. The granular convoluted tubules were lined by tall columnar cells with basal rounded nuclei and numerous apical acidophilic secretory granules (Fig. 2). STZ group displayed loss of the normal architecture with irregular acini, cytoplasmic vacuolization, and pyknotic nuclei in acinar and ductal cells, thickened connective tissue septa with leukocytic infiltration, and congested vessels in both glands. STZ + AA group showed morphological improvement. The acini and ducts mostly restored the normal architecture with regular epithelial lining (Fig. 3).![Representative photographs of salivary glands’ histology; H&E-stained, magnification X400. [A & C] parotid of control and AA groups, respectively, [B & D] submandibular gland of control and AA groups, respectively, showing ill-defined lobules separated by thin septa, blood vessels (B.V), serous acini (arrows) lined with pyramidal basophilic cells with rounded nuclei, striated ducts (SD) lined with cuboidal to columnar basally striated cells, granular convoluted tubules (GCT) in submandibular gland lined by columnar cells with basal rounded nuclei and numerous apical acidophilic secretory granules.](/content/185/2022/34/7/img/10.1016_j.jksus.2022.102273-fig3.png)
Representative photographs of salivary glands’ histology; H&E-stained, magnification X400. [A & C] parotid of control and AA groups, respectively, [B & D] submandibular gland of control and AA groups, respectively, showing ill-defined lobules separated by thin septa, blood vessels (B.V), serous acini (arrows) lined with pyramidal basophilic cells with rounded nuclei, striated ducts (SD) lined with cuboidal to columnar basally striated cells, granular convoluted tubules (GCT) in submandibular gland lined by columnar cells with basal rounded nuclei and numerous apical acidophilic secretory granules.
![Representative photographs of salivary glands’ histology; H&E-stained, magnification X400. [A & C] parotid of the STZ group, [B & D] submandibular gland of the STZ group showing irregular acini, vacuolated cytoplasm (V) of cells lining serous acini and ducts, pyknotic nuclei (arrowheads), thickened connective tissue septa (stars), leukocytic infiltration (circle), and congested vessels (B.V). [E & F] parotid and submandibular glands of the STZ + AA group, respectively, showing the acini (arrows) and ducts (arrowheads) mostly restored the normal architecture with regular epithelial lining.](/content/185/2022/34/7/img/10.1016_j.jksus.2022.102273-fig4.png)
Representative photographs of salivary glands’ histology; H&E-stained, magnification X400. [A & C] parotid of the STZ group, [B & D] submandibular gland of the STZ group showing irregular acini, vacuolated cytoplasm (V) of cells lining serous acini and ducts, pyknotic nuclei (arrowheads), thickened connective tissue septa (stars), leukocytic infiltration (circle), and congested vessels (B.V). [E & F] parotid and submandibular glands of the STZ + AA group, respectively, showing the acini (arrows) and ducts (arrowheads) mostly restored the normal architecture with regular epithelial lining.
3.3 Effect of STZ-induced diabetes and/or AA on histopathological scoring
At p ≤ 0.05, the STZ group showed a significant statistical ascent compared to control and AA groups regarding all assessed parameters in parotid and submandibular glands. However, the STZ + AA group showed a statistically significant reduction in all parameters compared to the STZ group in both glands (Fig. 4) and S2 Table.![Histopathological scoring for parotid and submandibular glands in all groups. [a] irregular acini, [b] vacuolated cells, [c] leukocytic infiltration, and [d] congested vessels. * significant from control, + significant from AA group, # significant from STZ group at p ≤ 0.05.](/content/185/2022/34/7/img/10.1016_j.jksus.2022.102273-fig5.png)
Histopathological scoring for parotid and submandibular glands in all groups. [a] irregular acini, [b] vacuolated cells, [c] leukocytic infiltration, and [d] congested vessels. * significant from control, + significant from AA group, # significant from STZ group at p ≤ 0.05.
3.4 Effect of STZ-induced diabetes and/or AA on collagen content
At p ≤ 0.05, collagen deposition between lobules in both glands increased significantly in STZ group compared to control and AA groups. Whereas collagen decreased significantly in STZ + AA group in both glands compared to STZ group (Fig. 5) and S4 Table.![Masson’s trichrome staining in salivary glands, magnification X 400. [A & C] parotid of the control and AA groups, respectively, [B & D] submandibular gland of control and AA groups, respectively, showing mild collagen staining (arrows). [E & F] Parotid and submandibular glands of STZ group, respectively, showing marked increased collagen staining (arrows). [G & H] parotid and submandibular gland of STZ + AA group, respectively, showing moderate collagen staining (arrows). * significant from control, + significant from AA group, # significant from STZ group at p ≤ 0.05.](/content/185/2022/34/7/img/10.1016_j.jksus.2022.102273-fig6.png)
Masson’s trichrome staining in salivary glands, magnification X 400. [A & C] parotid of the control and AA groups, respectively, [B & D] submandibular gland of control and AA groups, respectively, showing mild collagen staining (arrows). [E & F] Parotid and submandibular glands of STZ group, respectively, showing marked increased collagen staining (arrows). [G & H] parotid and submandibular gland of STZ + AA group, respectively, showing moderate collagen staining (arrows). * significant from control, + significant from AA group, # significant from STZ group at p ≤ 0.05.
3.5 Effect of STZ-induced diabetes and/or AA on glycoprotein content
At p ≤ 0.05, the glycoprotein content in PAS-stained sections of both glands showed a significant regression in STZ group compared to control and AA groups, while the STZ + AA group showed a significant rise compared to STZ group (Fig. 6) and S4 Table.![PAS staining in salivary glands, magnification X 400. [A & C] parotid of the control and AA groups, respectively, [B & D] submandibular gland of the control and AA groups, respectively, showing strong PAS reaction (arrows). [E & F] parotid and submandibular glands of the STZ group, respectively, showing weak PAS reaction (arrows). [G & H] parotid and submandibular glands of the STZ + AA group, respectively, showing moderate PAS reaction (arrows). * Significant from control, + Significant from AA group, # Significant from STZ group at p ≤ 0.05.](/content/185/2022/34/7/img/10.1016_j.jksus.2022.102273-fig7.png)
PAS staining in salivary glands, magnification X 400. [A & C] parotid of the control and AA groups, respectively, [B & D] submandibular gland of the control and AA groups, respectively, showing strong PAS reaction (arrows). [E & F] parotid and submandibular glands of the STZ group, respectively, showing weak PAS reaction (arrows). [G & H] parotid and submandibular glands of the STZ + AA group, respectively, showing moderate PAS reaction (arrows). * Significant from control, + Significant from AA group, # Significant from STZ group at p ≤ 0.05.
3.6 Effect of STZ-induced diabetes and/or AA on immunohistochemical results
At p ≤ 0.05, Bcl-2 expression in both glands showed statistically significant regression in STZ group compared to control and AA groups and statistically significant ascent in STZ + AA group compared to STZ group (Fig. 7) and S4 Table.![Immunohistochemical staining for Bcl-2 in salivary glands, magnification X 400. [A & C] parotid of control and AA groups, respectively, showing strong positive Bcl-2 expression in acinar cells (arrows). [B & D] submandibular gland of control and AA groups, respectively, showing strong positive cytoplasmic Bcl-2 expression in acinar and ductal cells (arrows). [E & F] parotid and submandibular glands of STZ group respectively showing weak cytoplasmic Bcl-2 expression (arrows). [G & H] parotid and submandibular glands of STZ + AA group respectively showing moderate positive Bcl-2 expression (arrows). * significant from control, + significant from AA group, # significant from STZ group at p ≤ 0.05.](/content/185/2022/34/7/img/10.1016_j.jksus.2022.102273-fig8.png)
Immunohistochemical staining for Bcl-2 in salivary glands, magnification X 400. [A & C] parotid of control and AA groups, respectively, showing strong positive Bcl-2 expression in acinar cells (arrows). [B & D] submandibular gland of control and AA groups, respectively, showing strong positive cytoplasmic Bcl-2 expression in acinar and ductal cells (arrows). [E & F] parotid and submandibular glands of STZ group respectively showing weak cytoplasmic Bcl-2 expression (arrows). [G & H] parotid and submandibular glands of STZ + AA group respectively showing moderate positive Bcl-2 expression (arrows). * significant from control, + significant from AA group, # significant from STZ group at p ≤ 0.05.
4 Discussion
The intricate physiological and biochemical processes relating to the consumption and digesting of food depend heavily on saliva and greatly affect life quality and social interaction (Pedersen et al., 2018). Xerostomia, dysgeusia, oral candidiasis, and cancer, besides periodontitis and dental caries, are all thought to be more common in people with DM (Verhulst et al., 2019).
Our study aimed to investigate DM's deleterious structural and functional effects on major salivary glands and the impact of AA administration as a powerful antioxidant to moderate these effects.
In the current work, STZ group showed lost normal glandular architecture with irregular acini, cytoplasmic vacuolization in glandular cells with apoptotic nuclei, thickened connective tissue septa with leukocytic infiltration, and congested vessels. This was approved by (Chen et al., 2020; Cui et al., 2021), who reported serous acinar cell deformation and atrophy with diminished secretory granules, vacuolation, and leukocytic infiltration in diabetic rats’ salivary glands. In the herein study, the changes in blood vessels could be attributed to the inflammatory process associated with DM, as stated by (Ror et al., 2018).
In diabetics with chronic hyperglycemia, the non-enzymatic antioxidants, as well as antioxidant enzymes, are significantly reduced in many tissues resulting in sustained ROS formation, which aggravates oxidative stress (Ighodaro, 2018; Singh et al., 2022).
(Ambudkar, 2016) suggested that DM is associated with reduced salivary gland function due to increased lipids peroxidation, intracellular Ca+2 accumulation, increased Ca+2 mobilizing ability of muscarinic receptors, and reduced Ca+2 stores within the smooth endoplasmic reticulum.
In this work, diabetic rats treated with AA showed improved histologic architecture. AA is a natural antioxidant scavenger playing an essential role in cellular defense against oxidative stress via neutralizing intracellular ROS, preventing lipid oxidation and mitochondrial permeability, and upregulation of repair enzymes (Kaźmierczak-Barańska et al., 2020).
By using the tunnelling mechanism, AA and glucose compete with one another to prevent the other from accessing the cell membrane. Additionally, AA acted to conserve the cells from injury by suppressing the discharge of arachidonic acid from the plasma membrane (Ceriello, 2003).
In the current work, we noticed a statistically significant increased collagen deposition in diabetic rats, while AA treated rats showed a statistically significant decreased collagen deposition in both glands. These results are per (El Sadik et al., 2018), who reported many collagen fibers among acini and encircling the striated ducts in the salivary glands of diabetic rats. In contrast, (Cui et al., 2021) stated that the connective tissue component of the submandibular gland was not obviously increased in diabetic rats.
Interestingly, (Rasheed et al., 2021) stated that other antioxidants, such as quercetin flavonoid, have lessened the amount of collagen deposited in between cardiomyocytes in high-fat diet-fed rats.
In our study, the diabetic group showed a statistically significant decreased PAS reaction in acinar and ductal cells. (El Sadik et al., 2018) explained this functional derangement by the reduction of polysaccharide concentration in acini.
However, (Nicolau et al., 2005) observed that enhanced glycogen synthase activity and decreased glycogen phosphorylase activity resulted in glycogen buildup in the salivary glands of diabetic rats.
In AA-treated rats, glycoprotein content showed a statistically significant increase which indicates the ability of AA to protect the functional and physiological integrity of the glands. The effectiveness of salivary gland function depends not only on the amount of secretion but on the precise composition of that secretion as well. Both T1DM and T2DM can alter the salivary function in the form of composition derangement of the secretory product, mainly the glycoprotein (Pappa et al., 2021) and polysaccharide contents (Korac et al., 2021).
In our study, immune-stained sections from the STZ group showed a weak cytoplasmic Bcl-2 expression in ductal and acinar cells of both glands.
This finding was linked by (Monteiro et al., 2017; Jung, 2021) to hyperglycemia, with subsequent oxidative stress and ROS production, which leads to nuclear apoptosis and cellular degeneration in both parotid and submandibular glands; thus, the normal function of salivary acinar cells is not achieved.
In the herein study, the Bcl-2 expression in acini and ducts of salivary glands in AA-treated rats increased significantly. This finding may be explained by AA's ability to prevent apoptosis by increasing Bcl-2 protein, lowering Bax protein, and preventing oxidative mitochondrial DNA damage (Han et al., 2007). Other researchers reported that the administration of Vitamin C before methotrexate injection decreased the number of apoptotic cells in the testicular tissue and improved the histological manifestations (Sayılmaz et al., 2016). (Rasheed et al., 2022) declared that dietary antioxidants like quercetin had upregulated the level of the antiapoptotic protein Bcl-2 in pancreatic and renal tissues in high-fat diet-fed rats, which is crucial for maintaining the viability of live cells.
5 Conclusion
Our study reported the histopathological and functional complications of DM on the major salivary glands and the putative protective role of ascorbic acid through its powerful antioxidant and antiapoptotic properties. Hence, Vitamin C should be supplemented as a protective natural product for the salivary glands in diabetic individuals to improve the quality of their life. However, more thorough research and clinical trials are required to investigate the molecular processes behind ascorbic acid's inhibitory effects on apoptosis.
Acknowledgements
The authors extend their appreciation to the Researchers supporting project number (RSP-2021/189), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Histological study on the protective role of ascorbic acid on cadmium induced cerebral cortical neurotoxicity in adult male albino rats. J Microsc Ultrastruct.. 2016;4(1):36-45.
- [CrossRef] [Google Scholar]
- The ameliorative role of Physalis pubescens L. against neurological impairment associated with streptozotocin induced diabetes in rats. Metab. Brain Dis.. 2021;36(6):1191-1200.
- [CrossRef] [Google Scholar]
- Calcium signalling in salivary gland physiology and dysfunction. J. Physiol.. 2016;594(11):2813-2824.
- [CrossRef] [Google Scholar]
- Thymoquinone Defeats Diabetes-Induced Testicular Damage in Rats Targeting Antioxidant, Inflammatory and Aromatase Expression. Int. J. Mol. Sci.. 2017;18(5):919.
- [CrossRef] [Google Scholar]
- The hematoxylin and eosin., connective and mesenchymal tissues with their stains. In: Suvarna S.K., Layton C., Bancroft J.D., eds. Bancroft’s Theory and Practice of Histological Techniques (7th ed.). Philadelphia: Churchill Living one; 2013. p. :173-212. ch 10 and 11
- [Google Scholar]
- New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetes Care. 2003;26(5):1589-1596.
- [CrossRef] [Google Scholar]
- Decreased basal and stimulated salivary parameters by histopathological lesions and secretory dysfunction of parotid and submandibular glands in rats with type 2 diabetes. Exp. Ther. Med.. 2020;19(4):2707-2719.
- [CrossRef] [Google Scholar]
- Insulin on changes in expressions of aquaporin-1, aquaporin-5, and aquaporin-8 in submandibular salivary glands of rats with Streptozotocin-induced diabetes. Int. J. Clin. Exp. Pathol.. 2021;14(2):221-229.
- [Google Scholar]
- Saliva A review of its role in maintaining oral health and preventing dental disease. BDJ Team. 2015;2(1):11-13.
- [CrossRef] [Google Scholar]
- Postnatal changes in the development of rat submandibular glands in offspring of diabetic mothers: Biochemical, histological and ultrastructural study. PLoS ONE. 2018;13(10)
- [CrossRef] [Google Scholar]
- Salivary gland proteins alterations in the diabetic milieu. J. Mol. Histol.. 2021;52(5):893-904.
- [CrossRef] [Google Scholar]
- Principles for valid histopathologic scoring in research. Vet. Pathol.. 2013;50(6):1007-1015.
- [CrossRef] [Google Scholar]
- Rosinidin flavonoid ameliorates hyperglycemia, lipid pathways and proinflammatory cytokines in streptozotocin-induced diabetic rats. Pharmaceutics. 2022;14(3):547.
- [CrossRef] [Google Scholar]
- Stability of a type 2 diabetes rat model induced by high-fat diet feeding with low-dose streptozotocin injection. J Zhejiang Univ. Sci.. 2018;19(7):559-569.
- [Google Scholar]
- Protective effects of ascorbic acid against lead-induced apoptotic neurodegeneration in the developing rat hippocampus in vivo. Brain Res.. 2007;1185:68-74.
- [CrossRef] [Google Scholar]
- Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother.. 2018;108:656-662.
- [CrossRef] [Google Scholar]
- Hyperglycemia induced apoptosis changes in salivary gland cells of mice. J. Biosci Med.. 2021;9:143-157.
- [CrossRef] [Google Scholar]
- Mitochondrial ROS formation in the pathogenesis of diabetic cardiomyopathy. Front. Cardiovasc. Med.. 2020;7:12.
- [CrossRef] [Google Scholar]
- Two faces of vitamin C-antioxidative and pro-oxidative agent. Nutrients. 2020;12(5):1501.
- [CrossRef] [Google Scholar]
- Redox changes in obesity, metabolic syndrome, and diabetes. Redox Biol.. 2021;42:101887
- [CrossRef] [Google Scholar]
- Long- and short-term diabetes mellitus type 1 modify young and elder rat salivary glands morphology. Arch. Oral Biol.. 2017;73:40-47.
- [CrossRef] [Google Scholar]
- Altered glycogen metabolism in the submandibular and parotid salivary glands of rats with streptozotocin-induced diabetes. J. Oral Sci.. 2005;47(2):111-116.
- [CrossRef] [Google Scholar]
- Immunohistochemical localization of osteoblast activating peptide in the mouse kidney. Acta Histochem.. 2018;120(4):323-328.
- [CrossRef] [Google Scholar]
- Downregulation of salivary proteins, protective against dental caries, in type 1 diabetes. Proteomes. 2021;9(3):33.
- [CrossRef] [Google Scholar]
- Salivary functions in mastication, taste and textural perception, swallowing and initial digestion. Oral Dis.. 2018;24(8):1399-1416.
- [CrossRef] [Google Scholar]
- The possible ameliorative Influence of quercetin on cardiac muscle changes induced by high fat diet in adult male albino rats: light and electron microscopic study. Egypt. J. Histol. 2021
- [CrossRef] [Google Scholar]
- Quercetin mitigates the adverse effects of high fat diet on pancreatic and renal tissues in adult male albino rats. J. King Saud Univ. Sci.. 2022;34(4):101946.
- [CrossRef] [Google Scholar]
- Oral manifestations in patients with diabetes mellitus. World J Diabetes.. 2019;10(9):485-489.
- [CrossRef] [Google Scholar]
- N-acetylcysteine., anti-CD4/CD8 antibodies., and physical exercise reduces histopathological damage in salivary glands of spontaneously diabetic mice. Austin Endocrinol. Diab. Case Rep.. 2018;3(1):1013-1018.
- [Google Scholar]
- Role of ascorbic acid in diabetes mellitus: A comprehensive review. Med. Biol. 2017
- [CrossRef] [Google Scholar]
- The histopathological evaluation of healing effects of vitamin C administered before methotrexate therapy on testicular injury induced by methotrexate. Turk. J. Urol.. 2016;42(4):235-239.
- [CrossRef] [Google Scholar]
- Ascorbic acid supplementation in type 2 diabetes mellitus: A protocol for systematic review and meta-analysis. Medicine. 2020;99(45):e23125.
- [Google Scholar]
- Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2. Diabetes. 2022;27(3):950.
- [CrossRef] [Google Scholar]
- Evaluating all potential oral complications of diabetes mellitus. Front. Endocrinol.. 2019;10:56.
- [CrossRef] [Google Scholar]
- Evaluation of parotid salivary glucose level for clinical diagnosis and monitoring type 2 diabetes mellitus patients. Biomed Res. Int.. 2017;2017:1-5.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102273.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







