Translate this page into:
Application of Candida tropicalis MK-160 for the production of xylanase and ethanol
⁎Corresponding author. msohail@uok.edu.pk (Muhammad Sohail)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Xylanase is the group of enzymes that catalyze the hydrolysis of 1, 4-β-d-xylosidic linkages in xylan, the most abundant hemicellulose moiety in plant cell-wall. Bacteria and filamentous fungi are considered as efficient xylanases producers, whereas, yeasts have scarcely been reported for their xylanolytic potential. In the present study, out of 225 indigenous yeast strains, 84 strains were able to produce xylanase in xylan supplemented agar medium, amongst which 40 strains yielded higher titers (>0.2 IU mL−1) in broth medium. The parameters for xylanase production from the most promising strain, MK-160, were optimized as temperature, 40 °C; pH, 7; and substrate concentration, 0.5%. However, the characterization of xylanase activity in cell-free culture supernatant revealed that the enzyme displayed optimal activity under different set of conditions i.e. at 40 °C, at acidic pH and in presence of 2% xylan. The strain was identified on the basis of morphological and cultural characteristics as Candida tropicalis MK-160. The strain showed the ability to grow on sugarcane-bagasse and wheat bran with the concomitant production of higher titers of xylanase and low levels of endoglucanase (EG). It was able to produce 5.45% ethanol in glucose supplemented medium. Furthermore, the cells of C. tropicalis MK-160 removed more than 90% of an azo-dye, congo red from aqueous solution. The data showed that the strain can further be evaluated for its potential to use for waste management and some other biotechnological processes.
Keywords
Xylanase
Endoglucanase
Candida tropicalis
Xylan
Ethanol
Sugarcane bagasse
1 Introduction
Lignocellulose (LC) is composed of three main components: (38–50%) cellulose, (17–32%) hemicelluloses and (15–30%) lignin (Ritter, 2008). Xylan, the major hemicellulosic polysaccharide and the second most abundant natural biopolymer (Timell, 1967), is comprised of xylopyranosyl residues linked by β-1, 4- glycosidic bonds (Flores et al., 1997). The complete hydrolysis of xylan requires concerted actions of few enzymes where endo-1, 4-β-xylanase and β- xylosidase play pivotal roles (Polizeli et al., 2005). Endo-1, 4-β-xylanase cleaves the internal chain of xylosidic linkages and β- xylosidase yields xylosyl residues by attacking on xylooligosaccharides (Subramaniyan and Prema, 2002).
Xylan degrading enzymes or xylanases are applied in food and beverage industries for the production of hydrolysates (Sreenath et al., 1996). These are used to improve digestibility of nutrients in animal feed (Ahmad et al., 2013). One of the major applications of xylanase is in bleaching of kraft pulp in pulp and paper industry (Patel and Savanth, 2015). Moreover, their role in the production of a natural sweetener, xylitol, has also been reported (Usama et al., 2013).
Mostly, filamentous fungi (Adesina and Onilude, 2013; Cacais et al., 2001) and bacteria (Rajagopalan et al., 2013; Subramaniyan and Prema, 2002) are employed for the production of xylanase. Whereas, xylanolytic yeasts have not been cited frequently (Lara et al., 2014; Lopes et al., 2011); few of the notable examples of xylanolytic yeasts include Cryptococcus albidus (Biely et al., 1978), Aureobasidium pullulans (Leathers et al., 1984), Pichia stiptis (Lee et al., 1987), Cryptococcus, Fellomyces (Gomes et al., 2015) and Candida (Otero et al., 2015). Yeasts provide some advantages, such as they grow rapidly than filamentous fungi and do not produce hyphae, hence rheological problems can be avoided. Moreover, xylanase can be obtained as a by-product of single cell protein. Yeasts are considered as a model systems for studying the mechanisms of gene expression (Bastawde et al., 1994). Therefore, it is imperative to isolate and screen indigenous yeast strains for their xylanolytic potential and to explore some biotechnological application, particularly for the utilization of LC substrates. The present study describes the isolation of xylanolytic yeasts, optimization of parameters for the production of xylanase from a promising strain, Candida tropicalis MK-160, and its ability to utilize sugarcane-bagasse (SB) and wheat bran (WB). Previously, the strain MTCC 6192 of C. tropicalis was cultivated on corn-cob hydrolysate for the production of xylitol (Kumar et al., 2017) whereas, the strain HNMA-1 was grown on SB hydrolysate for bioethanol production (Nouri et al., 2017). However, the indigenous strain MK-160 was found to produce higher titers of xylanase on SB and WB, produced ethanol and its cells were able to remove an azo-dye.
2 Experimental section
2.1 Isolation and screening of yeast strains
Different environmental and food samples including garden soil, plant parts, grapes, lemon, green chili and orange juice were used for the isolation of indigenous yeast strains. Ten-fold serial dilutions were prepared and diluted aliquots were plated on Sabouraud’s dextrose agar (SDA), Potato dextrose agar (PDA), yeast extract glucose peptone agar and Oxytetracycline glucose yeast extract agar. All media were purchased from Oxoid, UK. Colonial characteristics were noted and yeasts-like colonies were maintained on SDA and preserved with 60% (v/v) glycerol. In addition to the isolated yeasts, some strains were also retrieved from the lab of corresponding author, Department of Microbiology, University of Karachi, Pakistan, and maintained on SDA.
The isolated and retrieved strains (225) were screened for the production of xylanase by cultivating on beechwood xylan supplemented mineral salt medium (MSM; g L−1, KH2PO4 20, (NH4)2SO4 14, MgSO4.7H2O 3, CaCl2 3, protease peptone 10, FeSO4.7H2O 0.5, MnSO4.H2O 0.16, ZnSO4.7H2O 0.29, CoCl2.6H2O 0.29) agar plates. Qualitative and quantitative screening were carried according to Sohail et al. (2009).
2.2 Cultural and morphological identification
The selected strain, MK-160, was identified on the basis of cultural, morphological and biochemical tests including colonial morphology, acid production from glucose, fermentation of carbohydrates, tolerance to 1% acetic acid, germ tube test and growth on CHROM agar (Kurtzman and Fell, 1998).
2.3 Optimization of factors affecting xylanase production and activity
Various parameters affecting xylanase production by the strain MK-160 were optimized by adopting one-factor-at-a-time (OFAT) approach. Three different media compositions namely, MSM, Yeast extract-peptone medium (YEP) and Peptone medium (PM) were used for the cultivation of the strain and xylanase activity was determined in cell-free culture supernatant (CFCS). The effect of incubation temperature on xylanase production was determined by cultivating the strain in the most suitable medium at varying temperatures (25°, 30°, 35° and 40 °C) followed by performing xylanase assay (Kumari et al., 2017). Similarly, optimum pH for the xylanase production was investigated by cultivating the strain in the medium with pH adjusted to 4.0, 5.0, 6.0 or 7.0 at the optimum temperature. Likewise, the effect of carbon source (glucose, maltose, sucrose, xylose and xylan) and nitrogen source (yeast extract, peptone and urea), concentration of xylan (0.5–2%) and inoculum size (1% to 5%) on the xylanase production were studied, one after another. To investigate growth and enzyme production kinetics, the strain was cultivated in the suitable medium for 72 h by keeping all the parameters set at optimum level, as previously investigated. Aliquots were taken intermittently and OD600 (optical density at 600 nm) was noted. CFCS obtained from aliquots were assayed for the xylanase activity (Kumari et al., 2017). Xylanase produced by the strain MK-160 was characterized for the effect of temperature, pH, substrate concentration and presence of metallic ions (Sohail et al., 2011).
2.4 Cultivation on sugarcane-bagasse and wheat-bran
Sugarcane-bagasse (SB) and Wheat-bran (WB) obtained from local market, were dried and ground to 100 mesh size. Alkali and Hydrogen peroxide were used separately for pretreatment. Briefly, the substrates were pretreated with 1% KOH (in solid liquid ratio 1:4) and with 2% solution of hydrogen peroxide (0.5 gm H2O2/gm biomass) for 1 h at room temperature. The substrates were washed thoroughly with distilled water until pH of the wash-through became neutral and were dried at 50 °C until constant mass. The pretreated substrates (1%) were added in the MSM medium and sterilized by autoclaving at 121 °C for 20 min. Inoculum (5%) of C. tropicalis MK-160 was inoculated and cultivated under submerged conditions with previously known optimized factors for 3 days. CFCS were obtained and assayed for the xylanase activity.
2.5 Ethanol production and dye-adsorption
The ability of the yeast strain MK-160 to tolerate ethanol was studied with different concentration of ethanol ranging from 0% to 16% in growth media. The strain was inoculated to the medium with ethanol and incubated for 72 h at 30 °C. The growth was monitored spectrophotometrically by taking optical density (OD) at 600 nm. For ethanol production, the strain was cultivated in complex medium for 72 h and ethanol production was determined qualitatively, as well as, quantitatively (Shukla et al., 2011). Briefly, medium after cultivation was centrifuged and supernatant was distilled. The reagent (0.5 mL H2SO4 and 1 mL K2Cr2O7) was added in 1 mL of distilled solution, the mixture was incubated at 60 °C for 15 min and volume was made up to 3 mL with distilled water. Blue green color indicated the presence of ethanol. Quantitative estimation of ethanol was carried out by adopting the same method but taking OD at 600 nm (OD600) and compared with standard curve of different concentration of ethanol (Caputi et al., 1968).
Dye absorption ability of the yeast cells was studied using yeast grown in SDB until OD600 reached to 1.0 and transferred to congo red solution with a final concentration of 20–100 ppm. The experiment was conducted in 100 mL Erlenmeyer flask with a working volume of 50 mL. Dye absorption was investigated by taking OD497 intermittently.
3 Results and discussion
Xylanase along with cellulase are inevitably required for the LC based biorefineries to convert complex substrates. The production of xylanase from filamentous fungi limit the exploitation of the same strain for the diversified nature of biorefinery processes. However, xylanase producing yeasts can be employed for subsequent steps in biorefineries and hence are remained subject of intense research. In this context, new and novel strains are studied for possible biotechnological applications.
3.1 Isolation and screening of xylanase producing yeast strains
From 11 environmental samples, total 101 yeast strains were isolated. Together with the isolated strains, 124 strains were retrieved from the departmental culture collection. Out of 225 strains, only 84 strains showed zones of clearance around the colonies on xylan supplemented medium stained with Congo red. Plate screening with congo red is a popular method in which the dye remains attached to the polymer and the halo produced by hydrolysis of cellulose or xylan is directly related to the region of action of the corresponding enzymes (Florencio et al., 2012). Relatively less abundance of xylanolytic yeasts has also been reported by Otero et al. (2015) where only 23 strains were found positive out of 119 yeast strains. In this study, the isolates found positive in initial screening were cultivated in MSM broth containing beechwood xylan as sole source of carbon and CFCS was assayed for xylanase activity. The results showed that only 40 strains produced more than 0.2 IU mL−1 xylanase. On the basis of stability and titers, one of the strains, MK-160, was selected for further studies and was identified on cultural, morphological and biochemical grounds (Table 1) as Candida tropicalis.
Characteristics
C. tropicalis
Colony color
Cream
Colony shape
Round
Size
0.1–0.4 mm approx.
Texture
Smooth
Margin
Entire
Elevation
Raised
Cell type
Budding
Germ tube
Negative
Sugar fermentation
Glucose, Galactose, Maltose, Sucrose
Tolerance of 1% acetic acid
Negative
Acid production from glucose
Negative
CHROM agar colony color
Dark Blue
Hyphae/Pseudohyphae
Varied/positive
3.2 Optimization of factors affecting xylanase production from C. Tropicalis MK-160
Initially, the effect of media composition on xylanase production from C. tropicalis MK-160 was investigated and the highest level of xylanase (12.3 IU mL−1) was obtained when the strain was grown in YEP medium (Fig. S1). The effect of carbon and nitrogen source on the production of xylanase was studied by replacing the sources of carbon and nitrogen. It was observed that beechwood xylan appeared as a potent inducer of xylanase (Fig. S2) that was in agreement with the findings of Azeri et al. (2010) for xylanase production from various strains of Bacillus sp. Indeed higher titers of xylanase were also yielded from MK-160 in the presence of glucose which can be exploited in the designing of inexpensive production medium. Subsequently, the amount of xylan in the medium was optimized to 0.5% (Fig. S3) for xylanase production. There was drastic decrease in the xylanase titers when the concentration of the substrate was increased to 2%. Among nitrogen sources tested, presence of peptone enhanced the production of xylanase from the strain MK-160 (Fig. S4). The effect can be linked with higher growth obtained in presence of peptone (Nathan et al., 2014), however, the utilization of cost effective nitrogenous compounds would offer cost competitiveness.
When the effect of temperature on the xylanase production from MK-160 was studied, it was observed that there was not much variation in the titers of xylanase with the variation in temperature from 25 to 40 °C (Fig. S5). However, slightly higher titers of xylanase were obtained at 40 °C when the pH of the medium was adjusted to 7 (Fig. S6). Production of xylanase and cellulase from C. tropicalis at a wide range of temperature was also reported by Mattam et al. (2016). This property can be exploited to produce enzyme without investing on equipment to control the temperature.
Xylanase production from yeasts is generally initiated by a smaller inoculum size as reported by Thomas et al. (2015) for the production recombinant xylanase from Kluyveromces lactis. Similar observation was made for the strain MK-160 where an inoculum size of 4% was found as optimal for xylanase production (Fig. S7). It might be attributed to the fact that higher inoculum size hinders access to the nutrient and hence adversely affects metabolic activities (Bhatt et al., 2012).
Nonetheless, after optimization of various factors (Table 2), the xylanase titers as high as 23.6 IU mL−1 were obtained from MK-160 that showed an increase by two folds from the initial experiments.
Production parameter
Optimized conditions
Production media
YEP media
Temperature
40 °C
Carbon source
Beechwood Xylan
Nitrogen source
Peptone
pH
7
Substrate concentration
0.5%
Inoculum size
4%
Under optimized conditions, the growth and xylanase production kinetics from MK-160 was studied in YEP-xylan medium by taking aliquots periodically during cultivation. There was a continuous increase in OD600 accompanied with the production of the enzyme showed growth-linked expression of xylanase. The amount of reducing sugars in the medium was also increased concomitantly indicating the utilization of substrate by the strain. In the beginning of the experiment, pH of the production medium was slightly decreased to 6.4 but during 52–72 h there was an increase and it reached to 7.5 (Fig. 1) that can be linked with the autolysis of the cells and release of amines or amides.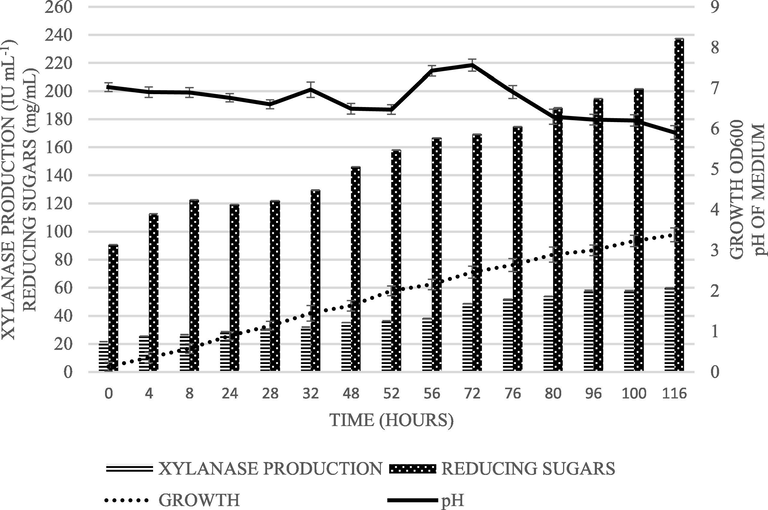
The strain MK-160 was cultivated in YEP-xylan broth and aliquots were collected intermittently. Growth, reducing sugars, xylanase activity and pH were monitored.
3.3 Optimization of conditions for the xylanase activity from C. Tropicalis MK-160
The xylanase activity in CFCS was characterized for temperature optima and it was revealed that xylanase retained its activity over a wide range of temperature ranging from 30 to 50 °C with an optimum at 45 °C (Fig. S8) that was very close to the optimum temperature (50 °C) reported earlier for the xylanase activity from Trichoderma inhamatum (Silva et al. 2015).
Literature reports describe varying pH as optimum for xylanase activity from filamentous fungi and yeasts. For instance, pH 2.0 was found as optimum for xylanase from Cryptococcus sp. S-2 (Lefuji et al., 1996), whereas xylanase from Cladosporium oxysporum exhibited maximum activity at pH 8.0 (Guan et al., 2016). Xylanase from MK-160 showed an interesting feature being most active at pH 2.5 with a gradual decrease in activity till the pH 4.5 followed by an increase in the activity (Fig. S9). It can be attributed to the formation of isozyme by this strain.
Xylanase from MK-160 showed comparable activity (22.4–22.7 IU.mL−1) in presence of either 0.5% or 2% beechwood xylan (Fig. S10), and activity reached to its maximum levels within 5 min (Fig. S11) that may be attributed to the affinity of this enzyme for its substrate.
Hemicellulolytic enzymes are frequently applied along with cellulase to saccharify the crude agro-industrial waste materials which are usually contaminated with various chemicals. The chemicals, particularly, mineral ions exert multifaceted actions on enzyme. Some of the ions can stabilize the structure of protein by interacting with the amino acids, on the other hand, some ions can disrupt non-covalent interactions among amino acids. Therefore, xylanase activity and stability from MK-160 was determined in presence of different metal solutions and chemicals at two different concentration i.e. 1 mM and 5 mM (Table 3). The presence of PO4−2, Co+2, Cu+2, or Ca+2 enhanced xylanase activity, whereas, Na+, Mg+2, Mn+2 and K+ appeared as inhibitors. Earlier, activation of xylanase from T. harzianum 1073 D3 from few divalent ions was reported by Isil and Nilufer (2005). The difference in the observation during the present study from the previous studies may be linked with the differences in the structural features of the enzymes from filamentous fungi and yeasts.
Chemicals
Xylanase activity (%) in presence of chemicals at concentration
1 mM
5 mM
Control
100
100
NaCl
88.81
78.94
MgSO4.7H2O
98.68
78.94
KH2PO4
130.92
116.44
CaCl2
105.26
113.48
MnSO4.5H2O
84.86
88.157
KCl
8.81
86.84
CoCl2
115.13
108.55
EDTA
84.21
106.57
UREA
106.57
105.263
CuCl2
91.44
103.28
3.4 Cultivation of MK-160 on sugarcane-bagasse and wheat-bran
Pakistan is an agricultural country that produces millions of tons of agro-wastes, most of which remain under-utilized. Utilization of these waste materials to produce value added products and enzymes can boost the country’s agricultural and commercial sectors. The utilization of sugarcane-bagasse (SB) and wheat bran (WB), two of the most abundant agro-wastes, was evaluated for the production of xylanase from MK-160. As the crude substrates were rich in cellulose, therefore, co-production of endoglucanase (EG) was also studied. The data inferred that there was a significant difference in the production of xylanase in SB and WB. Untreated SB proved to be a better substrate (Fig. 2) for xylanase production (20.424 IU mL−1) followed by alkali pretreated (17.152 IU mL−1) and H2O2 pretreated (16.64 IU mL−1) SB. It might be due to the release of inhibitors during the pretreatment of SB. Nonetheless, the cost of pretreatment of SB can be avoided if the strain finds application on industrial scale. Whereas, the alkaline pretreatment given to a different substrate, WB, proved to better, as cultivation on this substrate yielded higher titers (9.11 IU mL−1) than that of untreated WB (5.8 IU mL−1) and H2O2 pretreated WB (3.924 IU mL−1). The production of very low titers of EG was noted during cultivation of MK-160 on SB or WB, therefore, the strain can be regarded as true-xylanolytic strain. Moreover, the production of xylanase by the strain MK-160 was higher than that of some literature reports, such as, Bacillus safensis P20 produced 4.06 U mL−1 of xylanase at 20 °C in four days in a medium containing 1.5% SB, lactose and urea (Rahmani et al., 2014).
Xylanase (Xyl) and endoglucanase (EG) production (IU mL−1) from Candida tropicalis MK-160 under submerged fermentation of sugarcane bagasse and wheat bran.
3.5 Ethanol production and dye adsorption
The strain-MK160 was found to grow in presence of 8% of alcohol. The cultivation of this strain in commercial medium yielded an amount equivalent to 5.45% (v/v) ethanol that was greater than obtained by O’Leary (1977) from Kluyveromyces fragilis, but lower than that was obtained from Saccharomyces cerevisiae RL-11 (Mussato et al., 2012) and S. stipitis CBS-6054 (Scordia et al., 2012) i.e. 14.8% (or 11.7 g/L) and 10.4% (8.2 g/L), respectively. Nonetheless, studies on optimization of conditions to produce ethanol by cultivating the strain MK-160 on crude substrate are required to ascertain future prospects of this strain.
Yeast cells have been reported to absorb dyes present in effluent. In Pakistan, textile industries export products of worth millions of dollars and generate a huge revenue. However, textile effluent mostly contains certain classes of dyes which are not biodegradable. As a pretreatment step in industries, adsorption techniques are widely used for the removal of dyes (Crini, 2006). In this context, the strain MK-160 was also studied for its ability to adsorb an azo-dye, congo red. The results showed that 96% of the dye in aqueous solution was removed by the yeast cells after 29 h at 30 °C and 150 rpm, which shows that this organism has interesting feature to remove azo dye.
The ability of the strain to produce xylanase by utilizing crude substrates and to produce ethanol along with the ability to remove an azo-dye, merits its candidature for future biotechnological application.
4 Conclusions
This study concluded relatively less abundance of xylanolytic yeasts in environment. After optimizing the parameters to produce xylanase by the strain MK-160 of C. tropicalis, the titers of xylanase were increased to 23.6 IU mL−1. The strain also produced higher levels of xylanase when cultivated on untreated or pretreated sugarcane-bagasse or wheat-bran. It also showed ability to produce ethanol in commercial medium. Furthermore, the strain was also found effective in removing an azo-dye from solution and hence can find various biotechnological applications.
Acknowledgement
Authors are grateful to Third World Academy of Sciences (TWAS) for supporting this work vide grant number 10-134 to MS.
Statement of Conflict of interest
The authors do not declare any conflict of interest.
References
- Isolation, identification and screening of Xylanase and glucanase-producing microfungi from degrading wood in Nigeria. Afr. J. Agric. Res.. 2013;8:4414-4421.
- [Google Scholar]
- Effect of oral application of Xylanase on some hematological and serum biochemical parameters in broilers. Pak. Vet. J.. 2013;33:388-390.
- [Google Scholar]
- Thermoactive cellulase free Xylanase production from alkaliphilic Bacillus strains using various agro-residues and their potential in biobleachng of kraft pulp. Afr. J. Biotechnol.. 2010;9:63-72.
- [Google Scholar]
- Optimization of cellulase-free xylanase production by a novel yeast strain. J. Indust. Microbiol.. 1994;13:220-224.
- [Google Scholar]
- Production of Xylanase by Aspergillus flavus FPDN1 on Pearl millet bran: optimization of culture conditions and application in bioethanol production. Int. J. Res. Chem. Env.. 2012;2:204-210.
- [Google Scholar]
- Xylan degrading activity in yeast: growth on xylose, xylan and hemicelluloses. Folia Microbiol.. 1978;27:366-371.
- [Google Scholar]
- Production of xylan-degrading enzymes by a Trichoderma harzianum strain. Braz. J. Microbiol.. 2001;32:141-143.
- [Google Scholar]
- Spectrophotometric determination of ethanol in wine. Am. J. Enol. Vitic.. 1968;19:160-165.
- [Google Scholar]
- Non-conventional low-cost adsorbents for dye removal: a review. Bioresour. Technol.. 2006;97:1061-1085.
- [Google Scholar]
- Correlation between Agar Plate screening and solid-state fermentation for the prediction of cellulase production by Trichoderma Strains. Enzyme Res. 2012
- [CrossRef] [Google Scholar]
- β-Xylosidase and Xylanase characterization and production by streptomyces sp.CH-M-1035. Lett. Appl. Microbiol.. 1997;24:410-416.
- [Google Scholar]
- The diversity and extracellular enzymatic activities of yeasts isolated from water tanks of Vriesea minarum, an endangered bromeliad species in Brazil, and the description of Occultifur brasiliensis f.a., sp. Nov. Antonie. Van. Leeuwenhoek.. 2015;107:597-611.
- [Google Scholar]
- Production and partial characterization of an Alkaline Xylanase from a novel fungus cladosporium oxysporum. Res. Int. BioMed 2016
- [CrossRef] [Google Scholar]
- Investigation of factors affecting xylanase activity from Trichoderma harzianum 1073 D3. Braz. Arch. Biol. Technol.. 2005;48:187-193.
- [Google Scholar]
- Efficient detoxification of corn cob hydrolysate with ion-exchange resin for enhanced xylitol production by Candida tropicalis MTCC 6192. Bioresour. Technol. 2017
- [CrossRef] [Google Scholar]
- Plant cell wall hydrolyzing enzymes from indigenously isolated fungi grown on conventional and novel natural substrates. Pak. J. Bot.. 2017;49:745-750.
- [Google Scholar]
- The Yeast, a Taxonomic study. Amsterdam: Elsevier; 1998.
- Identification and characterization of xylanolytic yeasts isolated from decaying wood and sugarcane bagasse in Brazil. Antonie. Van. Leeuwenhoek.. 2014;105:1107-1119.
- [Google Scholar]
- Overproduction and regulation of xylanase inAureobasidium pullulans andCryptococcus albidus. Biotechnol. Bioeng. Symp.. 1984;14:225-240.
- [Google Scholar]
- Some properties of extracellular acetylxylan esterase produced by the yeastRhodotorula muciloginosa. Appl. Environ. Microbiol.. 1987;53:2831-2834.
- [Google Scholar]
- Acid Xylanase from Yeast Cryptococcus sp. S-2: Purification, Characterization, Cloning, and Sequencing. Biosci. Biotechnol. Biochem.. 1996;60:1331-1338.
- [CrossRef] [Google Scholar]
- Thermo-stable Xylanase s from non-conventional yeasts. J. Microbiol. Biochem. Technol.. 2011;3:36-42.
- [Google Scholar]
- Cellulolytic enzyme expression and simultaneous conversion of lignocellulosic sugars into ethanol and Xylitol by a new Candida tropicalis strain. Biotechnol. Biofuels.. 2016;9:157.
- [CrossRef] [Google Scholar]
- Sugar metabolism and ethanol production by different yeast strains from coffee industry wastes hydrolysates. Appl. Energy.. 2012;92:763-768.
- [Google Scholar]
- Process optimization and production kinetics for cellulase production by Trichoderma viride VKF3. SpringerPlus. 2014;3:92-103.
- [Google Scholar]
- Xylan-hydrolyzing thermotolerant Candida tropicalis HNMA-1 for bioethanol production from sugarcane bagasse hydrolysate. Ann. Microbiol.. 2017;67:633-641.
- [CrossRef] [Google Scholar]
- Alcohol production by selected yeast strains in lactase-hydrolyzed acid whey. Biotechnol. Bioeng.. 1977;19:1019-1035.
- [Google Scholar]
- Screening of yeasts capable of producing cellulase-free Xylanase. Afr. J. Biotechnol.. 2015;14:1961-1969.
- [Google Scholar]
- Review on fungal Xylanase s and their applications. Int. J. Adv. Res.. 2015;3:311-315.
- [Google Scholar]
- Xylanase s from fungi: properties and industrial applications. Rev. Appl. Microbiol. Biotechnol.. 2005;67:577-591.
- [Google Scholar]
- Optimization of production Xylanase from marine bacterium bacillus safensis P20 on sugarcane baggase by submerged fermentation. Int. J. Adv. Sci. Eng. Info. Technol.. 2014;4:31-34. ISSN: 2088-5334
- [Google Scholar]
- Production, purification and characterization of a Xylooligosaccharides forming Xylanase from high butanol producing strain Clostridium sp. BOH3. Bioenerg. Res.. 2013;6:448-457.
- [Google Scholar]
- Bioconversion of giant reed (Arundo donax L.) hemicellulose hydrolysate to ethanol by Schefferssomyces stipitis CBS6054. Biomass Bioenergy. 2012;39:296-305.
- [Google Scholar]
- Biotechnological potential of bacterial flora from Cheend juice: alcoholic beverage from Bastar, India. Nat. Sci.. 2011;9:62-66.
- [Google Scholar]
- Purification and characterization of Xylanase s from Trichoderma inhamatum. Electronic J. Biotechnol.. 2015;18:307-313.
- [Google Scholar]
- Production of cellulases from Alternaria sp. MS28 and their partial characterization. Pak. J. Bot.. 2011;43:3001-3006.
- [Google Scholar]
- Cellulase production from Aspergillus niger MS28: effect of temperature and pH. New Biotecnol.. 2009;25:437-441.
- [Google Scholar]
- Enzymatic polishing of jute/cotton blended fabrics. J. Ferment. Bioeng.. 1996;81:18-20.
- [Google Scholar]
- Biotechnology of microbial Xylanase s: enzymology, molecular biology and application. Cri. Rev. Biotechnol.. 2002;22:33-46.
- [Google Scholar]
- Production of an alkaline xylanase from recombinant Kluyveromyces lactis (KY1) by submerged fermentation and its application in bio-bleaching. Biochem. Eng. J.. 2015;102:24-30.
- [Google Scholar]
- Recent progress in the chemistry of wood hemicelluloses. Wood Sci. Technol.. 1967;1:45-70.
- [Google Scholar]
- Ethanol and Xylitol production from Xylanase broth of Thermomyces lanuginosus grown on some lignocellulosic wastes using Candida tropicalis EMCC2. Life. Sci. J.. 2013;10:968-978.
- [Google Scholar]
- Xylitol production by Candida tropicalis MTCC 6192.https://doi.org/10.1016/j.biortech.2017.11.039
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jksus.2018.04.009.
Appendix A
Supplementary data







