Translate this page into:
Antioxidant and antiplasmodial activities of Malus sikkimenmsis bioactives and their role in the suppression of LPS-induced neuroinflammation in glial cells
⁎Corresponding author. shasdixit@rediffmail.com (Shalini Dixit)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
As a part of our continued effort to isolate and identify the pharmacological properties of polyphenols from Malus Sikkimensis, here in this work we have evaluated the neuroprotective effects of these polyphenols along with their antioxidant and antiplasmodial properties. The antioxidant potential of these bioactives was estimated using enzymatic and non-enzymatic methods of free radical generation, where phloretin (PT) displayed better antioxidant potential under both the conditions. Additionally, PT also exhibited significant antiplasmodial activity. Phloridzin (PZ) and phloretin (PT) were also studied for their effect on lipopolysaccharide (LPS) induced neuro-inflammation in rat (C6) glioma cells. Our results showed that pre-treatment of PZ and PT at 5 and 10 µg/ml concentrations significantly (p < 0.05) suppressed the release of pro-inflammatory cytokines (TNF-α and IL-6) in a dose dependent manner. Additionally, PT exhibited more potent anti-inflammatory activity than PZ without any cytotoxic effects, as estimated using MTT assay. These findings suggest that PT possessed promising anti-neuroinflammatory potential along with antioxidant and antiplasmodial activities, which may be enhanced further by structural modification and chemical derivatization.
Keywords
Malus sikkimensis
Neuroinflammation
Glial cells
Antioxidant
Antiplasmodial
Phloretin
Phloridzin
1 Introduction
Apples are very significant source of phytochemicals among which flavonoids are the major one. Phytochemicals including flavonoids may restrain cancer cell proliferation, regulate inflammatory and immune response and act against lipid oxidation (Liu, 2003). Malus sikkimensis or Sikkim crab apple native to Arunanchal Pradesh, India is a good and known source of various polyphenols. Shao et al. (2008) examined juice and fruit tissues from 321 species of Malus genus and reported highest phenolic content in case of M. sikkimensis. Industrial values of fruit have long been recognized, hence leaves seems to be of immense and untapped potential because of the presence of significant amount of polyphenols. According to a study carried out by Petkovsek et al., (2010), apple leaves contain significant amounts of phloridzin (phloretin 2′-O-glucoside or phlorizoside) which is a phenolic compound and an important member of dihydrochalcones also having potential for the treatment of many physiological disorders (Gosch et al., 2009). Phloridzin's produces renal glycosuria and block intestinal glucose absorption by inhibiting sodium-glucose symporters. Benefits of phloridzin and its analogs have been also explored for the treatment of diabetes, obesity stress and hyperglycemia (Ehrenkranz et al., 2005).
Neuroinflammation is generally marked by brain inflammation initiated by the activation of microglial cells (Block et al., 2007). Microglial cells under normal physiological conditions maintain and regulated the central nervous system. However activation of microglial cells in response to injury, harmful toxins, infection or inflammation, supports the release of a variety of inflammatory mediators including interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α), nitric oxide (NO), prostaglandin E2 (PGE2), chemokines, and the generation of reactive oxygen/nitrogen species (Hirsch and Hunot, 2009). Neuroinflammation mechanisms are well observed in the pathology of neurodegenerative and acute diseases (Glass et al., 2010). In homeostatic conditions, microglial cells remain at resting phase and play role in protecting central nervous system (CNS) as well as brain against various insults such as infection, injury or disease (Perry and Teeling, 2013). Microglia sense injury and infection through toll-like receptors (TLR) that regulate the action of AP-1, NF-kappa B and signalling-dependent transcription factors leading to inflammatory signalling cascade (Bi et al., 2005). Its activation leads to efficient immune responses but the inflammatory mechanism also has the potential to cause neuronal dysfunction if the responses are not appropriately resolved. Inflammatory agents such as lipopolysaccharide (LPS) are effective activators of the immune response stimulating the migration and activity of mononuclear phagocytes (Guerra et al., 2011). LPS promotes changes in protein expression triggered by its binding to Toll like receptor 4 (TLR4) in astrocytes. Exposure to LPS leads to the translocation of nuclear factor kappa B (NFκB) into the nucleus, inducing the transcription of inflammatory genes, release of nitric oxide (NO), and the overproduction of ROS (Block et al., 2007; Wagay et al., 2019). Natural products bearing potential antioxidant properties have been also found to possess antimalarial properties thus are also in use traditionally for the treatment of malaria in endemic zones (Singh et al., 2017). Phlorizin (phloretin-2-beta-glucoside) has been also reported to possess anti-parasitic properties (Kutner et al., 1987) hence we have also estimated the antimalarial potential of bioactives isolated from M. sikkimensis. In recent years, studies are constrained to identify the novel agents as antioxidant molecule, since oxidative stress was notified to accelerate the events responsible for the onset of inflammation (Khansari et al., 2009). Oxidative stress along with inflammation may mediate most of the chronic diseases including cancer, diabetes, cardiovascular, neurological and pulmonary diseases (Willcox et al., 2004). Therefore chemotypes bearing both antioxidant and anti-inflammatory properties seems a novel approach to minimize ailments burden as well as precaution to various severe health ailments.
In the present study, we have isolated two major bioactives form Malus sikkimensis and studied their antioxidant and antiplasmodial potential as well as their effect on LPS induced neuroinflammation on C6 rat glial cell. The results of the present study under in vitro conditions clearly suggest that phloridzin and phloretin possesses significant antioxidant, antiplasmodial and anti-inflammatory properties, hence suggestive for further detailed study using in vivo models and chemical derivatization of these phytomolecules.
2 Materials and methods
2.1 Plant material and isolation of bioactives
Leaves of Malus sikkimensis were collected from Lachung of Gangtok, Sikkim in April 2014. The taxonomical authentication was done by Dr. L K Rai of G. B. Pant Himalayan Institute, Gangtok, India. A specimen voucher (gbp-sikkim/46a) was deposited to the herbarium of institute. The shade dried plant material was stored at room temperature following good storage practices. Dried and finely powdered plant material (100 gm) was kept for different extraction process ice cold (kept overnight), hot (100 °C), microwave (15 min at 50 °C, 350 W) and for sonication (15 min 60 °C) assisted extraction using different solvents such as hexane, chloroform, methanol and water to quantify the level of reference compounds in the plant extract.
The dried methanolic extract (4.5 g) was subjected to fractionation on a silica gel (60–120 mesh) column and eluted with a mixture of solvents with gradually increasing polarity, starting from hexane followed by ethyl acetate and methanol. Fractions no 15–20 (eluted with hexane:ethyl acetate (30:70, v/v), were again purified by semi-preparative HPLC using mobile phase (acetonitrile: water: 25:75, v/v) with a flow rate of 3 ml/min. The detection at 280 nm was resulted into the purified marker compound PT (Rt 11.28 min). Additionally, fractions no 22–32 were eluted with hexane:ethyl acetate (10:90, v/v) and further purified through the same methods as mentioned above, which resulted into purified compound PZ (Rt 3.58 min). The structure elucidation has been performed using established identity of PT and PZ, and mentioned in detail in our previous publication (Dixit et al., 2019), and their structures are depicted in Figs. 1A and 1B.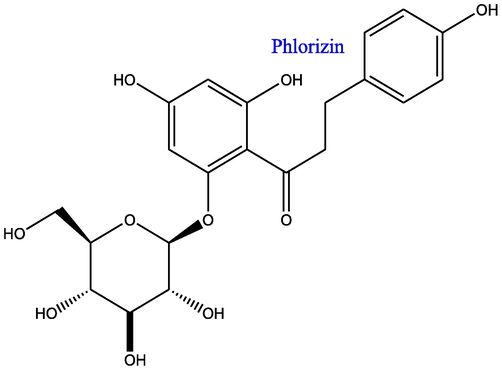
Chemical structure of Phloridzin.
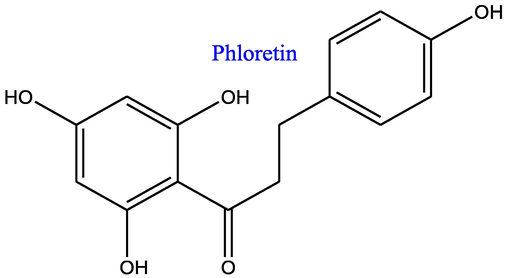
Chemical structure of Phloretin.
2.2 Chemicals and reagents
LPS (Escherichia coli 055:B5), HAM’s nutrient mixture F12 and dimethyl sulphoxide (DMSO) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Rat specific TNF-α, IL-6 Enzyme Immune Assay Kit (EIA) procured from BD Biosciences, USA. Fetal bovine serum (FBS), penicillin, streptomycin and other reagents for cell culture were obtained from Life Technologies Inc. (Grand Island, NY, USA). HPLC grade solvents were purchased from Merck, India where trifluoroacetic acid was purchased from SRL, Mumbai. Prior to use, all the solvents were filtered through a 0.45 µ membrane filter (Millipore, Billerica, MA, USA). Stock solution containing a mixture of standards (1 mg/mL) was prepared and kept at 4 °C.
2.3 Cell lines
Rat C6 glioma cells were obtained from the National Centre for Cell Science (NCCS), Pune, India and used over a passage range of 65–74. Cells were cultured in 75-cm2 culturing flasks in HAM’s nutrient mixture F12 which consisted of 10% Fetal Bovine Serum and antibiotics (100 U/mL of penicillin and 100 µg/mL of streptomycin). The cells were maintained at 37 °C in a humidified atmosphere with 5% CO2 in air and the medium was changed after each alternate day.
2.4 Cell culture and treatment
Effect of PZ and PT on neuroinflammation was studied using rat C6 glioma cells. Cells were seeded (2.5 × 105 cells/mL) into 24-well plates and maintained in HAM’s nutrient mixture F10 containing 10% fetal bovine serum at 37 °C in a humidified atmosphere of 5% CO2. The cells were pretreated with PZ and PT (5, and 10 μg/mL) and the standard anti-inflammatory drug dexamethasone (5 μg/mL) was also added 30 min before LPS stimulation.
2.5 Quantification of pro-inflammatory cytokines
The amount of TNF-α and IL-6 release in cell culture supernatant was carried out with ELISA Kit following the manufacturer’s protocol and our previous studies (Singh et al., 2012). Rat-specific TNF-α, and IL-6 (ELISA) Kits were procured from BD Biosciences, USA. Briefly, the ELISA plates (96 well) were coated (100 μL/ well) with rat-specific TNF-α and IL-6 capture antibody, respectively, and incubated overnight at 4 °C. The plate was blocked with 200 μL/well assay diluents. Culture supernatant and standard (100 μL) were added into the appropriate coated wells and incubated for 2 h at room temperature (22 ± 3 °C). After incubation, the plates were washed thoroughly five times with wash buffer. 100 µL of detecting solution (detection antibody and streptavidin HRP) was added in to each well. The plate was sealed and then incubated for 1 h at room temperature and then washed thoroughly five times with a wash buffer again. 100 µL of tetramethylbenzidine (TMB) substrate solution was added to each well and the plate (without plate sealer) was incubated for 30 min at room temperature in dark. 50 µL of stop solution (2 N H2SO4) was then added to each well. The color density was measured at 450 and 570 nm using a microplate reader (Molecular Devices, USA). The absorbance at 570 nm was subtracted from the absorbance at 450 nm. The values of cytokines were expressed as picograms per millilitre of culture supernatant.
2.6 Cell viability assay
The cells were seeded in 96-well plates and cell viability was determined by using MTT (3-(4,5-dimethylthazol-2-yl)-2,5-diphenyltetrazolinum bromide) assay (Meerloo et al., 2011). C6 Cells were treated (5, 10 and 30 µg/mL) and incubated for 24 h at 37 °C in 5% CO2 atmosphere. After treatment, 20 μL aliquots of MTT solution (5 mg/mL in PBS) were added to each well and left for 4 h. Then, MTT containing medium was carefully removed and the formazan crystals formed were solubilised in DMSO (100 µL) for 10 min. The culture plate was placed on a micro-plate reader (Spectramax; Molecular Devices, USA) and the absorbance was measured at 550 nm. The amount of color produced is directly proportional to the number of viable cells. Cell cytotoxicity was calculated as the percentage (%) of viability = (mean treated absorbance/mean untreated absorbance × 100).
2.7 Determination of antioxidant activity of M. sikkimensis bioactives
2.7.1 Enzymatic method
The two bioactives (Phloretin and Phloridzin) were assessed in both enzymatic and non enzymatic systems of free radical generation. In enzymatic method, superoxide anions were generated enzymatically in a system composed of Xanthine (0.122 mg/ml), Xanthine oxidase (12 μL/ml) and nitrobluetetrazolium (NBT) (0.74 mg/ml) in the presence and absence of test samples (50, 100 and 200 µg/ml). The generated formazones were measured spectrophotometrically as a result of reduction of NBT by O2− at 560 nm (Shrivastava et al., 2013). In non-enzymatic method, the in vitro system employed for non-enzymatic generation of O2− comprised of phenazine methosulphate (PMS) (0.28 mg/ml), NADH (1.65 mg/ml) and NBT (1.286 mg/ml) in the absence or presence of test samples (50, 100 and 200 µg/ml). After the incubation, the amount of formazone formed was measured as above.
2.7.2 Non-enzymatic method
Additionally, the effect of phytomolecules on the generation of hydroxyl free radicals (OH•) was also measured non-enzymatically (Buettner and Need, 1985). Briefly, OH• were generated non-enzymatically in a system consisted of FeSO4 (0.276 mg/ml), sodium ascorbate (1.9 mg/ml), H2O2 (5%) and deoxyribose (0.94 mg/ml) in absence or presence of test samples (50, 100 and 200 µg/ml). Samples were incubated for 2 h at 37 °C and after the incubation 1 ml of TBA (1%) and 1 ml of TCA (2%) was added to samples. Samples were placed in a boiling water bath for 10 min and after cooling the malondialdehyde produced was measured spectrophotometrically at 532 nm.
2.8 In vitro antiplasmodial and hemolytic activity
The in vitro antiplasmodial activity of PT and PZ was also evaluated against human malaria parasite strain P. falciparum (NF-54- chloroquine sensitive strain). Stock concentration of compounds was prepared in DMSO at 10 mM concentration. Two-fold serial dilutions of test compounds were made in 96 well plates and incubated with 2.0% red blood cells suspension containing 1.0% parasitaemia (mostly ring stages). The plates were incubated at 37 °C in CO2 incubator in an atmosphere of 5% CO2. Plates were incubated for next three days (72 h) and later on 100 µL of lysis buffer containing 2x concentration of SYBR Green-I (Invitrogen) was added in each well and again incubated for one hour at 37 °C. The plates were examined at 485 ± 20 nm of excitation and 530 ± 20 nm of emission for relative fluorescence units (RFUs) per well using the fluorescence plate reader (FLX800, BIOTEK). Chloroquine was used as the reference antimalarial drug. The percent inhibition in treated group (drug treated) was calculated with respect to RFU of control groups (without drug). The data was expressed in term of IC50 inhibitory concentration which was calculated using Logit regression analysis of dose response curves (Srivastava et al., 2017). Effect of PT and PZ on erythrocytes integrity was also studied through in vitro hemolytic assay, by using standard protocol mentioned earlier (Robert et al., 2010).
2.9 Statistical analysis
Results are presented as the mean ± SEM of triplicate observations. One-way analysis of variance (ANOVA) followed by Turkey’s test was used to determine statistical significance for multiple comparisons. The value P < 0.05 was considered statistically significant. The statistical computation was performed using Graph Pad Prism version 8.1.
3 Results
3.1 Isolation of PT and PZ from Malus sikkimenmsis
By using semi-preparative HPLC with mobile phase of acetonitrile:water, PT and PZ were isolated with high purity (>95%). UV maxima for PT and PZ were recorded at 282 and 283 nm, respectively. The authenticity of PZ and PT was confirmed by its spectral characteristics and detailed in our previous publication (Dixit et al., 2019). The PZ content in the bark and leaves was found to be higher (13 mg/100 mg of dried plant material) than fruits. PT was only present in the leaves (0.57 mg/100 mg of dried material).
3.2 Effect of M. sikkimensis bioactives on production of proinflammatory cytokines in LPS stimulated C6 glial cells
Both PZ and PT at the studied concentrations of 5 and 10 μg/mL showed significant reduction in the production of pro-inflammatory cytokines in LPS-induced neuroinflammation in C6 cells. Interestingly, PZ showed 26.27 ± 1.33 and 30.77 ± 0.94% inhibition and PT showed 17.10 ± 1.19 and 42.13 ± 3.54% inhibition of TNF-α release at the 5 and 10 µg/ml concentrations, respectively. Similarly, PZ exhibited 10.48 ± 1.38 and 19.04 ± 1.56% inhibition and PT showed 22.09 ± 2.14 and 29.83 ± 0.85% inhibition of IL-6 cytokine release, respectively. The percent inhibition of pro-inflammatory cytokines by PZ and PT is depicted in (Fig. 2 and Table 1).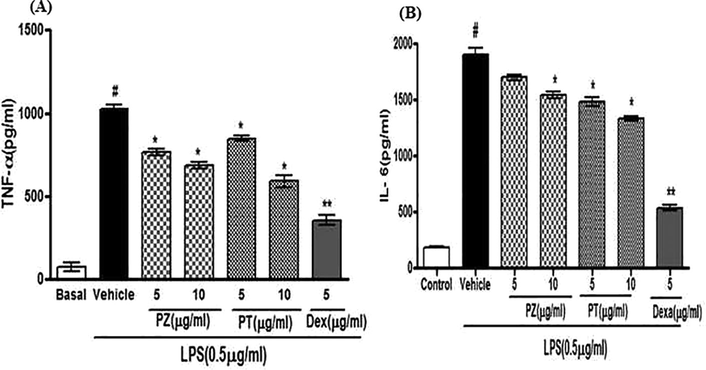
Inhibitory effect of PZ and PT on inflammatory cytokines (A. TNF-α; B. IL-6) production in LPS induced neuro-inflammation in C6 glial cells. Values are Mean ± SE where n = 4.
Compounds
Dose (μg/ml)
TNF-α
IL-6
PZ
5
26.27 ± 1.33
10.47 ± 1.38
10
30.77 ± 0.94
19.04 ± 1.56
PT
5
17.10 ± 1.19
22.09 ± 2.14
10
42.13 ± 3.54
29.83 ± 0.85
Dexamethasone
5
65.27 ± 2.89
71.82 ± 1.39
10
71.14 ± 1.50
77.84 ± 0.79
3.3 Effect of M. sikkimensis bioactives on cell viability assay of C6 glial cells
The in-vitro effect of PZ and PT on cell viability in C6 glial cells was also evaluated using MTT assay. The insignificant change in percent dead cell population was observed (p > 0.05) at all the concentrations of drugs when compared with normal cells (Table 2).
Compounds
Dose
(μg/ml)% of Cell viability
(Mean ± SE)
PZ
0
100
5
97.84 ± 0.16
10
98.51 ± 0.60
30
98.60 ± 0.99
PT
0
100
5
97.46 ± 0.23
10
99.41 ± 0.76
30
98.42 ± 0.23
3.4 Effect of M. sikkimensis bioactives on antioxidant activity
The antioxidant potential of PT and PZ was also evaluated by generating free radicals (Super oxide radicals and hydroxyl radicals) in vitro in the presence and absence of these compounds. As shown in Table 3, PT showed an excellent antioxidant activity by inhibiting the generation of Superoxide (O2−) radicals (62.12 ± 3.66%) and hydroxyl (OH−) radicals (35.07 ± 1.19%) in non-enzymatic system at 200 µg/ml concentration. PZ, a glycoside derivative of PT showed a weaker antioxidant effect comparing PT. In enzymatic system of radical generation both PT and PZ showed a concentration dependent inhibition of superoxide radical generation (O2−), 49.15 ± 1.14% and 38.65 ± 3.77% at 200 µg/ml. Standard drug Allopurinol caused 55.25% inhibition of enzymatic generation of superoxide radicals (O2−) while in non-enzymatic system standard drug Ascorbic acid caused 94.12 ± 2.30% and 75.98 ± 3.47% inhibition of Superoxide (O2−) radicals and hydroxyl (OH−) radicals, respectively at 200 µg/ml concentration. These results indicated that both the molecules possess significant antioxidant property.
Compounds
Concentration
(µg/ml)Percentage inhibition
(Mean ± SE)
Enzymatic
Superoxide anions (O2−)Non-Enzymatic
Superoxide anions (O2−)Non-Enzymatic
Hydroxyl ions (OH−)
PZ
50
10.05 ± 1.24
38.25 ± 3.65
25.12 ± 0.14
100
25.69 ± 2.47
69.62 ± 2.32
41.49 ± 2.87
200
49.15 ± 1.14
91.57 ± 4.25
73.17 ± 2.58
PT
50
5.65 ± 0.98
29.25 ± 2.15
12.75 ± 1.69
100
19.35 ± 1.55
41.34 ± 1.25
24.12 ± 2.32
200
38.65 ± 3.77
62.12 ± 3.66
35.07 ± 1.19
(Standard drug)
200
55.25 ± 3.89
(Allopurinol)94.12 ± 2.30
(Ascorbic acid)75.98 ± 3.47
(Ascorbic acid)
3.5 Effect of PT and PZ on malaria parasite growth
In this study, PT and PZ exhibited significant antiplasmodial activity against P. falciparum NF-54 (CQ sensitive) strain, where the results are presented in Table 4. Results demonstrated that PT exhibited an IC50 value of 5.65 ± 0.27 µg/ml which was found to be better then PZ, which exhibited an IC50 value of 27.98 ± 0.88 µg/ml. Chloroquine which was used as reference antimalarial exhibited its antiplasmodial activity with an IC50 value of 0.025 µg/ml. Additionally, PT and PZ did not exhibited any hemolytic property upto 100 µg/ml concentration hence showing their selectivity towards malaria parasite in the host erythrocytes.
Compounds
In-vitro antiplasmodial activity
IC50 (µg/ml)
PZ
27.98 ± 0.88
PT
5.65 ± 0.27
Chloroquine
0.025
4 Discussion
In our previous report, we have isolated phloridzin (PZ) and phloretin (PT) from Malus sikkimensis. These bioactives were isolated with a good level of purity and we have also developed a systematic validated method for the determination of these bioactives in the leaves of Sikkim crabapple (Malus sikkimensis), using HPLC-Photo Diode Array (PDA). Hence in the present study, we have estimated the pharmacological and medicinal aspects of these bioactives using cell based assays. PZ and PT exhibited significant anti-inflammatory activity on LPS-induced neuroinflammation, which is known to be associated with the excess production of pro-inflammatory cytokine. Glial cells are the primary central nervous system immune effector cells whereas neuroinflammation is an altered situation which plays a significant role in various chronic and acute pathological conditions of the central nervous system anomalies. Our results showed that the stimulation of C6 cells with LPS (0.5 μg/mL) significantly increased the level of TNF-α and IL-6 in culture supernatant when compared with unstimulated cells. Interestingly, PT and PZ isolated from apples exhibited significant anti-inflammatory potential by suppressing the release of TNF-α and IL-6 cytokines in LPS stimulated glial cells. It is well established that glial cells are the resident innate immune cells of the central nervous system and play critical role in inflammation-mediated neurodegenerative disorders. Previous reports also suggested that the plant derived bioactives possess significant potential to protect neuroinflammation in C6 cells (Srivastava et al., 2014).
Additionally, natural products also possess protective effects against inflammation mediated increased oxidative stress which in turn make them more useful for the prevention and treatment of neuro-degenerative diseases (Uttara et al., 2009). We have also estimated the antioxidant potential of PT and PZ using enzymatic and non-enzymatic systems, where they exhibited potential antioxidant properties, in the scavenging of superoxide and hydroxyl radicals. It is well documented and we also found in our results that the antioxidant property of the flavonoids is mainly due to the aromatic –OH groups, thus the modification of 2-OH by a glucose moiety in PZ, might be responsible for the suppression of the antioxidant potential of PZ, as compared with PT by many folds (Flora, 2009). Considering the importance of the oxidative status in the onset of inflammation (Lugrin et al., 2014), the anti-inflammatory activity of Phloretin and Phloridzin in this study might be due to their antioxidant properties. Additionally, both PZ and PT did not affect the cell viability at the tested concentration thus having no direct toxic effect to the cells hence showing promising safety values for their therapeutic usage.
Moreover, the obtained antiplasmodial activity of PT and PZ also might be due the antioxidant potential of these bioactives. Malaria pathogenesis has been clearly identified to be associated with an increase in amount of free radicals and reduction in the acivity of antioxidant enzymes hence compounds bearing antioxidant properties have been found to be valuable for antimalarial combination therapies. Overall, phloretin and phloridzin isolated from Malus sikkimenmsis possess many therapeutic values hence should be studied in detailed. Interestingly, phloretin was found as a lead candidate of the present study which can be used as a template for further research by various researchers.
5 Conclusion
Here in this study, the isolation and validation of the dietary consumption of apple has been proved with their effects on lowering the incidence of degenerative diseases, which is further validated with their anti-inflammatory properties, antiplasmodial and anti-oxidant activities. PT and PZ also exhibited substantial protection against LPS stimulated neuroinflammation in C6 rat glial cells along with their ability to scavenge free radicals under both enzymatic and non-enzymatic methods. PT and PZ also exhibited antiplasmodial effects hence validating their medicinal efficacies. Above findings also concrete that phloretin (PT) may play a promising role in the treatment of degenerative diseases through inhibition of oxidative and pro-inflammatory markers. Therefore, this molecule can be used as a dual action pharmacophore for the development of anti-inflammatory agents since protecting the inflammatory as well as oxidative imbalance, generally seen effective in various health ailments.
Acknowledgements
The authors are thankful to Director CSIR-CIMAP, Lucknow & Govt. of India for funding the project under DST-Wos (A) fellowship program (Project number GAP 293). N.A. AlFaris, J.Z. Altamimi and T.S. Aldayel thanks the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia for the grant (RGP-1440-0021).
Declaration of Competing Interest
The authors have no conflict of interest concerning the work reported in this paper.
References
- Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide activated microglia. Int. Immunopharmacol.. 2005;5:185-193.
- [Google Scholar]
- Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. J.. 2007;8(1):57-69.
- [Google Scholar]
- Hydrogen peroxide and hydroxyl free radical production by hematoporphyrin derivative, ascorbate and light. Cancer Lett.. 1985;25(3):297-304.
- [Google Scholar]
- Quantitation of dietary dihydrochalcones in Indian crab apple (Malus sikkimensis) using validated high-performance liquid chromatography. J. Chromatogr. Sci.. 2019;57(8):79-687.
- [Google Scholar]
- Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid. Med. Cell. Longevity. 2009;2(4):191-206.
- [Google Scholar]
- Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918-934.
- [Google Scholar]
- Biosynthesis of phloridzin in apple (Malus domestica Borkh.) Plant Sci.. 2009;176:223-231.
- [Google Scholar]
- Lipopolysaccharide modulates astrocytic S100B secretion: a study in cerebrospinal fluid and astrocyte cultures from rats. J. Neuroinflammation. 2011;8(1)
- [CrossRef] [Google Scholar]
- Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol.. 2009;8(4):382-397.
- [Google Scholar]
- Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflammation Allergy Drug Discovery. 2009;3:73-80.
- [Google Scholar]
- Kutner, S., Breuer, W.V., Ginsburg, H., Cabantchik, Z.I., 1987. On the mode of action of phlorizin as an antimalarial agent in in vitro cultures of Plasmodium falciparum. Biochem Pharmacol, 1, 36(1), 123-9
- Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr.. 2003;78(3 Suppl):517S-520S.
- [Google Scholar]
- The role of oxidative stress during inflammatory processes. Biol. Chem.. 2014;395(2):203-230.
- [Google Scholar]
- Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol.. 2013;35(5):601-612.
- [Google Scholar]
- The influence of organic/integrated production on the content of phenolic compounds in apple leaves and fruits in four different varieties over a 2-year period. J. Sci. Food Agric.. 2010;90(14):2366-2378.
- [Google Scholar]
- Effects of leaf extracts from Croton zambesicus Müell. Arg. Hemost. J Ethnopharmacol.. 2010;128(3):641-648.
- [Google Scholar]
- Apple polyphenols, phloretin and phloridzin: new trapping agents of reactive dicarbonyl species. Chem. Res. Toxicol.. 2008;21(10):2042-2050.
- [Google Scholar]
- Lipid lowering and antioxidant effect of miglitol in triton treated hyperlipidemic and high fat diet induced obese rats. Lipids. 2013;48(6):597-607.
- [Google Scholar]
- HILIC quantification of oenotheralanosterol A and B from Oenothera biennis and their suppression of IL-6 and TNF-α expression in mouse macrophages. J. Ethnopharmacol.. 2012;141(1):357-362.
- [Google Scholar]
- Antimalarial activity and safety assessment of Flueggea virosa leaves and its major constituent with special emphasis on their mode of action. Biomed. Pharmacother.. 2017;89:761-771.
- [Google Scholar]
- Effect of Pluchea lanceolata bioactives in LPS-induced neuroinflammation in C6 rat glial cells. Naunyn-Schmiedeberg's Arch. Pharmacol.. 2014;387(2):119-127.
- [Google Scholar]
- Correlation between in vitro and in vivo antimalarial activity of compounds using CQ-sensitive and CQ-resistant strains of Plasmodium falciparum and CQ-resistant strain of P. yoelii. Parasitol. Res.. 2017;116(7):1849-1854.
- [Google Scholar]
- Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol.. 2009;7:65-74.
- [Google Scholar]
- Phenolic profiling and antioxidant capacity of Morchella esculenta L. by chemical and electrochemical methods at multiwall carbon nanotube paste electrode. Food Measure. 2019;13:1805-1819.
- [Google Scholar]
- Antioxidants and prevention of chronic disease. Crit. Rev. Food Sci. Nutr.. 2004;44(4):275-295.
- [Google Scholar]







