Translate this page into:
Antimicrobial evaluation of spirooxindolopyrrolidine engrafted indoles against multidrug resistant ESKAPE clinical pathogens
⁎Corresponding authors. anatarajan@ksu.edu.sa (Natarajan Arumugam), sinouvassane@utar.edu.my (Sinouvassane Djearamane)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The synthesis of a new class of effective antibiotics and antimicrobial agents with less toxicity is highly desirable due to bacterial resistance to antibiotics has increased dramatically. In this context, molecules that embedding a spiro moiety are attractive from a medicinal chemistry point of view as these spiro heterocycles show a vital part in the development of delivery systems for antimicrobial therapies. In the present study, the synthesis and antimicrobial evaluation of structurally attractive complex hybrid heterocycles comprising spriooxindolopyrrolidine and indole heterocycles was attained in quantitative yields by cycloaddition strategy. The new class of spirocompouds were unequivocally assigned through spectroscopic analysis and the antimicrobial efficacy were assessed against six microbial pathogens. Among them, compound 4a, bearing chlorine substituted derivative showed significant activity against tested ESKAPE pathogens. The maximum zone of inhibition observed against ESKAPE microbial pathogens causing infectious disease ranged from 6.75 ± 0.40 to 19.75 ± 1.15 mm, with MIC values ranging between 16.00 to > 256.00 µg/ml .
Keywords
Multidrug resistant
ESKAPE pathogens
Spiropyrrolidines
Cycloaddition reaction
Human health
1 Introduction
One of the biggest societal and public health problems is the resistance of harmful bacteria to antibiotics, since bacteria and fungi account for 80–87 % of all cases of health-related infections (HAIs) in the human population (Haque et al., 2018). The pathogenic potential of these bacteria is based on a number of virulence mechanisms, such as the enzymes, expression of adhesins, toxins and chemicals affecting the immune system, all of which are essential for annexation or intensification of infections (Waglechner et al., 2017; Palma et al., 2020). Current drug development process is not sufficient to support the complete eradication of antimicrobial infections (Reddy et al., 2019). Several pathogens are resistant to antibiotics and need to be treated with potentially detrimental drugs. As a result, drug discovery researchers and pharmaceutical companies have focused their efforts on finding new ways to target resistant microorganisms with lower toxicity profile (Sass et al., 2013). The preparation of novel small molecules with new mechanism of action to curing infectious disease is urgently needed.
In this context, oxygen and nitrogen containing heterocycles are very attractive in antimicrobial research as they present in substantial number of medicines as an active moiety (Stephen et al., 2015). Among them, heterocycles comprising spirooxindolopyrrolidine motif are crucial in the field of pharmaceutical chemistry since this motif are predominant in biologically potent natural products and synthetic compounds. These spirooxindole hybrids displayed diverse pharmaceutical properties viz. anticancer (Yang et al., 2016, Lotfy et al., 2017, Barakat et al., 2018), anti-bacterial (Chande et al., 2005), analgesic (Rajanarendar et al., 2013), local anesthetic (Kornet, et. al., 1976), anti-mycobacterial (Rajesh et al., 2011, Arumugam et al., 2021, Arumugam et al., 2021), and AChE inhibition activities (Arumugam et al., 2018, Kumar et al., 2018, Almansour et al., 2020). Owing to their unique structural profile such as inherent complex structure and three dimensionality natures, they exhibit high rigidity and the capability to expose functionality that provides a higher affinity to biological target. Finally, spiro core structures have the potential to improve solubility, a crucial attribute during the drug development process, by metabolic stability, modulating log P and having sp3 hybridization.
Recently, our research team designed and synthesized structurally diverse fused spirooxindolopyrrolidines employing multicomponent cycloaddition methodology (Arumugam et al., 2015, Arumugam et al., 2018, Arumugam et al., 2018) and these synthesized spiro compounds exhibited diverse biological profiles including antimicrobial activities. It is important to note that some of the synthesized spiropyrrolidine heterocycles exhibited excellent activities. Remarkably, a few of hybrid with spiro unit showed higher activity than the standard drug (Arumugam et al., 2020, Arumugam et al., 2021). The biological precedents mentioned above has led to further study on the synthesis of hybrid heterocycles that embedding spiro-oxindolopyrroldines and indole into a single compound which would be of great importance for medicinal value, since the indole moiety has a significant biological profile, anticipating spiro-pyrroldine with the indole motif will enhanced biological activity (Alaqeel et al., 2022). The present study described a one-pot, synthesis of complex dispirooxindolopyrrolidines integrated indole hybrids via an intermolecular [3 + 2] cycloaddition cascade reaction methodology. These synthesized complex molecules were tested for their antibacterial properties against Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. (ESKAPE) microbial pathogens.
2 Materials and methods
2.1 Synthesis of spirooxindolopyrrolidine fused indole, 4a-d
An equimolar ration of acrylate 3, and L-tryptophan 2 and indoline-2,3-dione 1 (1 mmol) was dissolved in 20 ml of MeOH and then the reaction mixture was refluxed for 2 h. Completion of the reaction was observed by TLC and the solvent was reduced under low temperature. The product was washed with Et2O to give the compound in quantitative yield.
Spirocompound 4a: Yield (89 %); Pale yellow solid; 1H NMR (500 MHz, CDCl3): δH 2.15 (t, J = 13.2 Hz, 1H), 2.85 (d, J = 13.2 Hz, 1H), 3.28 (d, J = 13.2 Hz, 1H), 3.45 (s, 3H), 3.69 (d, J = 13.2 Hz, 1H), 4.78–4.82 (m, 1H), 6.21 (s, 1H), 6.75–6.83 (m, 3H, ArH), 6.95 (t, J = 9.0 Hz, 1H), 7.03 (t, J = 9.0 Hz, 1H), 7.08 (s, 1H), 7.16–7.29 (m, 4H, ArH), 7.50 (s, 1H), 8.00 (d, J = 9.0 Hz, 1H), 10.26 (s, 1H), 10.33 (s, 1H), 10.67 (s, 1H); 13C NMR δC: 29.3, 41.4, 51.3, 62.3, 64.4, 64.7, 75.2, 109.2, 110.6, 110.9, 113.2, 117.8, 119.6, 120.5, 121.1. 123.2, 124.7, 125.6, 127.5, 128.2, 129.1, 131.1, 136.1, 142.7, 143.3, 172.3, 177.8, 182.3; Mass: m/z = 556 (M+); Anal. Calcd for C30H25ClN4O5: C, 64.69; H, 4.52; N, 10.06; Found C, 64.80; H, 4.64; N, 10.17.
Spirocompound 4b: Yield (87 %); Pale yellow solid; 1H NMR (500 MHz, CDCl3): δH 2.63–2.71 (m, 2H), 3.10 (s, 3H), 3.19 (d, J = 12.4 Hz, 1H), 4.6 (d, J = 1.4 Hz, 1H), 5.53–5.54 (m, 1H), 6.38 (m, 3H, ArH), 6.61–6.63 (m, 1H, ArH), 6.69–6.82 (m, 5H, ArH), 6.93–6.96 (m, 3H, ArH), 9.1 (s, 1H), 9.80 (s, 1H); 13C NMR δC: 28.4, 39.6, 50.7, 61.8, 62.6, 64.4, 74.7, 108.8, 109.6, 117.0, 120.7, 123.2, 127.3, 127.8, 127.9, 128.3, 128.6, 129.3, 132.6, 139.6, 141.9, 143.4, 171.5, 177.4, 179.2, 182.2; Mass:m/z = 606 (M+); Anal. Calcd for C31H25F3N4O6: C, 61.39; H, 4.15; N, 9.24; Found: C, 61.50; H, 4.27; N, 9.37.
2.2 Antibacterial activity of compound 4a-d
The well diffusion method established by CLSI (CLSI, 2012) was used to test four (4a-d) synthetic compounds for their antibacterial activity against ESKAPE pathogens. These bacterial pathogens were grown in nutrient broth and allowed to thrive for 24 h at 37 °C. Before, being kept on the MHA plates, the dissolved compounds were impregnated on a 6.00 mm blank sterile disc and dried under sterile conditions. McFarland standards (1.00 x108 CFU/mL) of each ESKAPE pathogen were swabbed on to MHA plates as microbial inoculum. The positive and negative control was amoxicillin (30 mcg) and DMSO, respectively. After that, an impregnated dry disc was placed on surface of the culture plates and incubated twenty four hour at 37 °C. The preliminary antibiotic test was performed in triplicate.
After preliminary screening, the significant compound 4a selected for it antibacterial activity evaluation by agar well diffusion method (Bauer et al., 1996, Bonev et al., 2008). 0.1 ml of the respective ESKAPE pathogens were streaked onto the plates containg MHA plates. 6 mm diameter wells were made in MHA plates using a sterilized steel drill that filled with 25.00, 50.00, 75.00 and 100.00 µl of the compound (4a). Amoxicillin and DMSO and were used as a positive and negative controls. The diameter of inhibition zone was calculated after 24 h of incubation.
2.3 MIC determination by broth microdilution assay
MIC values of compound 4a was evaluated using broth micro dilution method (Winn et al., 2006). MIC assay evaluations were completed by three times with potential lead compound 4a. The compound 4a was assayed for their growth control activity against ESKAPE pathogens and amoxicillin. The compound 4a was dissolved in DMSO for MIC assay. After 24 h of incubation at 37 °C, the ESKAPE pathogens were attained from Mueller Hinton broth (MHB). The inoculum of test ESKAPE pathogens were fixed to attain the MacFarland standard (0.5) turbidity of an inoculum size was 1.0x108 CFU/mL for MIC assays. The MIC test was performed with MHB at pH 7 using the doubling dilution technique. Microtiter well (last well) containing only inoculation broth was earmarked as a control, and no growth of ESKAPE pathogen was stated as the MIC value in µg/mL. The compound 4a and amoxicillin were diluted with MHB and arranged at concentrations of 2.00, 4.00, 8.00, 16.00, 32.00, 64.00, 128.00, 256.00 and 512.00 µg/mL, respectively (Abusetta et al., 2020). The experiment was repeated three times to find mean MIC value.
3 Results
3.1 Synthesis of spirooxindolopyrrolidine fused indole
The Baylis-Hillman adduct (BHA) such as methyl 2-(3-hydroxy-2-oxoindolin-3-yl) acrylate 3 was synthesized from isatin and acrylate, DABCO was used as catalyst through Baylis-Hillman reaction (Mi Chung et al., 2002). With the BHA in hand and it has been utilized as dipolarophile under optimized reaction conditions, we carried out the three-component cycloaddition of 3 with ylide generated from indoline-2,3-dione 1 and L-tryptophan under reflux condition. An equimolar mixture of 1, 2 and 3 in refluxing methanol (10 ml, 60 min) afforded the spirooxindolopyrrolidine tethered indole hybrids 4 as single product in good yield (86 %). The reaction was performed initially with different solvents system such as MeOH, EtOH, CH3CN, 1,4-Dioxane, toluene and reaction furnished the cycloadduct in 86, 79, 75, 74, 46 % yields respectively and found that methanol is appropriate solvent for this three-component reaction (Scheme 1). Consequently, the entire subsequent reaction was carried out under these similar optimized conditions.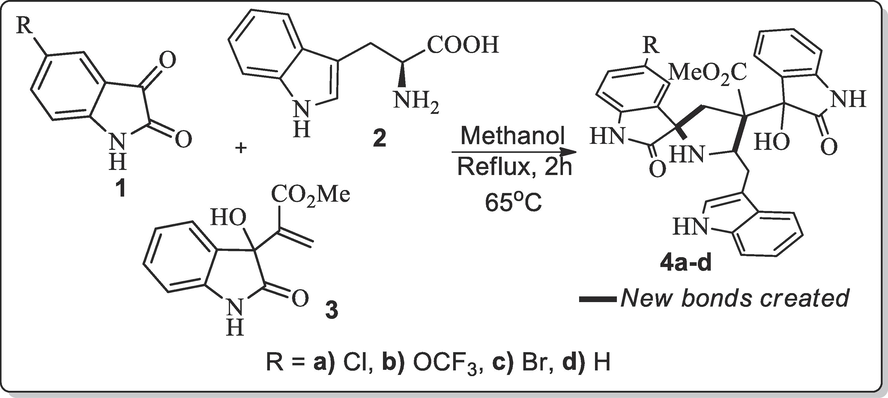
Preparation spirooxindolopyrrolidine incorporated indoles, 4a-d.
The structure of mono-spirooxindolopyrrolidine integrated indole hybrids 4 was elucidated by spectroscopic analysis as illustrated for a representative compound 4a. In the 1H NMR spectrum of 4a, a multiplet at δ 4.78–4.82 ppm is ascribable to H-5 hydrogen and two doublets at δ 3.28 ppm and δ 2.85 ppm were attributed to H-3 hydrogens of pyrrolidine rings. The triplet and doublet at δ 2.15 and δ 3.69 ppm were belong to indole adjacent hydrogens (H-6). A signal at δ 3.46 as singlet was assigned to ester methyl hydrogens. The aromatic signals as multiple in the region δ 6.75 to 8.00 ppm. The signals at δ 10.27 and 10.33 ppm were assigned to oxindole NH hydrogens and a signal at δ 10.67 ppm was assignable to indole hydrogen. The carbon signal at δ 75.2 ppm due to the OH group attached oxindole quaternary carbon (C-7). The signals at δ 64.7 and 41.4 ppm were assignable to spiro carbon (C-2) and methylene carbon (C-3) respectively. The carbon signals at 62.3 and 64.4 ppm were attributed to methylene (C-5) and quaternary carbons (C-4) respectively. The methylene carbon (C-6) resonated at δ 41.4 ppm. The ester methyl carbon exhibited at δ 51.3 ppm. The two oxindole carbonyl carbons resonated at δ 177.8 and182.3 ppm, respectively and the signal at 172.3 was assignable to ester carbonyl carbon.
A rational mechanism for the construction of spirooxindolopyrrolidines tethered indole 4 is depicted in Scheme 2. Firstly, the ylide created in situ by the reaction of isatin 1 and L-tryptophan 2 through iminium ion 5 and 6 via spontaneous dehydration and decarboxylation. Subsequently, ylide 7 adds to exocyclic double bond of 3 regioselectively to form compound 4 in good yields. Other possible regioiomer could not observed even in trace due to the polarization of the dipolarophile 3 that preferentially trap with the electron-rich carbon of the 7 furnishing 4 in good yields. The cycloaddition reaction generated up to four adjoined stereocenter out of four, one is spiro carbon and two quaternary carbons via two C–C and one C-N bonds in one-pot synthetic method.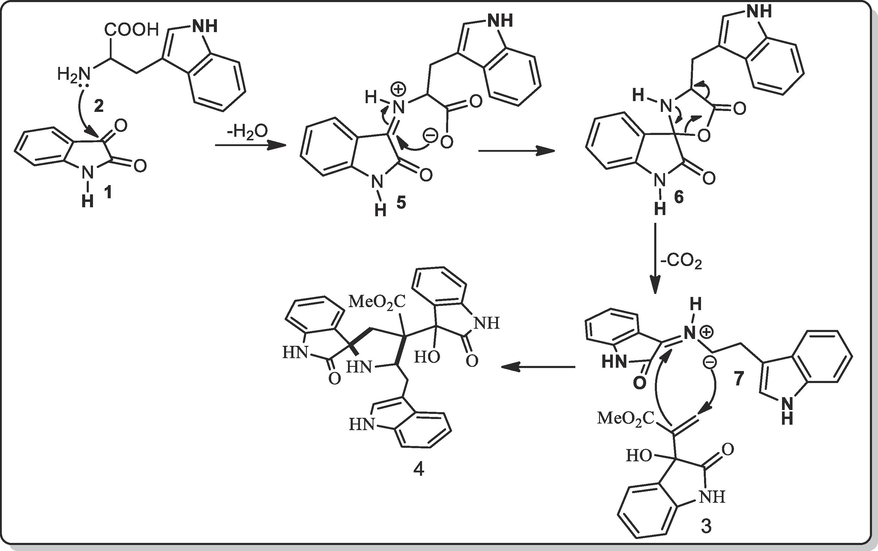
The formation of spirooxindolopyrrolidine engrafted indoles.
4 Discussion
4.1 Antibacterial activity
The above synthesized spirooxindolopyrrolidine integrated indole hybrids 4a-d were assayed for their antimicrobial potency against multidrug resistant microbial pathogens such as S. aureus, K. pneumoniae, E. faecium, A. baumannii, P. aeruginosa, and Enterobacter (ESKAPE) species using agar well diffusion method. Antibiotics sensitivity profile (ASP) of tested ESKAPE pathogens outcomes were presented in Table S1 (vide supplementary data). The overall ASP results indicated that the maximum resistant was observed in seven antibiotics and minimum of four antibiotics against tested microbial pathogens. Among the tested ESKAPE pathogens A. baumannii was resistant to seven tested antibiotics whereas, Enterobacter sp., showed only four antibiotics. But the E. faecium and K. pneumoniae displayed maximum of five antibiotics. The two antibiotics such as ampicillin and gentamycin were showed resistant to maximum numbers tested pathogens.
Compounds 4a-d showed efficient anti-bacterial activity against ESKAPE bacterial pathogens and amoxicillin was used as drug of standard. The results of the preliminary antimicrobial testing of the compounds (4a-d) are shown in Table 1. The overall, the inhibitory potency of synthesized compounds 4a-d showed efficient antibacterial activity against ESKAPE pathogens. Amongst, compound 4a which carrying chlorine atom on the phenyl ring showed significant inhibitory activities against tested ESKAPE pathogens (Table 2). The maximum and minimum inhibition zone was observed towards Enterobacter sp. (18.50 ± 1.05 mm) and A. baumannii (6.50 ± 0.25 mm), and results was compared with standard amoxicillin drug (19.75 ± 0.50 mm).
ESKAPE pathogens
Compounds concentration (mg/mL)/ Zone of inhibition (mm)
4a
4b
4c
4d
AMC$
E. faecium
14.30 ± 0.50
13.75 ± 0.30
11.20 ± 0.18
9.15 ± 0.30
17.00 ± 0.35
S. aureus
17.85 ± 1.00
14.60 ± 0.35
12.45 ± 0.70
11.00 ± 0.15
20.50 ± 0.75
K. pneumoniae
14.60 ± 0.18
13.05 ± 0.20
11.00 ± 0.50
8.50 ± 0.55
19.20 ± 0.30
A. baumannii
12.55 ± 0.08
10.75 ± 1.10
8.10 ± 0.40
6.50 ± 0.25
15.00 ± 1.25
P. aeruginosa
16.85 ± 0.25
13.00 ± 1.00
10.60 ± 0.35
9.00 ± 0.45
21.00 ± 1.85
Enterobacter sp.
18.50 ± 1.05
13.30 ± 0.15
12.65 ± 0.40
11.00 ± 1.25
19.75 ± 0.50
ESKAPE pathogens
Compound 4a concentrations (µg/mL)/ Zone of inhibition (mm)
25.00
50.00
75.00
100.00
AMC*
E. faecium
9.25 ± 0.25
10.15 ± 0.05
12.00 ± 0.15
14.70 ± 0.45
15.00 ± 0.15
S. aureus
6.75 ± 0.40
7.50 ± 0.30
14.05 ± 0.65
17.05 ± 0.20
17.00 ± 0.20
K. pneumoniae
0.00
7.85 ± 1.05
12.10 ± 0.10
13.80 ± 0.30
16.50 ± 0.35
A. baumannii
0.00
6.50 ± 0.35
11.25 ± 0.20
12.00 ± 0.40
14.15 ± 0.50
P. aeruginosa
7.05 ± 1.00
7.05 ± 1.00
15.35 ± 0.75
15.85 ± 0.65
18.00 ± 0.80
Enterobacter sp.
8.10 ± 0.25
8.10 ± 0.25
17.60 ± 0.55
19.50 ± 1.15
20.15 ± 0.45
4.2 MIC determination
The lowermost concentration of the compound 4a at which no growth of ESKAPE pathogens were detected upon manual examination after incubation at 37 °C for 24 h is deliberated the MIC value (Table 3). Even if the wells were clear of turbidity, pellets formed on the bottom of the wells were considered bacterial growth. The bottom most MIC value of 4a was observed at 16.00 µg/mL and the uppermost MIC value of > 256.00 µg/mL were detected against Enterobacter sp., and A. baumannii respectively (Table 3).
ESKAPE pathogens
MIC value (µg/mL)
4a
AMC
E. faecium
32.00
5.00
S. aureus
64.00
5.00
K. pneumoniae
128.00
10.00
A. baumannii
>256.00
15.00
P. aeruginosa
64.00
10.00
Enterobacter sp.
16.00
5.00
However, the most anti-microbial agents in therapeutic usage exert their antimicrobial activities by interfering with biosynthetic pathways such as protein, DNA and RNA synthesis and also disturb the cytoplasmic membrane (O'Neill et al., 2004). In this study, compound 4a exert its antimicrobial efficiency by disturbance cell membrane integrity (Oliva et al., 2004, Randall et al., 2013) thereby inhibiting the bacterial pathogens.
4.2.1 Molecular docking and characterization
Molecular docking studies of the selective antimicrobial target protein of RNA polymerase (6VJS) involved through biological transcription mechanism were executed to extend the observation and understanding the lead (chemical compound) interaction (Dhanaraj et al., 2021). The best resolution X-RD target protein structure of Escherichia coli RNA polymerase were downloaded and verified to confirm the structural characterization of primary and secondary and tertiary features. To recognize the atomic interaction and stearic binding site on the active biomolecule’s backbone and amino acid functional groups (Dhanaraj et al., 2018). The proposed ligand important biological medicinal properties can be predicted by using the docking results parameters viz. energy score, Emodel, hydrogen bonding, and G-score. The identified ligand and compound structure were optimized and minimized before it uses for the flexible glide docking. The structural ligand binding site were selected based on the existed pdb molecular properties and by literature reference active site were confirmed. The preprocessed macromolecule and optimized ligand were processed primary SP Glide docking followed by XP glide flexible docking was excecated. The G score values of the ligand protein were predicted as −7.804 with interacting energy of −37.492, glide evdw −29.702, glide ecoul −10.796, glide emodel score −44.655, XP hydrogen bonding score of −0.9 (Table 4). The protein active binding site amino acid molecule of PHE 1270, GLY 1271, LEU 1291, GLU 1272 were interacted with ligand by hydrogen bonding with the chemical bonding phenomenon of electron donating and acceptance. Interaction of molecules was revealed through gscore and other docking parameters with strong hydrogen bonding interaction (Fig.1).
S.No
Protein ID
Energy
glide gscore
glide evdw
glide ecoul
glide energy
glide emodel
XP HBond
Aminoacid interaction Interaction
Bonding Information
1
6VJS
−37.492
−7.804
−29.702
−10.796
–32.646
−44.655
−0.9
PHE 1270,GLY 1271,LEU 1291,GLU 1272
Hydrogen bond
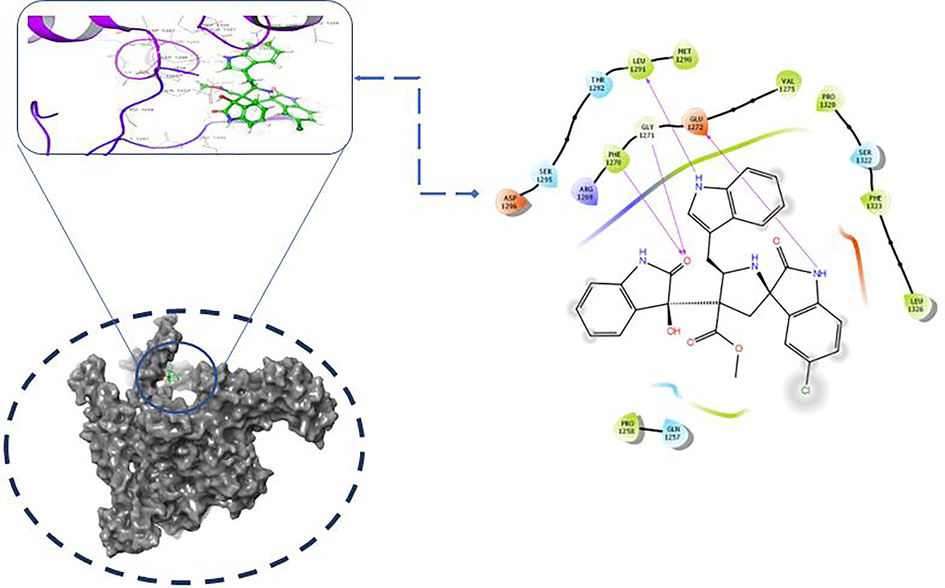
Molecular Binding interaction of RNA Ploymerase enzyme and ligand hydrogen bonding pose with binding site amino acids of (PHE 1270, GLY 1271, LEU 1291,GLU 1272).
5 Conclusions
A new class of dispirooxindolopyrrolidine integrated indole hybrids was obtained in good to excellent yields by the [3 + 2] cycloaddition cascade methodology. The rare class of non-stabilized ylide azomethine derived from L-tryptophan and isatin via decarboxylative/dehydration cycloaddition process and it is pertinent to note that the azomethine ylide are relatively meagre in literature. The synthesized spirooxindolopyrrolidines were displayed potent antibacterial efficacy against ESKAPE bacterial pathogens. Among them compound that bearing chlorine atom on the oxindole moiety had most effective activity against ESKAPE pathogens. The maximum inhibition zone against designated infectious disease-causing ESKAPE pathogens has been determined to range from 6.75 ± 0.40 to 19.75 ± 1.15 mm, with MIC values from 16.00 to>256.00 µg/ml.
Acknowledgement
The project was funded by Researchers Supporting Project number (RSP2023R143), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- De-sign, Synthesis, in Vitro Antibacterial Ac-tivity, and Docking Studies of New Rhoda-nine Derivatives. Open Journal of Medicinal Chemistry. 2020;10:15-34.
- [Google Scholar]
- Alaqeel, S.H., Arumugam, N., Almansour, A.I., Suresh Kumar, R., Ponmurugan, K., Al-Dhabi, N.A., Brindhadevi, K. Perumal, K. 2022. Synthesis and antimicrobial potential of spirooxindolopyrrolidine tethered oxindole heterocyclic hybrid against multidrug resistant microbial pathogens 114, 66-70.
- Design, synthesis and cholinesterase inhibitory activity of novel spiropyrrolidine tethered imidazole heterocyclic hybrids. Bioorg Med Chem Lett.. 2020;30(2):26789.
- [Google Scholar]
- An expedient regio- and diastereoselective synthesis of hybrid frameworks with embedded spiro[9, 10] dihydroanthracene [9,3′]-pyrrolidine and spiro[oxindole-3,2′-pyrrolidine] motifs via an ionic liquid-mediated multicomponent reaction. Molecules. 2015;20:16142-16153.
- [Google Scholar]
- Spiropyrrolidine/spiroindolizino[6,7-b]indole heterocyclic hybrids: Stereoselective synthesis, cholinesterase inhibitory activity and their molecular docking study. Bioorg Chem.. 2018;79:64-71.
- [Google Scholar]
- Regio- and diastereoselective synthesis of anticancer spirooxindoles derived from tryptophan and histidine via three-component 1,3-dipolar cycloadditions in an ionic liquid. Tetrahedron. 2018;74:5358-5366.
- [Google Scholar]
- Anti-tubercular activity of novel class of spiropyrrolidine tethered indenoquinoxaline heterocyclic hybrids Bioorg. Chem. 2020;99:103799
- [Google Scholar]
- A stereo, regioselective synthesis and discovery of antimycobaterium tuberculosis activity of novel b-lactam grafted spirooxindolopyrrolidine hybrid heterocycles. Arab. J. Chem.. 2021;14:102938
- [Google Scholar]
- Stereoselective synthesis and discovery of novel spirooxindolopyrrolidine engrafted indandione heterocyclic hybrids as antimycobacterial agents. Bioorg. Chem.. 2021;2021(110):104798
- [Google Scholar]
- Stereoselective synthesis and discovery of novel spirooxindolopyrrolidine engrafted indandione heterocyclic hybrids as antimycobacterial agents. Bioorg. Chem.. 2021;110:104798
- [Google Scholar]
- Arumugam, N., Abdulrahman I. A., Suresh Kumar, R., thamili1, D.A.D., Periyasami, G., Periasamy, V. S., Athinarayanan, J., Alshatwi, A.A., Mahalingam, S.M., Menéndez, J.C. 2018. Regio and stereoselective synthesis of anticancer spirooxindolopyrrolidine embedded piperidone heterocyclic hybrids derived from one-pot cascade protocol Chem. Cent. J. 12, 95.
- Arumugam, N., Almansour, A.I., Suresh Kumar, R., Mohammad, Al-Aizari, A., Alaqeel, S.I., Kansız, S., Krishna, V.S., Sriram, D. 2020. Dege N. Regio- and diastereoselective synthesis of spiropyrroloquinoxaline grafted indole heterocyclic hybrids and evaluation of their anti- Mycobacterium tuberculosis activity. RSC Adv. 10, 23522–23531.
- Substituted spirooxindole derivatives as potent anticancer agents through inhibition of phosphodiesterase 1. RSC Adv.. 2018;2018(8):14335-14346.
- [Google Scholar]
- Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol.. 1996;45(4):493-496.
- [Google Scholar]
- Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother.. 2008;61(6):1295-1301.
- [Google Scholar]
- Facile synthesis of active antitubercular, cytotoxic and antibacterial agents: A Michael addition approach. Eur. J. Med. Chem.. 2005;40:1143-1148.
- [Google Scholar]
- A comparative meta-genomic analysis of HPV strains: A step towards the design, synthesis and characterization of noval quenazoline derivative for antiviral activity. Comput. Biol. Chem.. 2018;73:213-220.
- [Google Scholar]
- Computational Studies on T2Rs Agonist-Based Anti–COVID-19 Drug Design. Front. Mol. Biosci.. 2021;8:637124
- [Google Scholar]
- Health care-associated infections - an overview. Infect Drug Resist.. 2018;11:2321-2333.
- [Google Scholar]
- Oxindole-3-spiropyrrolidines and -piperidines. Synthesis and local anesthetic activity. J. Med. Chem.. 1976;19:892-898.
- [Google Scholar]
- Venkatesh, S. Ionic liquid-enabled synthesis, cholinesterase inhibitory activity, and molecular docking study of highly functionalized tetrasubstituted pyrrolidines. Bioorg Chem.. 2018;77:263-268.
- [Google Scholar]
- Synthesis of new spirooxindole-pyrrolothiazole derivatives: Anti-cancer activity and molecular docking. Bioorg. Med. Chem.. 2017;254:1514-1523.
- [Google Scholar]
- Baylis-Hillman reaction of isatin derivatives: isatins as a new entry for the Baylis-Hillman reaction. Bulletin of the Georgian Academy of Sciences. Korean Chem. Soc.. 2002;23:1651-1654.
- [Google Scholar]
- National Committee for Clinical Laboratory Standards, 2012. Clinical and Laboratory Standards Institute ‘‘Performance Standards for Antimicrobial Susceptibility Testing’’; twenty-second informational supplement—11th edn. M100-S22. Standards, vol 32 No 3.
- Antistaphylococcal activity and mode of action of clofazimine. J. Antimicrob. Chemother.. 2004;53:435-440.
- [Google Scholar]
- Preclinical evaluation of novel antibacterial agents by microbiological and molecular techniques. Expert Opin. Invest. Drug.. 2004;13:1045-1063.
- [Google Scholar]
- Antimicrobial resistance in veterinary medicine: an overview. Int J Mol Sci.. 2020;21(6):2-21.
- [Google Scholar]
- A facile synthesis, anti-inflammatory and analgesic activity of isoxazolyl-2,3-dihydrospiro[benzo[f]isoindole-1,3'-indoline]-2',4,9-triones. Bioorg. Med. Chem. Lett. 2013;23:3954.
- [Google Scholar]
- Antimycobacterial activity of spirooxindolo-pyrrolidine, pyrrolizine and pyrrolothiazole hybrids obtained by a three-component regio- and stereoselective 1,3-dipolar cycloaddition Med. ChemCommun.. 2011;2:626.
- [Google Scholar]
- The silver cation (Ag+): antistaphylococcal activity, mode of action and resistance studies. J. Antimicrob. Chemother.. 2013;68:131-138.
- [Google Scholar]
- Pyranopyrazoles asefficient antimicrobial agents: green, one pot and multicomponent approach. Bioorg Chem. 2019;82:324.
- [Google Scholar]
- Sass, P., Br¨otz-Oesterhelt, H. 2013. Bacterial cell division as a target for new antibiotics. Curr Opin Microbiol. 16, 522–530.
- Non-toxic antimicrobials that evade drug resistance. Nat Chem Biol.. 2015;11(7):481-487.
- [Google Scholar]
- Antibiotic resistance: it’s bad, but why isn’t it worse? BMC Biol.. 2017;15(84):2-8.
- [Google Scholar]
- Winn, W., Allen, S., Janda, W., et al. 2006 In Color Atlas and Textbook of Diagnostic Microbiology, Lippincott Williams & Wilkins, Baltimore, Md, USA, 6th edition.
- Diversity-oriented one-pot multicomponent synthesis of spirooxindole derivatives and their biological evaluation for anticancer activities. Tetrahedron. 2016;72:8523-8536.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102996.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







