Translate this page into:
Antimicrobial and cytotoxic activities of novel pyrimidine-2,4-dione connected with 2H-thiopyran derivatives
⁎Corresponding author. idhayadhulla@nmc.ac.in (Idhayadhulla Akbar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The purpose of this study is to develop a new pyrimidine-2,4-dione hybrid with 2H-thiopyran molecules as a potential antibacterial and antifungal agents against clinical pathogens that cause infectious diseases, in addition to conducting the cytotoxic screening.
Methods
The synthesis of 2H-thiopyran connecting pyrimidine-2,4-dionederivatives was carried out in a medium consisting of water with an Mg(II) acetate catalyst. The antimicrobial activity of all synthesized compounds was tested against Gram-positive (Staphylococcus aureus (ATCC-25923), Enterococcus faecalis (clinical isolate), and Gram-negative (Klebsiella pneumoniae (clinical isolate), Escherichia coli (ATCC-2522), and Pseudomonas aeruginosa) bacteria. Antifungal activity was examined in vitro using Aspergillus niger, Candida albicans, Microsporum audouinii, and Cryptococcus neoformans as test organisms (clinical isolates). Cytotoxic assay was also performed in vitro at various concentrations.
Results
The highly active compound in this study was 3-((2,6-di(furan-2-yl)dihydro-2H-thiopyran-4(3H)-ylidene)amino)dihydropyrimidine-2,4(1H,3H)-dione which exhibited the lowest MIC value (8 µg/mL) with broad activity against one Gram-positive and three Gram-negative. The compound, 3-((2,6-di(furan-2-yl)dihydro-2H-thiopyran-4(3H)-ylidene)amino)dihydropyrimidine-2,4(1H,3H)-dione showed least MIC value (MIC: 0.25 µg/mL) against C. albicans. The compound 3-((2,6-bis(4-hydroxyphenyl)dihydro-2H-thiopyran-4(3H)-ylidene)amino)dihydro pyrimidine-2,4(1H,3H)-dione was highly active (GI50 0.03 µm) against HeLa cancer cell lines.
Conclusions
The overall results indicated that a successful preparation of a few of the promising molecules, which are antimicrobials well as cytotoxicity has been achieved.
Keywords
Piperidine-2,4-dione
2H-thiopyran cyclization method
Antibacterial
Antifungal
Cytotoxic activity
1 Introduction
Multidrug-resistant bacteria (MDR) continue to spread globally, posing serious healthcare challenges. The Infectious Disease Society of America has specifically identified six 'ESKAPE' pathogen species (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species). In addition to their potential multidrug resistance mechanisms and diversity of virulence factors, they are dangerous nosocomial pathogens. Thiopyrans are important molecules in medicinal chemistry and are most easily accessible. A thiopyran-containing molecule has high bioactivity and is commonly used in heterocyclic chemistry (Elnagdi et al., 2015). For example (Fig. 1), some important bioactive thiopyrans have been identified in recent years, including hamylomethylene-c-butyrolactone (1), and thiopyrano [2,3-d] thiazoles, which have antitrypanosomal (2), antimicrobial (3), and anticancer (4) activities (Dongxu et al., 2019). Alloxan and uracil, two important components of DNA and RNA, are examples of pyrimidines that can be found in nature as substituted and ring-fused derivatives (Olinski et al., 2021). Thiamine, riboflavin, and folic acid are vitamins that contain pyrimidine rings. The first barbiturate was barbitone, a pyrimidine derivative that features anticonvulsant properties and hypnotic effects. In the past two decades, various well-known and impressive pyrimidine-containing drugs have been introduced as chemotherapeutic agents with a broader scope of application.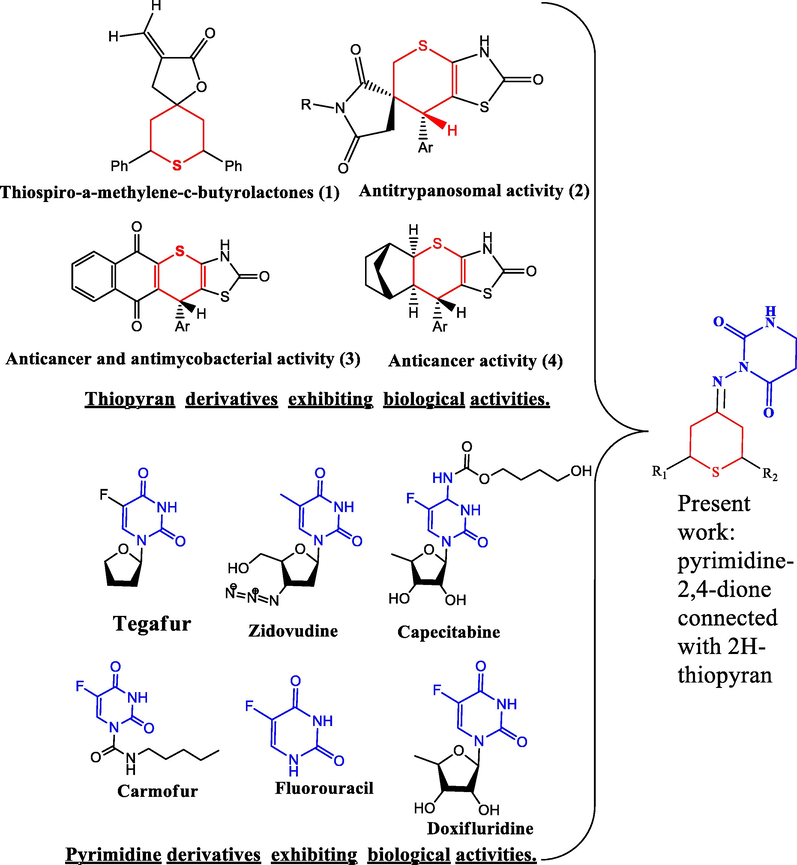
Bioactive compounds of Thiopyran and Pyrimidine.
For example, the antimicrobials trimethoprim, piribedil and phenobarbital, which are frequently used to treat epilepsy and seizures, and zidovudines, which are used in the chemotherapy of HIV (Tommasi et al., 2015). The emergence of MDR strains is a major setback to treating pathogens using existing drugs. In recent years, researchers have discovered only a few natural compounds that are the antibacterial agents. As a result of the restricted availability of natural products, it is critically required to make further efforts to produce innovative synthetic antibiotics that are effective against MDR bacteria (Maffioli et al., 2017). Pyrimidine analogues have previously been developed and used as anticancer and antiviral therapeutics (Seley-Radtke and Yates, 2018). A pyrimidine analogue is generally a small compound with a simple structure and excellent oral bioavailability. The most successful and most commonly used anticancer drug currently is 5-FU, which is one of these analogues (Kumar et al., 2022). One of the most important activities of this medicine is to induce thymine less death by competing with thymidylate synthetase (ThyA) and thymidine kinase (TK) for metabolism. Trimethoprim-sulfamethoxazole is responsible for thymine deficiency deaths because it prevents the delivery of folate, which is essential for the synthesis of thymidine (Chatterjee et al., 2008).
Magnesium can be used in the catalysis of fundamental asymmetric reactions; the limitations of magnesium catalysts in organic reactions are very few. Overall, many cyclization compounds can be used in the catalysis of fundamental asymmetric reactions. These compounds are created by using Lewis acid and another metal catalysis (Choi et al., 2019). In this article we demonstrate the role of Mg(II) catalysts in the chemical synthesis process. In addition to that, the antimicrobial and cytotoxic activities of these compounds are also analysed.
2 Materials and methods
2.1 Chemistry
The Shimadzu 8201pc was used to record FTIR spectra (between 4000 and 400 cm−1). The 1H and 13C NMR spectra of each compound were recorded with Bruker DRX instruments operating at 300 and 75 MHz, respectively. Thin-layer chromatography (TLC) on silica gel plates was used for the purpose of analysing all of the synthesised compounds.
2.1.1 Synthesis of 2-(2,6-di(furan-2-yl)dihydro-2H-thiopyran-4(3H)-ylidene)hydrazine carboxamide (2a)
Compound 1a, at a concentration of 0.01 mol, and semicarbazide, at a concentration of 0.1 mol, were combined and refluxed in ethanol (10 mL) for up to five hours. The mixture was then applied into the cold water. A precipitate was obtained through the process of filtration, and after that, it was washed with water. The final solid substance was recrystallized with the assistance of various alcohols that were suitable for the process. The scheme of compound synthesis was depicted in (Figure S1,S2,S3,S4,S5,S6,S7,S8,S9,S10,S11,S12,S13,S14,S15,S16).
Yield: 82 %; yellow solid; IR (KBr, cm−1): 3421, 2976, 1702, 1621, 672; 1H NMR (300 MHz) δ 10.01(s, 1H, NH), 7.32–7.30(d, 2H, J = 6.34 Hz, furan), 6.76 (s, 2H), 6.32–6.30 (2H, dd, J = 6.34 Hz, J = 7.14 Hz, furan), 6.04–6.02 (d, 2H, J = 7.14 Hz, furan), 3.76–3.71 (dd, 1H, 2C–H, J = 13.80 Hz, J = 11.84 Hz), 3.69–6.67 (2H, dd, J = 13.82 Hz, J = 11.83 Hz), 3.41–3.39 (2H, d, J = 11.82 Hz), 2.12–2.10 (1H, d, J = 11.21 Hz, 3C-Hax, 5C–Hax).
2.1.2 2-(2,6-diphenyldihydro-2H-thiopyran-4(3H)-ylidene)hydrazinecarboxamide(2b)
Light yellow solid; yield: 86 %; IR (KBr): 3441, 3058, 2962, 1710, 1679, 801, 640 cm−1; 1H NMR (300 MHz) δ: 10.08 (s, 1H, NH), 7.47–7.26 (m, 10H, Ar–H), 6.67 (s, 2H), 3.72–3.67 (1H, dd, J = 13.74 Hz, J = 11.80 Hz), 3.49–3.47 (2H, d, J = 11.67 Hz,), 2.18–2.15 (d, 2H, J = 11.37 Hz).
2.1.3 2-(2,6-bis(4-chlorophenyl)dihydro-2H-thiopyran-4(3H)-ylidene)hydrazinecarboxamide (2c)
Light yellow solid; yield: 80 %; IR (KBr): 3489, 3030, 2941, 1725, 1650, 839, 801, 655 cm−1; 1H NMR (300 MHz), δ: 10.02 (s, 1H, NH), 7.12–7.10 (d, 4H, J = 6.24 Hz, Ph), 7.09–7.05 (d, 4H, J = 6.24 Hz, Ph), 6.71 (s, 2H, NH2), 3.79–3.75 (dd, 1H, J = 13.74 Hz, J = 11.37 Hz), 3.59–3.56 (d, 1H, J = 11.35 Hz), 2.86–2.84 (d, 1H, J = 11.48 Hz).
2.1.4 2-(2,6-bis(4-hydroxyphenyl)dihydro-2H-thiopyran-4(3H)-ylidene)hydrazine carboxamide (2d)
Light yellow solid; yield: 89 %; IR (KBr): 3416, 2904, 1750, 1681, 1467, 809, 742 cm−1; 1H NMR (300Mhz): δ 10.18 (s, 1H, C⚌N–NH), 9.55(s, 2H, OH), 7.19–7.15 (d, 4H, J = 6.74 Hz, Ar), 6.65 (s, 2H), 6.43–6.40 (d, 4H, J = 6.78 Hz, Ar), 3.72–3.68 (dd, 2H, J = 13.71 Hz, J = 11.60), 3.30–3.28 (d, 2H, J = 11.64 Hz), 2.23–2.21 (d, 2H, J = 11.37 Hz).
2.1.5 2-(2,6-bis(4-nitrophenyl)dihydro-2H-thiopyran-4(3H)-ylidene)hydrazinecarboxamide (2e)
Light yellow solid; yield: 79 %; IR (KBr): 3409, 3021, 2910, 1713, 1657, 1535, 812, 674 cm−1; 1H NMR (300 MHz): δ 10.04(s, 1H), 8.46–8.40 (4H, d, J = 6.46 Hz, phenyl ring), 7.41–7.39 (d, 4H, J = 6.46 Hz, phenyl ring), 6.68 (s, 2H, NH2), 3.76–3.72 (dd, 2H, J = 13.71 Hz, J = 11.65), 3.71–3.68 (2H, dd, J = 13.74 Hz, J = 11.67), 3.33–3.30 (2H, d, J = 11.64 Hz), 2.09–2.7 (2H, d, J = 11.36 Hz, 3C–Hax, 5C–Hax).
2.1.6 2-(2,6-bis(4-methoxyphenyl)dihydro-2H-thiopyran-4(3H)-ylidene) hydrazine carboxamide (2f)
Light yellow solid; yield: 90 %; IR (KBr): 3439, 2910, 1784, 1607, 811, 697 cm−1; 1H NMR(300 MHz): δ 10.19 (s, 1H, NH), 7.23–7.20 (d, 4H, J = 6.46 Hz, phenyl ring), 6.77 (s, 2H, NH2), 6.37–6.32 (d, 4H, J = 6.46 Hz, phenyl ring), 3.81 (s, 6H, –OCH3), 3.69–3.65(dd, 2H, J = 13.74 Hz, J = 11.67 Hz), 3.47–3.45 (d, 2H, J = 11.67 Hz), 2.17–2.13 (d, 2H, J = 11.39 Hz).
2.1.7 2-(2,6-di-p-tolyldihydro-2H-thiopyran-4(3H)-ylidene)hydrazinecarbox amide (2 g)
Light yellow solid; yield: 88 %; IR(KBr): 3424, 2910, 1721, 1684, 836, 672 cm −1; 1H NMR(300Mhz): δ 10.24(s, 1H), 7.45–7.40(4H, d, J = 6.89 Hz, phenyl ring), 7.29–7.27 (d, 4H, J = 6.89 Hz, phenyl ring), 6.67 (s, 2H, NH2), 3.70 (1H, dd, J = 13.70 Hz, J = 11.64 Hz, 2C–H), 3.72–3.70 (dd, 2H, J = 13.71 Hz, J = 11.64 Hz, 6C–H), 3.36–3.34 (d, 2H, J = 11.66 Hz), 2.24(s, 6H, 2CH3), 2.09–2.06 (d, 2H, J = 11.31 Hz, 3C–Hax, 5C–Hax).
2.1.8 2-(2,6-bis(4-(dimethylamino)phenyl)dihydro-2H-thiopyran-4(3H)-ylidene)hydrazine carboxamide (2 h)
Light yellow solid; yield: 81 %; IR (KBr): 3477, 2941, 1773, 1672, 804, 780 cm −1; 1H NMR (300Mhz): δ 10.17 (s, 1H), 7.09–7.06 (d, 4H, J = 6.89 Hz, phenyl ring), 6.56 (s, 2H, NH2), 6.45–6.42 (d, 4H, Ar), 3.67–3.62 (dd, 2H, J = 13.07 Hz, J = 11.98 Hz), 3.58–3.52 (2H, d, J = 11.41 Hz), 3.10 (12H, s, -N(CH3)2), 2.34–2.32 (d, 2H, J = 11.27 Hz, 3C–Hax, 5C–Hax).
2.1.9 3-((2,6-di(furan-2-yl)dihydro-2H-thiopyran-4(3H)-ylidene)amino)dihydropyrimidine-2,4(1H,3H)-dione (3a)
Compound 2a (0.1 mol), ethyl acrylate (0.1 mol), and Mg (II) acetate (0.03 mol) (4.1 g) were heated in ethanol while being in a state of reflux for seven hours. After the completion of the reaction, the liquid was put into the cold water to prevent any further reaction from taking place. The solid material that had been obtained was cleaned with water and then re-crystallized using appropriate alcohols. TLC analysis was used to determine what the final product was. This approach was also utilised in the production of other compounds (3b–4 h).
Light white color; yield: 76 %; IR (KBr): 3088, 1785, 1625, 678 cm−1; 1H NMR (300 MHz): δ 7.24 (2H, d, J = 7.21 Hz, furan), 6.48–6.53 (dd, 2H, J = 7.21 Hz, J = 7.29 Hz, furan), 6.29–6.26 (d, 2H, J = 7.29 Hz, furan), 6.23(1H, s, NH), 3.93 (2H, s, CH2N), 3.74–3.70 (2H, dd, J = 13.80 Hz, J = 11.85 Hz), 3.46–3.42 (2H, d, J = 11.85 Hz,), 2.14–2.12 (2H, d, J = 11.21 Hz,); 13C NMR (75 MHz): δ 170.7, 161.1, 158.3, 152.1, 142.8, 112.2, 109.6 (8C, furyl ring), 46.9 (CH2N), 39.2 (C2, C6), 34.7 (C3,C5); EI-Ms(m/z): 356.75 (M+, 15 %).
2.1.10 3-((2,6-diphenyldihydro-2H-thiopyran-4(3H)-ylidene)amino)dihydropyrimidine-2,4(1H,3H)-dione (3b)
Light white color; yield: 80 %; IR (KBr): 3024, 3030, 1752, 1627, 852, 661 cm−1; 1H NMR (300 MHz): δ 7.44–7.22 (m, 10H, phenyl ring), 6.29 (1H, s, NH), 3.87 (s, 2H), 3.612–3.59 (dd, 2H, J = 13.73 Hz, J = 11.57 Hz), 3.46–3.42 (d, 2H, J = 11.57 Hz), 2.18–2.14 (d, 2H, J = 11.45 Hz); 13C NMR (75 MHz): δ 172.5, 167.2, 157.1, 141.3, 129.2, 127.2, 126.3 (12C, Ph), 38.4 (C2, C6), 48.7 (CH2N), 35.1 (C3, C5); EI-Ms(m/z): 365.12 (M+, 5 %).
2.1.11 3-((2,6-bis(4-chlorophenyl)dihydro-2H-thiopyran-4(3H)-ylidene)amino)dihydro pyrimidine-2,4(1H,3H)-dione(3c)
Light white color; yield: 85 %; IR(KBr): 3088, 3024, 1742, 1681, 862, 829, 671 cm−1; 1H NMR (DMSO‑d6): δ 7.47–7.42 (d, 4H, J = 6.12 Hz, J = 7.22 Hz, phenyl ring), 7.38–7.22 (d, 4H, J = 6.12 Hz, J = 7.22 Hz, Ar), 6.25(s, 1H, NH), 3.96(2H, s, CH2N), 3.77–3.75 (dd, 2H, J = 13.71 Hz, J = 11.32 Hz), 3.55–3.53 (2H, d, J = 11.31 Hz), 2.87–2.86 (2H, d, J = 11.42 Hz); 13C NMR(75 MHz): δ 171.8, 166.1, 156.2, 142.4, 137.4, 131.8, 128.9 (12C, Ph), 48.1, 35.1 (C3,C5), 31.8 (C2, C6); EI-Ms, m/z: 434.20 (M+, 15 %).
2.1.12 3-((2,6-bis(4-hydroxyphenyl)dihydro-2H-thiopyran-4(3H)-ylidene)amino)dihydro pyrimidine-2,4(1H,3H)-dione(3d)
Light white color; yield: 82 %; IR (KBr): 3045, 1779, 1641, 1452, 820, 618 cm−1; 1H NMR (300Mhz): δ(ppm): 9.51 (s, 2H), 7.19–7.10 (dd, 4H, J = 6.16 Hz, J = 7.29 Hz, Ar), 6.70–6.62 (4H, dd, J = 6.16 Hz, J = 7.29 Hz, Ar), 6.28 (s, 1H, NH), 3.87 (2H, s, CH2N), 3.75–3.67 (dd, 2H, J = 13.74 Hz, J = 11.61 Hz), 3.29–3.27 (2H,d, J = 11.66), 2.20–2.18 (d, 2H, J = 11.39 Hz); 13C NMR(75Mhz): δ 174.8, 168.3, 156.4, 155.8, 133.8, 117.4, 127.1, 46.738.4, 35.1; EI-Ms(m/z): 397.11 (M+, 22 %).
2.1.13 3-((2,6-bis(4-nitrophenyl)dihydro-2H-thiopyran-4(3H)-ylidene)amino)dihydropyrimidine-2,4(1H,3H)-dione (3e)
Light white color; yield: 82 %; IR(KBr): 3071, 3027, 1712, 1685, 1537, 817, 650 cm−1; 1H NMR (300Mhz): δ 8.45–8.40 (d, 4H, J = 6.12 Hz, J = 7.22 Hz, Phenyl ring), 7.29–7.23 (d, 4H, J = 6.12 Hz, J = 7.20 Hz, Ar), 6.26 (1H, s, NH), 3.94 (s, 2H), 3.77–3.76 (dd, 2H, J = 13.70 Hz, J = 11.47 Hz), 3.30–3.28 (2H, d, J = 11.47 Hz), 2.12–2.10 (2H, d, J = 11.39 Hz); 13C NMR (75Mhz): δ 174.5, 167.1, 157.7, 146.0, 145.2, 129.6, 124.0 (12C,Ph), 46.3 (CH2N), 36.8 (C2, C6), 31.1(C3, C5); EI-Ms(m/z): 455.44 (M+, 5 %).
2.1.14 3-((2,6-bis(4-methoxyphenyl)dihydro-2H-thiopyran-4(3H)-ylidene)amino)dihydropyr imidine-2,4(1H,3H)-dione(3f)
Light white color; yield: 86 %; IR (KBr, cm−1): 3081, 1737, 1677, 802, 665; 1H NMR (300 MHz): δ 7.22–7.18 (d, 4H, J = 6.12 Hz, J = 7.22 Hz, Phenyl ring), 6.35–6.31 (d, 4H, J = 6.12 Hz, J = 7.22 Hz, Ar), 6.21 (s, 1H, NH), 3.92(2H, s, CH2N), 3.81 (s, 6H, –2OCH3), 3.74–3.67 (dd, 2H, J = 13.74 Hz, J = 11.67 Hz), 3.47–3.45 (d, 2H, J = 11.67 Hz), 2.18–2.14 (2H, d, J = 11.43 Hz); 13C NMR (75 MHz): δ 174.5, 168.2, 158.6, 157.9, 132.2, 127.8, 114.4 (12C, Ph), 55.8 (2C, C–OCH3), 46.9 (CH2N), 38.4 (C2, C6), 38.1 (C3, C5); EI-Ms(m/z): 425.34 (M+, 16 %).
2.1.15 3-((2,6-di-p-tolyldihydro-2H-thiopyran-4(3H)-ylidene)amino)dihydropyrimidine-2,4-(1H,3H)-dione (3 g)
Light white color; yield: 82 %; IR (KBr): 3090, 1762, 1651, 822, 675 cm−1; 1H NMR (300 MHz): δ 7.45–7.32 (d, 4H, J = 6.19 Hz, J = 7.28 Hz, Ar), 7.29–7.19 (d, 4H, J = 6.19 Hz, J = 7.28 Hz, Ar), 6.21(1H, s, NH), 3.90 (2H, s, CH2N), 3.72–3.69 (dd, 2H, J = 13.74 Hz, J = 11.61 Hz, 2C–H, 6C–H), 3.34–3.32 (d, 2H, J = 11.61 Hz, 3C–Heq, 5C–Heq), 2.26 (s, 6H, 2CH3), 2.05–2.03 (d, 2H, J = 11.36 Hz, 3C–Hax, 5C–Hax); 13C NMR (75 MHz):δ 177.8, 167.8, 157.4, 179.1, 136.5, 135.7, 126.5 (12C, Ph), 46.2(CH2N), 38.4 (C3, C5), 31.7 (C2, C6), 21.2(2C); EI-Ms(m/z): 393.45 (M+, 12 %).
2.1.16 3-((2,6-bis(4-(dimethylamino)phenyl)dihydro-2H-thiopyran-4(3H)-ylidene)amino)dihydropyrimidine-2,4(1H,3H)-dione(3 h)
Light white color; yield: 86 %; IR (KBr): 3046, 1782, 1620, 808, 665 cm−1; 1H NMR (300 MHz): δ 7.19–7.14 (d, 4H, J = 6.45 Hz, J = 7.32 Hz, Ar), 6.42–6.36 (d, 4H, J = 6.45 Hz, J = 7.34 Hz, Ar), 6.26 (1H, s, NH), 3.92 (2H, s, CH2N), 3.69–3.65 (dd, 2H, J = 13.27 Hz, 2CH, 6C–H), 3.56–3.54 (d, 2H, J = 11.46 Hz), 3.11 (s, 12H, –2N(CH3)2), 2.30–2.28 (d, 2H, J = 11.20 Hz, 3C–Hax, 5C–Hax); 13C NMR (75 MHz): δ 173.4,168.6, 159.4, 149.0, 132.6, 131.2, 113.5, (12C, Ph), 47.1(CH2N), 40.8 (4C, –N(CH3)2), 38.4 (C2, C6), 33.6 (C3, C5); EI-Ms(m/z): 451.89 (M+, 22 %).
2.2 Biological activity
2.2.1 In vitro antimicrobial screening
The compounds were assessed for their antibacterial and antifungal activities as per our previous study (Barathikannan et al., 2016; Valsalam et al., 2019). Briefly, the selected bacterial strains (Staphylococcus aureus (ATCC-25923), Enterococcus faecalis (clinical isolate), and Gram-negative (Klebsiella pneumoniae (clinical isolate), Escherichia coli (ATCC-2522), and Pseudomonas aeruginosa) cultured in Mueller Hinton Broth medium for 18 h at 37 °C in an orbital shaker incubator at 150 rpm. Whereas, the selected fungal strains (Aspergillus niger, Candida albicans, Microsporum audouinii, and Cryptococcus neoformans) were cultured in potato dextrose broth medium (Himedia, Mumbai, India) at 28 °C for 72 h in an orbital shaker incubator at 150 rpm. The inoculum was diluted appropriately with Mueller Hinton broth medium and spread on Mueller Hinton Agar plates. About 10 µL samples were loaded and incubated for 24 h for bacteria and 72 h for fungi. After 72 h, the zone of inhibition was analyzed (Ahamed et al., 2018a).
2.2.2 Cytotoxic activity
The cytotoxic activity of each substance was examined. The specifics of the technique utilized to determine the cytotoxic activity are described in our prior study. Briefly, HeLa, MCF-7, and HepG2 were cultured in a cell culture medium and incubated in a CO2 incubator. The sample was loaded at various concentrations and incubated for 24 h. Doxorubicin was used as the standard (Ahamed et al., 2018b).
2.2.3 Statistical analysis
Using the Statistical Package for Social Services, version 25, the mean of the results (LD50 values) was determined based on at least 3 independent assessments, and the standard deviations (SD) were also calculated.
3 Results
3.1 Synthesis of pyrimidine-2,4-dione hybrid with 2H-thiopyran molecules
The chemicals were analyzed using a variety of methods, including FTIR, 1H and 13C NMR, mass spectrometry, and elemental analyses, amongst others. Absorption bands at 3421–3489, 2976–2904, 1621–1684, 1702–1784, and 680–640 cm−1 can be seen in the IR spectrum of 2a–h. These absorption bands correspond to the NH2, NH, -C⚌N, and -C⚌O groups, respectively. The 1H NMR spectra of 2a–h show that the peaks at δ10.01–10.27 and δ 6.77–6.56 correspond to C⚌N–NH and NH2, respectively. The peak at δ 3.79–3.33 shows that proteins are present at positions 3C–Heq and 5C–Heq on the thiopyran moiety; however, the peak at δ2.08–2.86 shows that proteins are present at positions 3C–Hax and 5C–Hax on the thiopyran moiety, which is confirmed by the formation of compounds 2a–h.
Absorption bands at 3088–3024 cm−1, 1685–1620 cm−1, 1785–1712 cm−1, and 678–618 cm−1 were observed in the infrared spectra of 3a–h. These absorption bands were consistent with NH, C⚌N, C⚌O, and C—S—C groups, respectively. The 1H NMR spectra of 3a–h showed a peak at δ 6.21–6.29, which corresponds to NH in the imidazolidine-2,4-dione ring. The peak at δ 3.96–3.90 corresponds to the CH2N protein present in the imidazolidine-2,4-dione ring. The peaks at δ 3.55–3.29 and δ 2.05–2.87 are attributed to proteins 3C–Heq, 5C–Heq, 3C–Hax, and 5C–Hax in the thiopyran moiety. The 13C NMR spectra of 3a–h have peaks at δ 177.7–170.7, 156.4–158.1, and 46.9–48.7 consistent with C⚌O, C⚌N, and CH2N respectively. The peaks at δ 31.8–39.2 and 34.7–38.4 correspond to C2, C6 and C3, and C5 carbon positions, respectively. The mass spectrum revealed the molecular ion peak of chemical 3a at m/z 356.75; other compounds 3b–h was also confirmed by mass spectrometry. Based on spectral characterization, the compounds were confirmed by pyrimidine-2,4-dione connected with 2H-thiopyran derivatives, these compounds were analyzed for antibacterial, antifungal and cytotoxic activity.
3.2 Antibacterial activity
The above observation of biological activity results shows that antibacterial activity, Compounds 2a–h have only much low activities compared to others. The synthesized compound, 3c exhibits the broadest activities against all isolates. It is to be noted that compound, 3c displayed enhanced activity (MIC: 8 μg/mL) against K. pneumoniae than the standard, ciprofloxacin (MIC: 16 μg/mL). At the same time compound 3c exhibited identical potency for the standard, in the case of E. coli, P. aeruginosa and E. faecalis. Ccompounds 3c, 3d, and 3e performed equally well against P. aeruginosa (MIC: 8 μg/mL) and the reference also showed similar activity (MIC: 8 μg/mL) (Table 1).
Compounds
Gram Positive
Gram Negative
S. aureus
E. faecalis
K. pneumoniae
E.coli
P. aeruginosa
2a
64
>100
>100
>100
>100
2b
64
>100
>100
>100
>100
2c
64
>100
>100
>100
>100
2d
64
64
64
>100
>100
2e
64
64
64
64
64
2f
64
64
64
64
64
2 g
64
64
64
64
64
2 h
32
64
64
>100
>100
3a
32
16
16
32
16
3b
16
8
16
32
16
3c
8
16
8
8
8
3d
32
32
8
8
8
3e
16
8
16
8
8
3f
8
16
32
8
16
3 g
8
32
16
16
32
3 h
16
32
32
16
32
Ciprofloxacin
4
16
16
8
8
3.3 Antifungal activity
Compounds 3c, 3d and 3f showed remarkable activity (MIC: 0.25 to 4 μg/mL) against C.albicans compared to other compounds. It is to be emphasized that, compound 3c is excellent in curbing the growth of C. albicans compared to the standard. Likewise, compounds, 3e and 3f showed identical patterns of activity (MIC: 4 μg/mL) against M. audounii to the standard (MIC: 4 μg/mL). In addition, compounds 3d and 3e are also active (MIC: 4 μg/mL) against C. neoformans, showing moderate activity to the standard. The compounds 3a and 3 h showed promising efficacy against C. neoformans (MIC: 1 and 2 g/mL, respectively) (Table 2).
Compounds
A. niger
C. albicans
M. audouinii
C. neoformans
2a
>100
>100
>100
>100
2b
32
>100
32
>100
2c
>100
>100
>100
>100
2d
64
>100
64
>100
2e
64
>100
>100
64
2f
64
64
64
64
2 g
>100
64
64
>100
2 h
>100
32
>100
>100
3a
16
16
32
2
3b
8
8
32
16
3c
8
0.25
8
8
3d
16
4
8
4
3e
8
8
4
4
3f
16
4
4
16
3 g
16
16
8
16
3 h
16
8
16
1
Clotrimazole
1
0.5
4
2
3.4 Cytotoxicity analysis
The compound 3d exhibited remarkable activity against HeLa cells (GI50: 0.03 μm) than the standard doxorubicin (GI50: 0.05 μm). The compound 3c exhibited high activity against MCF-7(GI50: 0.05 μm). The compounds 3e and 3f were moderately effective against HeLa cells (GI50: 0.12 and 0.21 μm). Cytotoxicity results are given in Table 3.
Compounds
HepG2
MCF-7
HeLa
GI50
(µM)TGI
(µM)LC50
(µM)GI50
(µM)TGI
(µM)LC50
(µM)GI50
(µM)TGI
(µM)LC50
(µM)
2a
45.1 ± 0.21
89.1 ± 0.08
>100
42.9 ± 0.09
81.2 ± 0.25
>100
43.2 ± 0.12
89.4 ± 0.34
>100
2b
43.3 ± 0.48
84.2 ± 0.18
>100
40.1 ± 0.18
85.0 ± 0.18
>100
41.0 ± 0.14
87.2 ± 0.18
>100
2c
41.2 ± 0.36
88.1 ± 0.54
>100
48.2 ± 0.17
86.6 ± 0.49
>100
40.5 ± 0.36
88.1 ± 0.08
>100
2d
40.9 ± 0.41
81.6 ± 0.62
>100
45.9 ± 0.29
87.5 ± 0.14
>100
42.2 ± 0.18
82.5 ± 0.15
>100
2e
44.9 ± 0.12
83.2 ± 0.41
>100
41.9 ± 0.18
87.0 ± 0.28
>100
44.3 ± 0.20
81.0 ± 0.32
>100
2f
43.3 ± 0.13
85.9 ± 0.28
>100
42.2 ± 0.15
80.3 ± 0.32
>100
48.1 ± 0.28
87.1 ± 0.17
>100
2 g
41.4 ± 0.17
82.8 ± 0.30
>100
41.5 ± 0.36
86.3 ± 0.17
>100
46.1 ± 0.32
84.7 ± 0.09
>100
2 h
40.7 ± 0.31
82.4 ± 0.14
>100
40.6 ± 0.29
80.1 ± 0.14
>100
41.0 ± 0.31
82.6 ± 0.12
>100
3a
15.9 ± 0.26
33.9 ± 0.19
63.8 ± 0.25
11.9 ± 0.14
27.6 ± 0.95
57.0 ± 0.14
32.8 ± 0.39
61.0 ± 0.05
>100
3b
16.3 ± 0.19
35.3 ± 0.16
61.2 ± 0.14
15.2 ± 0.27
30.1 ± 0.36
61.2 ± 0.32
12.1 ± 0.14
27.1 ± 0.24
65.3 ± 0.18
3c
15.4 ± 0.03
32.5 ± 0.20
62.5 ± 0.10
0.05 ± 0.36
0.19 ± 0.14
0.32 ± 0.17
20.2 ± 0.45
44.7 ± 0.22
92.8 ± 0.18
3d
11.7 ± 0.41
22.1 ± 0.27
5.2 ± 0.12
02.6 ± 0.31
05.5 ± 0.08
12.4 ± 0.21
0.03 ± 0.04
0.32 ± 0.32
1.90 ± 0.32
3e
15.9 ± 0.36
33.9 ± 0.21
62.4 ± 0.21
11.9 ± 0.29
24.6 ± 0.12
48.0 ± 0.14
0.12 ± 0.19
0.24 ± 0.38
0.65 ± 0.30
3f
16.3 ± 0.48
35.3 ± 0.29
61.2 ± 0.11
15.2 ± 0.20
30.1 ± 0.19
62.1 ± 0.51
0.21 ± 0.21
0.46 ± 0.49
0.95 ± 0.41
3 g
15.4 ± 0.95
32.5 ± 0.31
62.5 ± 0.31
13.5 ± 0.26
26.9 ± 0.45
53.5 ± 0.32
10.3 ± 0.28
24.7 ± 0.62
43.8 ± 0.32
3 h
01.7 ± 0.15
02.1 ± 0.04
04.7 ± 0.11
12.6 ± 0.21
24.5 ± 0.32
48.4 ± 0.15
12.8 ± 0.91
24.6 ± 0.18
46.5 ± 0.12
Doxorubicin
0.01 ± 0.07
0.13 ± 0.15
0.58 ± 0.51
0.02 ± 0.31
0.21 ± 0.20
0.74 ± 0.14
0.05 ± 0.18
0.41 ± 0.24
0.88 ± 0.04
4 Discussion
The synthesis of 2H-thiopyran connecting pyrimidine-2,4-dione derivatives was carried out in a medium consisting of water with an Mg(II) acetate catalyst. It has not been previously reported that Mg(II) acetate can be useful in the synthesis of products involving 2H-thiopyran and piperidine-2,4-dione derivatives, as far as our research has been able to determine. Fig. 2 depicts the synthesis method, while Fig. 3 depicts the reaction mechanism. The preparative routes of 1a–h and 2a–h were as per the previously reported method (Ahamed et al., 2018). It is a novel one, and there are no published reports on the use of Mg (II) acetate for the preparation of five-member and six-member cyclization reaction products. Compounds 3a–3 h were prepared by cyclization method in a water medium using Mg (II) acetate as the catalyst.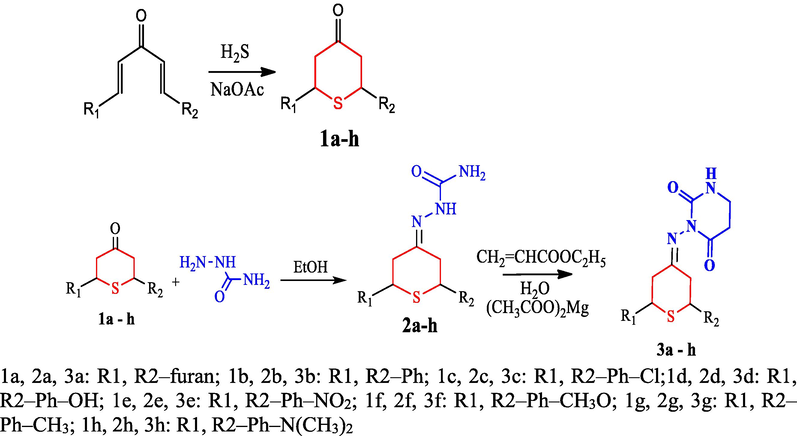
Synthesis route for the preparation of 3a–h.
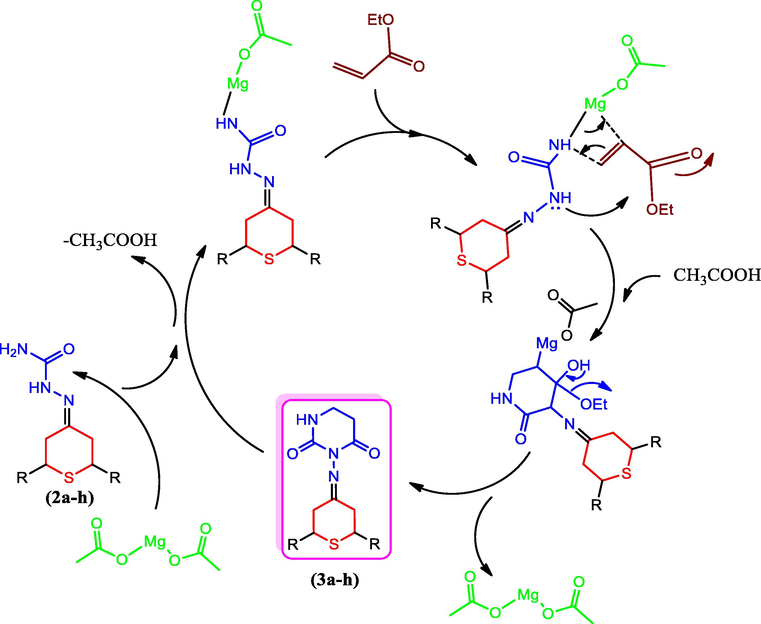
Synthesis mechanism of compounds 3a–3 h.
The synthesised compounds showed antibacterial activity against various drug-resistant bacteria. The antimicrobial activity of synthesis compounds as described previously. Previously, mutant Staphylococcus aureus strains with aberrant ThyA function have been identified in patients receiving long-term TMP-SMX treatment (Kriegeskorte et al., 2014). According to the findings of several studies, TMP-SMX resistance mutants transform into thymidine-dependent variations with decreased pathogenicity and growth rate. Tegafur is a chemotherapeutic agent that is derived from 5-FU and is used as part of the treatment process for various cancers. The compound is present in the combination drug tegafur/uracil. 5-FU is formed after metabolization (Cherazard et al., 2017). The compound 3d was highly effective against all the Gram-negative bacteria (MIC: 8 μg/mL). The compound 3e was also effective (MIC: 8 μg/mL) against various Gram-negative (E. coli and P. aeruginosa) and Gram-positive (E. faecalis) bacterium.
Intravenous administration is the treatment method of choice for cancers such as colon, oesophageal, stomach, pancreatic, breast, and cervical. Moore and colleagues discovered that a cream containing 5-FU was effective in treating actinic keratosis, basal cell carcinoma, and skin warts (Moore, 2009). The synthesized compounds showed cytotoxic activity against various cancer cell lines. Doxifluridine can be metabolized into 5-FU by enzymes thymidine phosphorylase and pyrimidine nucleoside phosphorylase (Fatfat et al., 2022). Additionally, it is derived from capecitabine. Several types of cancers exhibit increased expression of pyrimidine-nucleoside phosphorylase and thymidine. The active metabolite of 5-FU results in cell death by inhibiting DNA synthesis. The antineoplastic drug, carbofur (1-hexylcarbamoyl-5-fluorouracil) is a pyrimidine analogue that is used for treating cancer. It is possible to administer a lipophilic-masked analogue of 5-FU, a fluorouracil derivative, by oral administration. Thymidine phosphorylase and dihydropyrimidine dehydrogenase were active against human tumour cell lines (Ogata et al., 2007).
5 Conclusions
The new series of hybrids of piperidine-2,4-diones synthesized were tested for antimicrobial and cytotoxic activities. Compound 3d (GI50: 0.03 µM) exhibited potential cytotoxicity against the HeLa cell lines compared to doxorubicin. Overall results revealed that only a pair of the tested compounds has considerable inhibitory potency compared to the respective controls for antibacterial, antifungal, and cytotoxicity. Therefore, thiopyran connected with piperidine-2,4-dione may serve as lead molecules and further in-depth studies are underway for the development of antimicrobial and anticancer drugs at the in vivo level.
6 Authors’ contribution
AA and IAA conducted all biological experiments while RS, and AI conceived the research idea and designed the study. Allauthors read and approved the manuscript.
Acknowledgements
The authors are thankful to the Department of Science and Technology, Government of India for providing financial support (Instrumentation facility) to Nehru Memorial College, Puthanampatti, Tamil Nadu, India under the FIST program-2019, New Delhi, India. This research was supported by Researchers Supporting Project number (RSPD2023R718), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis of novel pyridine-connected piperidine and 2H-thiopyran derivatives and their larvicidal, nematicidal, and antimicrobial activities. Mex. Chem. Soc. 2018;62(4):135-147.
- [Google Scholar]
- Antimicrobial, anticoagulant, and cytotoxic evaluation of multidrug resistance of new 1, 4-dihydropyridine derivatives. Saud. J. Biol. Sci.. 2018;25(6):1227-1235.
- [Google Scholar]
- Chemical analysis of Punica granatum fruit peel and its in vitro and in vivo biological properties. BMC Complimnetary and Alternative Medicine.. 2016;16:264.
- [Google Scholar]
- In vivo mutations of thymidylate synthase (encoded by thyA) are responsible for thymidine dependency in clinical small-colony variants of Staphylococcus aureus. J. Bacteriol.. 2008;190:834-842.
- [Google Scholar]
- Antimicrobial resistant Streptococcus pneumoniae: prevalence, mechanisms, and clinical implications. Am. J. Therapeut.. 2017;24(3):e361-e369.
- [Google Scholar]
- A divergent synthetic pathway for pyrimidine-embedded medium-sized azacycles through an N-quaternizing strategy. Chem. Sci.. 2019;10:569-575.
- [Google Scholar]
- Recent developments in utility of green multi-component reactions for the efficient synthesis of polysubstituted pyrans, thiopyrans, pyridines, and pyrazoles. Mol. Diversity. 2015;19(3):625-651.
- [Google Scholar]
- Micelles as potential drug delivery systems for colorectal cancer treatment. World J. Gastroenterol.. 2022;28(25):2867.
- [Google Scholar]
- Inactivation of thyA in Staphylococcus aureus attenuates virulence and has a strong impact on metabolism and virulence gene expression. MBio. 2014;5(4):e01447-e10514.
- [Google Scholar]
- An Overview of the Synthetic Route to the Marketed Formulations of Pyrimidine: A Review. Mini Rev. Med. Chem.. 2022;22(6):884-903.
- [Google Scholar]
- Antibacterial nucleoside-analog inhibitor of bacterial RNA polymerase. Cell. 2017;169(7):1240-1248.
- [Google Scholar]
- Clinical applications for topical 5-fluorouracil in the treatment of dermatological disorders. J. Dermatol. Treatment.. 2009;20:328-335.
- [Google Scholar]
- Significance of thymidine phosphorylase in metronomic chemotherapy using CPT-11 and doxifluridine for advanced colorectal carcinoma. Anticancer Res.. 2007;27:2605-2611.
- [Google Scholar]
- Genomic Uracil and Aberrant Profile of Demethylation Intermediates in Epigenetics and Hematologic Malignancies. Int. J. Mol. Sci.. 2021;22(8):4212.
- [Google Scholar]
- The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold. Antiviral Res.. 2018;154:66-86.
- [Google Scholar]
- ESKAPEing the labyrinth of antibacterial discovery. Nat. Rev. Drug Discovery. 2015;14(8):529-542.
- [Google Scholar]
- Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. Journal of Photochemistry & Photobiology, B: Biology. 2019;191:65-74.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102588.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







