Translate this page into:
Anticancer effects of pH- sensitive carvacrol zinc oxide quantum dots on DMBA induced mammary carcinoma in female sprague dawley rats
⁎Corresponding author at: Department of Biochemistry and Biotechnology, Faculty of Science, Annamalai University, India. bionalini@gmail.com (Nalini Namasivayam) manojsrinivasan1002@gmail.com (Nalini Namasivayam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
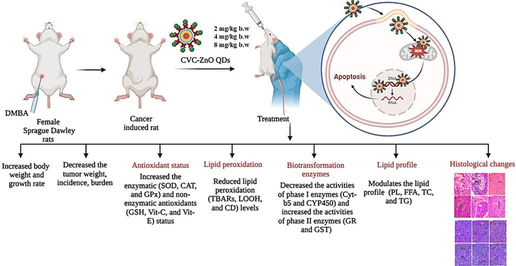
pH-sensitive CVC-ZnO QDs show significant anticancer effects on DMBA-induced mammary carcinoma. They reduce tumor growth, improve antioxidant status, and lower lipid peroxidation. CVC-ZnO QDs modulate biotransformation enzyme activities and positively impact lipid profiles. They prevent DMBA-induced tissue damage in mammary and kidney tissues, indicating its potential as a safe and effective anticancer agent for mammary carcinoma.
Abstract
Objectives
The current research explores the anticancer effects of pH sensitive CVC-ZnO QDs (Carvacrol-loaded Zinc Oxide Quantum Dots) on DMBA induced mammary carcinoma in rats.
Methods
Female SD rats were used, and mammary cancer was induced by chemical carcinogen via subcutaneous injection near the mammary gland. Different concentrations of CVC-ZnO QDs were orally supplemented to evaluate the optimum dose. We assessed the growth rate, body weight changes, tumor volume, tumor incidence and tumor burden in both the inducer and treatment groups. We also evaluated the biochemical parameters (antioxidant status, lipid peroxidation, detoxification enzymes, and lipid profile) and histopathological changes in the kidney and mammary tissues.
Results
Our findings indicate that CVC-ZnO QDs treated rats significantly decreased the tumor weight, incidence, burden, lipid peroxidation levels, phase I detoxification enzyme activities and increased the body weight, phase II detoxification enzyme activities, and antioxidant status compared to the DMBA alone treated rats. CVC-ZnO QDs treatment also altered the lipid profile of plasma and mammary tissue. Furthermore, histopathological results confirmed that the CVC-ZnO QDs protect against DMBA-mediated damage to the mammary and kidney.
Conclusion
The findings indicate that the CVC-ZnO QDs administered at 4 mg/kg b.w exhibited a significant anticancer effect against DMBA-induced mammary cancer.
Keywords
Antioxidant
Carvacrol
Detoxification enzymes
DMBA
Mammary cancer
ZnO QDs
1 Introduction
Breast cancer begins in the breast tissue and is the most prevalent cancer impacting women globally. Based on the latest statistics, breast cancer represents around 30 % of newly diagnosed cancers among women (Cao et al., 2021). While there have been notable strides in diagnosing and treating breast cancer, it remains a pressing public health issue, underscoring the demand for innovative and efficient methods in both therapy and diagnosis (Anwar et al., 2020).
Quantum dots (QDs) represent nanocrystals possessing exceptional optical and electronic characteristics, rendering them highly prospective for various biomedical purposes such as cancer detection and treatment. QDs are composed of semiconductor materials that emit bright and stable fluorescent signals when excited by light, making them ideal for imaging and sensing applications (He et al., 2019). Recent investigations have highlighted the potential of QDs for breast cancer therapy and imaging. For instance, QDs have been used for imaging breast cancer cells, which allows the detection of cancerous tissues with high accuracy and specificity (Rajitha et al., 2021). In addition, QDs have also been utilized to deliver therapeutic drugs to breast cancer cells, showing promising results in preclinical studies (Tagde et al., 2020). Edis et al. (2021) reported using QDs to deliver chemotherapeutic agents to mammary cancer cells, showing reduced toxicity and improved efficacy compared to conventional chemotherapy.
DMBA is a potent chemical carcinogen used to induce mammary tumors in animal models. The DMBA induced breast cancer model in female SD (Sprague Dawley) rats has been widely used to study the molecular and cellular mechanisms underlying the progression and development of mammary cancer (Kerdelhué et al., 2016). This ideal has additionally been employed to assess the effectiveness of different chemotherapy drugs and identify new therapeutic targets for treating breast cancer.
Carvacrol (CVC), a phenolic compound found in oregano, has been shown to possess anticancer properties (Li et al., 2021). Zinc oxide quantum dots (ZnO QDs), on the other hand, are nanomaterials that have been investigated for their potential use in chemotherapy because of their characteristics, such as optical attributes and a significant (Jiang et al., 2018).
From our initial investigation, it has been noted that nanoformulations containing CVC-ZnO QDs exhibit pH-responsive behavior, leading to their release specifically in the acidic pH conditions found within the tumor microenvironment. Consequently, this targeted drug delivery approach primarily directs therapeutic agents towards cancer cells while having a lesser impact on normal cells. The aim of this study is to explore the anticancer potential of pH-sensitive CVC-ZnO QDs in female SD rats with DMBA-induced mammary carcinoma. This investigation entails an examination of various parameters, including tumor growth rate, biochemical analysis in both plasma and mammary tissue, as well as histological alterations in the kidney and mammary tissue.
2 Materials and methods
2.1 Chemicals
Carvacrol and DMBA were acquired from Sigma Aldrich Chemical Pvt. Ltd., while all other chemicals utilized in this study were of analytical quality.
Synthesis of CVC-ZnO QDs
ZnO QDs were prepared from zinc acetate dihydrate and sodium hydroxide precursors. They were formed by mixing the precursors in anhydrous ethanol at 60 °C for 5 h, followed by centrifugation and dissolution in anhydrous ethanol. CVC was loaded onto the QDs during 12 h of continuous stirring in a colloidal suspension. The prepared CVC-ZnO QDs had spherical shape with an average crystal size of 7.64 nm.
2.2 Animal model
We purchased 36 female SD rats from the Biogen Laboratory Animal Facility in Bangalore, India, registered under CPCSEA (Reg No: 971/PO/RcBiBt/S/2006/CPCSEA). The rats were between 6 and 8 weeks old and weighed 100 g and 120 g. These rats were housed in the Central Animal House, RMMC (Rajah Muthiah Medical College), Annamalai University. Prior to initiating the experiment, we ensured compliance with the guidelines by securing the necessary approval from the Institutional Animal Ethics Committee for the Supervision and Administration of Experimental Animals (IAEC Proposal No: AU-IAEC/1321/6/22). The rats were acclimated to the laboratory conditions, which included controlled humidity (50 ± 10 %), a temperature of 24 ± 2˚C and a 12 h (light/dark cycle). They were provided with stranded feed (Pellet diet- cereals, animal and vegetal proteins vitamins & minerals) and water throughout the study.
2.3 Induction mammary carcinoma and CVC-ZnO QDs preparation
Mammary carcinoma was induced in rats by administering a single dose of DMBA (25 mg/rat) through an emulsion of physiological saline (0.25 ml) and sunflower oil (0.75 ml). CVC-ZnO QDs were suspended in 0.1 % DMSO.
2.4 Experimental design
Thirty-six female SD rats were randomly divided into 6 groups, each consisting of six. The experimental procedure conducted in this study is illustrated in Fig. 1. After a study duration of 13 weeks, all animals were euthanized through cervical dislocation. Several parameters were measured and calculated, including animal body weight, growth rate, tumor weight, and tumor characteristics. Blood was collected for biochemical analysis and subjected to centrifugal force for 15 min at 1000g to separate the plasma. Mammary tissue was removed from each rat, processed by homogenization with a suitable buffer, and then subjected to centrifugation. The resulting supernatant was utilized for biochemical analyses. Additionally, kidney and mammary tissues were preserved (10 % formalin) for histological studies.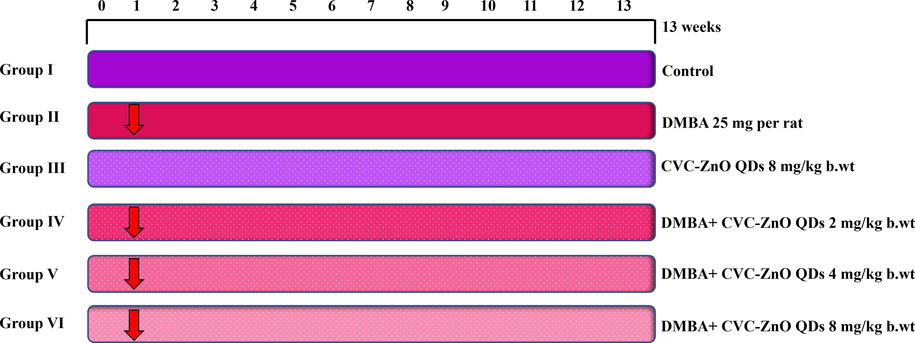
Experimental design.
2.5 Biochemical analysis
The biochemical analysis was performed in the control and experimental rat plasma and mammary tissue. The Table 1 shows the biochemical parameters and their methods.
Biochemical parameters
Method
Antioxidant status
SOD (superoxide dismutase)
Kakkar et al. (1984)
CAT (catalase)
Sinha (1972)
GPx (Glutathione peroxidase)
Rotruck et al. (1973)
GSH (reduced glutathione)
Beutler and Kelly (1963)
Vit-E (Vitamin-E)
Omaye et al. (1979)
Vit-C (Vitamin-C)
Palan et al. (1991)
Lipid peroxidation
TBAR (Thiobarbituric acid reactive substances)
Yagi (1987) in plasma
Ohkawa et al. (1979) in mammary tissue
LOOH (lipid hydroperoxide)
Jiang et al. (1992)
CD (Conjugated dienes)
Rao and Recknagel (1968)
Phase I enzymes
CYP450 (cytochrome-p450)
Omura and Sato (1964)
Cyt-b5 (cytochrome b5)
Omura and Sato (1964)
Phase II enzymes
GST (Glutathione s-transferase)
Habig et al. (1974)
GR (Glutathione reductase)
Carlberg and Mannervik (1985)
Lipid profile
TC (Total cholesterol)
Zlatkis et al. (1953)
TG (Triglycerides)
Foster and Dunn (1973)
PL (Phospholipid)
Zilversmit and Davis (1950)
FFA (Free fatty acids)
Falholt et al. (1973)
2.6 Histopathological studies
The control and experimental rats kidney and mammary tissues were examined histopathologically. After fixation in 10 percent buffered formalin, the tissues were regularly treated, embedded in paraffin, and sliced into 2–3 mm sections using a rotary microtome. The resulting tissue slices were placed on glass slides and subjected to H&E (hematoxylin and eosin) staining.
2.7 Statistical analysis
The mean ± SD is used to represent experimental values. One-way ANOVA (analysis of variance) was used to compare the mean values across groups, and DMRT was used for multiple comparisons. And the significance was set at p ≤ 0.05.
3 Results
3.1 Effect of CVC-ZnO QDs on growth rate, body weight, and tumor characteristics
Table 2 represents the growth rate, body weight, and tumor characteristics of the control and experimental rats. The body weight was assessed based on the differences observed in the initial and final stages between the control and experimental rats. Rats treated with DMBA alone exhibited a notable reduction in growth rate and body weight. Nevertheless, administering CVC-ZnO QDs orally at various doses resulted in a substantial enhancement of growth rate and body weight in cancer bringing rats (DMBA-induced). In contrast to the control group of rats, the group administered CVC-ZnO QDs showed no notable variances in their growth rate and body weight. Tumor volume was measured using the formula V = 4/3π (D1/2) (D2/2) (D3/2), where D1, D2 and D3 are the three diameters (in mm) of the tumor; (n) indicates total number of rats bearing tumors. Tumor burden was calculated by multiplying the tumor volume and total number of tumors. Values are expressed as mean ± SD for six rats in each group. Values not sharing a common superscript (a,b,c) differ significantly at p < 0.05 (DMRT).
Groups
Initial body weight (g)
Final body weight
Growth rate (%)
Total number of tumors (n)
Tumor weight (g)
Tumor incidence (%)
Tumor volume
(mm3/rat)
Tumor burden (mm/rat)
Control
111.92 ± 6.1
197.00 ± 6.3a
76.01a
0/6
–
–
–
DMBA
116.99 ± 3.1
157.90 ± 7.9b
34.96b
6/6
7.53
100
33.46 ± 3.44a
200.77 ± 20.65a
CVC-ZnO QDs (8 mg)
115.26 ± 4.7
205.40 ± 9.3a
78.2a
0/6
–
–
–
DMBA + CVC-ZnO QDs (2 mg)
109.40 ± 5.3
174.81 ± 7.6c
59.78c
3/6
6.35
50
15.75 ± 0.59b
47.26 ± 1.79b
DMBA + CVC-ZnO QDs (4 mg)
112.05 ± 4.2
186.62 ± 5.9a
66.55a
2/6
5.49
33.33
8.78 ± 0.5c
17.57 ± 1.05c
DMBA + CVC-ZnO QDs (8 mg)
112.80 ± 2.72
195.13 ± 4.05a
72.98a
0/6
–
–
–
–
Tumor burden and volume in rats were typically evaluated by measuring the size of tumors using calipers and calculated the tumor volume using the formula V = 4/3π (D1/2) (D2/2) (D3/2). In the DMBA alone-induced rats, 100 % tumor development was observed, with tumor burden (200.77 mm3) and tumor volume (33.46 mm3). Oral supplementation with CVC-ZnO QDs to cancer-induced rats markedly decreased tumor volume, burden and incidence. In control and CVC-ZnO QDs alone animals, no tumors were seen. Hence, among the 2, 4, and 8 mg/kg b.w doses, the activity of CVC-ZnO QDs at 4 mg/kg b.w had an impact in the DMBA-induced rats.
3.2 Effect of CVC-ZnO QDs on antioxidant status
The enzymatic and non-enzymatic antioxidant activities of control and experimental rats are shown in Tables 3 and 4. The presence of antioxidant in the plasma and mammary tissues of rats treated with DMBA alone was notably reduced when compared to the control group. The addition of CVC-ZnO QDs at various concentrations notably enhanced antioxidant activities in comparison to rats induced with DMBA alone. Particularly observed outcomes were evident in rats that received supplementation of CVC-ZnO QDs at a dose of 4 mg/kg b.w. Data are expressed as the mean ± SD for six rats in each group. Values not sharing a common superscript (a,b,c) differ significantly at p < 0.05 (DMRT). Units for SODX, CATY and GPXZ are expressed as the amount of enzyme required to inhibit 50 % of NBT reduction, micromoles of H2O2 utilized/second, and micromoles of glutathione utilized/minute, respectively. Data are expressed as the mean ± SD for six rats in each group. Values not sharing a common superscript (a,b,c) differ significantly at p < 0.05 (DMRT). Units for SODX, CATY and GPXZ are expressed as the amount of enzyme required to inhibit 50 % of NBT reduction, micromoles of H2O2 utilized/second, and micromoles of glutathione utilized/minute, respectively.
Groups
SOD UX/mL
CAT UY/mL
GPx UZ/mL
GSH mg/dL
Vit C mg/dL
Vit E mg/dL
Control
5.23 ± 0.78a
3.12 ± 0.41a
57.67 ± 3.34a
18.42 ± 1.03a
6.67 ± 0.92a
4.84 ± 0.84a
DMBA
2.73 ± 0.56b
1.61 ± 0.24b
28.69 ± 2.71b
9.87 ± 0.82b
2.52 ± 0.76b
1.67 ± 0.61b
CVC-ZnO QDs (8 mg)
5.29 ± 0.71a
3.18 ± 0.38a
57.71 ± 2.43a
18.47 ± 0.75a
6.72 ± 0.43a
4.88 ± 0.72a
DMBA + CVC-ZnO QDs (2 mg)
4.68 ± 0.57c
1.99 ± 0.27c
41.67 ± 3.52c
14.45 ± 0.94c
5.43 ± 0.84c
3.47 ± 0.42c
DMBA + CVC-ZnO QDs (4 mg)
5.02 ± 0.42a
2.96 ± 0.24a
56.28 ± 2.84a
17.99 ± 0.72a
6.26 ± 0.64a
4.55 ± 0.75a
DMBA + CVC-ZnO QDs (8 mg)
5.21 ± 0.28a
3.09 ± 0.26a
57.64 ± 2.76a
18.39 ± 0.93a
6.64 ± 0.52a
4.81 ± 0.48a
Groups
SOD UX/mg of protein
CAT UY/mg of protein
GPx UZ/mg of protein
GSH mg/100 g wet tissue
Vit C
mg/100 g wet tissue
Vit E
mg/100 g wet tissue
Control
13.65 ± 0.78a
61.04 ± 2.45a
9.65 ± 0.52a
11.86 ± 2.63a
6.42 ± 0.34a
5.63 ± 0.24a
DMBA
7.82 ± 0.63b
38.95 ± 2.84b
4.81 ± 0.49b
5.47 ± 1.55b
2.13 ± 0.29b
1.94 ± 0.26b
CVC-ZnO QDs (8 mg)
13.71 ± 0.71a
61.08 ± 3.10a
9.68 ± 0.53a
11.89 ± 2.61a
6.46 ± 0.32a
5.67 ± 0.30a
DMBA + CVC-ZnO QDs (2 mg)
10.64 ± 0.62c
49.38 ± 2.78c
7.24 ± 0.48c
9.85 ± 2.01c
5.68 ± 0.33c
4.86 ± 0.23c
DMBA + CVC-ZnO QDs (4 mg)
13.23 ± 0.64a
59.06 ± 2.64a
9.37 ± 0.56a
11.54 ± 2.42a
6.09 ± 0.27a
5.28 ± 0.27a
DMBA + CVC-ZnO QDs (8 mg)
13.62 ± 0.66a
60.99 ± 2.45a
9.61 ± 0.55a
11.79 ± 2.46a
6.39 ± 0.29a
5.61 ± 0.21a
3.3 Effect of CVC-ZnO QDs on lipid peroxidation
The lipid peroxidation levels in the plasma and mammary tissue of both control and experimental rats are detailed in Table 5. The findings suggest that the lipid peroxidation levels were significantly elevated in both the plasma and mammary tissues of rats subjected to DMBA treatment alone when compared to the control group. Oral supplementation of CVC-ZnO QDs to DMBA-traded rats significantly reduced lipid peroxidation levels. In comparison with control rats, CVC-ZnO QDs alone treated demonstrated no significant variation in lipid peroxidation within the plasma and mammary tissue. Data are expressed as the mean ± SD for six rats in each group. Values not sharing a common superscript (a,b,c) differ significantly at p < 0.05 (DMRT).
Plasma
Mammary tissue
Groups
TBARs
(n mol/ml)
LOOH (mmol/ml)
CD (µg/ml)
TBARs (n mol/mg protein)
LOOH (mmol/mg protein)
CD (mg/g protein)
Control
2.56 ± 0.24a
2.57 ± 0.22a
0.94 ± 0.11a
1.92 ± 0.13a
0.96 ± 0.11a
1.67 ± 0.09a
DMBA
5.32 ± 0.22b
4.41 ± 0.28b
2.46 ± 0.18b
3.44 ± 0.11b
1.84 ± 0.14b
2.94 ± 0.11b
CVC-ZnO QDs (8 mg)
2.49 ± 0.27a
2.51 ± 0.21a
0.90 ± 0.12a
1.89 ± 0.16a
0.94 ± 0.14a
1.69 ± 0.09a
DMBA + CVC-ZnO QDs (2 mg)
3.51 ± 0.22c
2.28 ± 0.22c
1.32 ± 0.17c
2.36 ± 0.16c
1.40 ± 0.17c
2.27 ± 0.12c
DMBA + CVC-ZnO QDs (4 mg)
2.61 ± 0.21a
2.73 ± 0.29a
1.01 ± 0.12a
2.09 ± 0.14a
0.99 ± 0.11a
1.84 ± 0.11a
DMBA + CVC-ZnO QDs (8 mg)
2.60 ± 0.27a
2.61 ± 0.27a
0.97 ± 0.19a
1.95 ± 0.16a
0.98 ± 0.16a
1.71 ± 0.14a
3.4 Effect of CVC-ZnO QDs on detoxification enzymes
Table 6 displays the enzymatic detoxification activities observed in the mammary tissues of both control and experimental groups of rats. The rats treated with DMBA alone exhibited significantly higher phase I enzyme activity than the control rats. Supplementation with CVC-ZnO QDs reduced DMBA-metabolizing phase I enzyme activities. Compared to the control rats the rats treated with DMBA alone showed a notable decrease in the enzyme activities of phase II. Conversely, treatment through CVC-ZnO QDs significantly improved the phase II enzymes activities in the DMBA-treated rats. Notably, the medium dose of CVC-ZnO QDs at 4 mg/kg b.w demonstrated a more pronounced effect than the other two. Cytochrome P450 reductase—nmol/mg of microsomal protein, Cytochrome b5 reductase—nmol/mg of microsomal protein, Glutathione S-transferase–µmol of CDNB-GSH conjugate formed/mg microsomal protein/min, Glutathione reductase–µmol of NADPH oxidized/mg microsomal protein/min. Values are expressed as mean ± SD for six rats in each group. Values not sharing a common superscript (a,b,c) differ significantly at p < 0.05 (DMRT).
Groups
Cytochrome P450
Cytochrome b5
GST
GR
Control
0.96 ± 0.09a
0.49 ± 0.03a
1.68 ± 0.24a
4.67 ± 1.65a
DMBA
1.67 ± 0.16b
0.98 ± 0.09b
0.78 ± 0.08b
1.57 ± 0.97b
CVC-ZnO QDs (8 mg)
0.94 ± 0.07a
0.48 ± 0.04a
1.71 ± 0.26a
4.77 ± 1.87a
DMBA + CVC-ZnO QDs (2 mg)
1.29 ± 0.08c
0.76 ± 0.03c
0.98 ± 0.28c
2.99 ± 1.34c
DMBA + CVC-ZnO QDs (4 mg)
1.04 ± 0.08a
0.58 ± 0.05a
1.54 ± 0.27a
3.86 ± 1.48a
DMBA + CVC-ZnO QDs (8 mg)
0.98 ± 0.07a
0.51 ± 0.04a
1.64 ± 0.24a
4.65 ± 1.64a
3.5 Effect of CVC-ZnO QDs on lipid profile
Table 7 represents the lipid profile of the control and experimental rats in the plasma and mammary tissues. In the DMBA- alone induced rats, concentrations of PL, FFA, TC, and TG were significantly higher in the plasma compared to the control rats. However, in mammary tissues of DMBA alone rats the PL and FFA levels were decreased and the TC and TG levels were significantly increased. CVC-ZnO QDs administration to DMBA-induced rats brought back the levels to near those of the control values in both the plasma and mammary tissues. Values are expressed as mean ± SD for six rats in each group. Values not sharing a common superscript (a,b,c) differ significantly at p < 0.05 (DMRT).
Plasma (mg/dl)
Mammary tissue (mg/g)
Groups
TC
TG
PL
FFA
TC
TG
PL
FFA
Control
79.32 ± 6.57a
75.67 ± 6.12a
101.65 ± 9.3a
12.47 ± 1.51a
6.41 ± 0.42a
5.49 ± 0.37a
15.48 ± 0.98a
10.26 ± 0.81a
DMBA
120.41 ± 9.42b
128.34 ± 10.04b
147.68 ± 11.75b
17.92 ± 1.82b
15.74 ± 0.57b
10.57 ± 0.49b
9.82 ± 0.67b
6.78 ± 0.44b
CVC-ZnO QDs (8 mg)
78.75 ± 6.31a
75.10 ± 5.87a
100.97 ± 9.64a
11.97 ± 1.47a
6.37 ± 0.43a
5.37 ± 0.41a
15.73 ± 1.01a
10.31 ± 0.92a
DMBA + CVC-ZnO QDs (2 mg)
101.92 ± 7.57c
108.75 ± 7.68c
131.56 ± 10.57c
15.34 ± 1.58c
10.64 ± 0.47c
8.59 ± 0.39c
11.54 ± 0.92c
8.97 ± 0.86c
DMBA + CVC-ZnO QDs (4 mg)
93.24 ± 5.91a
88.64 ± 6.31a
110.37 ± 9.71a
13.72 ± 1.53a
6.83 ± 0.44a
5.92 ± 0.41a
14.99 ± 0.97a
10.04 ± 0.86a
DMBA + CVC-ZnO QDs (8 mg)
80.68 ± 6.82a
77.21 ± 6.27a
102.34 ± 9.27a
12.81 ± 1.51a
6.57 ± 0.43a
5.54 ± 0.36a
15.99 ± 0.89a
10.21 ± 0.89a
3.6 Histological changes
Figs. 2 and 3 shows the histological changes of kidney and mammary tissue of control and experimental rats. Histopathological examination of kidney tissue of DMBA-alone induced rats revealed several features, including tubular dilation, loss of nucleus, tubular atrophy, and glomerular injury. Control and CVC-ZnO QDs alone-rats revealed the normal architecture of the kidney tissue. CVC-ZnO QDs treated DMBA-induced rats showed mild acute tubular injury, inflammatory cell infiltration, and fibrosis. Histology of mammary tissue of DMBA induced rats demonstrated infiltrating malignant tumor. CVC-ZnO QDs treated rats showed mild tumor infiltration and fibrosis with near normal architecture. The control and CVC-ZnO QDs treated alone rats showed normal architecture of the mammary tissues.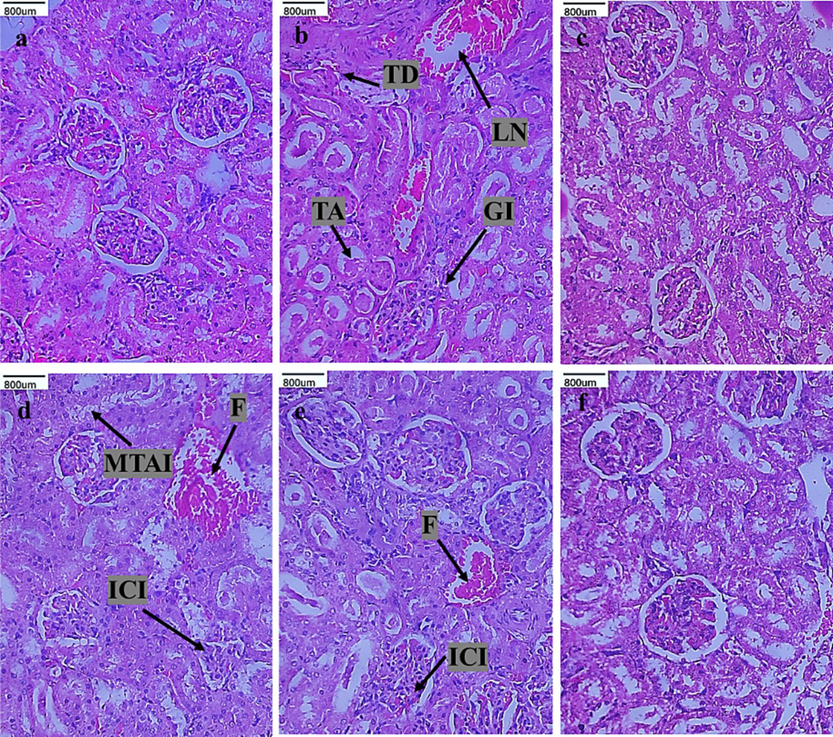
Histological changes of kidney tissue of control and experimental rats (H&E staining). Group I (a) control, group II (b) DMBA alone, group III (c) CVC-ZnO QDs alone, group IV (d) CVC-ZnO QDs (2 mg/kg b.w), group V (e) CVC-ZnO QDs (4 mg/kg b.w), and group VI (f) CVC-ZnO QDs (8 mg/kg b.w). Arrows indicate (TD) tubular dilation, (TA) tubular atrophy, (LN) loss of nucleus, (GI) glomerular injury, (ICI) inflammatory cell infiltration, (MATI) mild acute tubular injury, and (F) fibrosis.
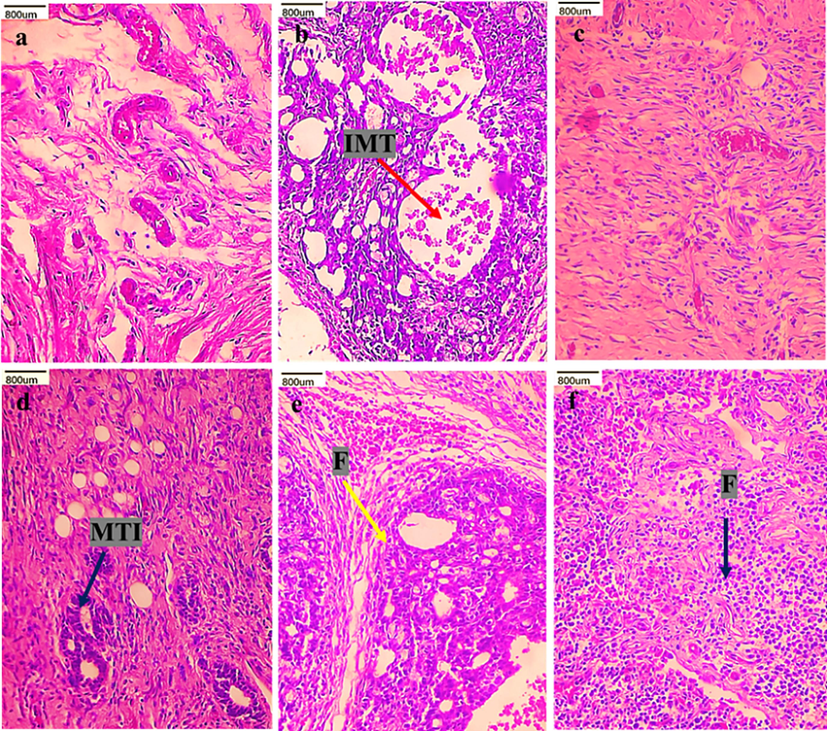
Histological changes of mammary tissue of control and experimental rats (H&E staining). Group I (a) control, group II (b) DMBA alone, group III (c) CVC-ZnO QDs alone, group IV (d) CVC-ZnO QDs (2 mg/kg b.w), group V (e) CVC-ZnO QDs (4 mg/kg b.w), and group VI (f) CVC-ZnO QDs (8 mg/kg b.w). Arrows indicate (IMT) infiltrating malignant tumor, (MTI) mild tumor infiltration, (F) fibrosis.
4 Discussion
Nanotechnology enhances cancer treatment by delivering targeted therapies at the molecular level, increasing precision and minimizing damage to healthy cells (Yao et al., 2020). QDs are nanocrystals with unique optical properties that can be used as carriers due to their small size and biocompatibility (Badıllı et al., 2020). There have been studies on drug-loaded QDs for the chemoprevention of cancerous breast cells. One study used folate receptor–targeted DOX-QD–loaded NPs, to treat female mice with breast cancer induced by a triple-negative cancer cell (Alibolandi et al., 2016). Carvacrol is a natural compound with anticarcinogenic activity (Sivaranjani et al., 2016). The previous study found that CVC caused breast cancer cells to undergo cell death and inhibited the cell proliferation genes involved in cancer (Li et al., 2021). DMBA induces mammary carcinogenesis by acting as a potent chemical carcinogen that causes DNA damage and mutations in mammary epithelial cells. It initiates and promotes the mammary tumors by disrupting the regulatory mechanisms of growth and proliferation of cell (Kerdelhué et al., 2016). The present study investigates the pH-sensitive CVC-ZnO QDs anticancer efficacy in DMBA-induced mammary carcinoma by its ability to scavenge free radicals and detoxify chemical carcinogens.
Antioxidants are essential in protecting cells from the damage caused by ROS (reactive oxygen species), which are highly reactive molecules that can induce oxidative harm to lipids, DNA, and proteins. Lipid peroxidation is a process that occurs when ROS attacks the PUFA (polyunsaturated fatty acids) in cell membranes, leading to the formation of highly reactive lipid radicals that can further damage cell structures (Yaman and Ayhanci, 2021).
Lipid peroxidation and ROS have been implicated in the progression and development of cancer, affecting the metabolisms that lead to weight loss (Davis and Kuttan,., 2001). In this research, the growth rate and body weight of DMBA alone rats were reduced because of the changes in biochemical processes during tumor development. In addition, elevated lipid peroxidation levels play a crucial role in tumor formation. CVC-ZnO QDs-supplemented rats show elevated body weight and growth rate, indicating decreased lipid peroxidation levels. Further, CVC-ZnO QDs decreased the quantity of tumors and the volume of tumors in rats induced with DMBA. This may be due to the targeted drug delivery of nanoparticles (Alibolandi et al., 2016).
Evidence suggests high lipid peroxidation levels are associated with cancer progression and development. This is because the oxidative stress caused by free radicals and ROS can damage DNA and other cellular components, leading to mutations and abnormal cell growth (Jelic et al., 2021). In this study, increased lipid peroxidation levels (TBARs, LOOH, and CD) in DMBA-induced rats were observbed. However, CVC-ZnO QDs treated rats show decreased lipid peroxidation levels.
Antioxidants work by neutralizing free radicals, which help to protect cells from damage (Jelic et al., 2021). SOD helps to convert the O2 (superoxide radical) into H2O2 (hydrogen peroxide) and O (oxygen). CAT converts H2O2 into H2O and O, thereby preventing the accumulation of H2O2. GPx uses glutathione as a cofactor to reduce H2O2 and lipid peroxides into less harmful molecules. GSH (tripeptide), Vit-C, and Vit-E donate electrons to ROS and free radicals, thereby neutralizing their damaging effects (Goodman et al., 2011). Previous research has shown that antioxidants (enzymatic and non-enzymatic) can reduce the severity and incidence of cancer burden in DMBA-induced animals (Latif et al., 2021). In this study, we observed that antioxidants decreased in the cancer-bearing rats. Vayalakkara et al. (2022) study showed that QDs conjugated with a drug antioxidant could selectively target cancer cells and induce cell death by reducing cellular antioxidant defenses. CVC-ZnO QDs treated DMBA-induced rats showed increased antioxidant levels due to reduced oxidative stress.
Detoxification enzymes play crucial roles in metabolizing and eliminating chemicals from the body. Phase I metabolism involves the oxidation of DMBA by enzymes such as Cyt-b5 and CYP450. Phase II enzymes, including GR and GST, can conjugate these reactive intermediates with glutathione and other molecules to facilitate their excretion from the body, thereby reducing cancer risk (Dhamodharan et al., 2021). According to Latif et al., (2021), rats induced with DMBA, increase the activity of phase I enzymes and decrease the activity of phase II enzymes. In this investigation, we noted an augmentation of phase I enzyme activity and a corresponding decrease in phase II enzyme activity in rats subjected to DMBA treatment. On the other hand, CVC-ZnO QDs treated DMBA-induced rats show significantly altered detoxification enzymes, possibly due to CVC delivery to breast cancer cells, potentially enhancing the bodies to eliminate carcinogens and prevent cancer development. A previous study observed that CVC could increase and decrease in phases I and II enzymes activity in DMH-induced colon carcinogenesis, respectively (Sivaranjani et al., 2016).
Lipid profile typically includes PL, FFA, TC, and TG measurements in the bloodstream. From a study by Dhamodharan et al. (2021), DMBA-induced mammary cancer in rats is associated with significant increases in plasma PL, FFA, TC, and TG levels. High PL, FFA, TC, and TG levels, on the other hand, are associated with increased cancer risk. This may be because cholesterol is a precursor for estrogen, a hormone that can promote the growth of mammary cancer. In our study, we noticed that lipid profile (PL, FFA, TC, and TG) were increased in the plasma of DMBA-induced rats, while in mammary tissues, the PL and FFA levels were decreased. This decrease may result from increased PL degradation, impairing membrane function. Another possible explanation for the reduced PL concentration could be the decreased FFA levels in the mammary tissues, as suggested by Van Hoeven and Emmelot. in 1973. Due to the strong antihyperlipidemic properties of CVC-ZnO QDs, supplemented DMBA-treated rats showed significantly decreased plasma PL, FFA, TC, and TG lipid profiles. In contrast, elevated levels of PL and FFA were observed in the mammary tissues.
Nandakumar and Balasubramanian (2011) reported that DMBA-treated rats showed renal tubular structure enlargement and loss of architecture. In this present study, DMBA-induced rats show significant kidney damage, including tubular dilation, loss of nucleus, tubular atrophy, and glomerular injury. Nanoparticles can be engineered to selectively target the kidneys, allowing for precise delivery of drugs to the affected area. This can be particularly useful in treating conditions such as kidney inflammation. In this context, Ozturk et al. (2018) suggested that CVC could reduce kidney injury induced by bilateral renal ischemia/reperfusion (I/R) in female SD rats. In our study, treatment with CVC-ZnO QDs at different doses significantly reduced these pathological changes, with the 4 mg /kg b.w. dose showing the greatest protective effect.
Histopathology of mammary tissue of DMBA-induced rats exhibited IMT. These results agree with Latif et al. (2021). On the contrary, rats treated with CVC-ZnO QDs displayed no indications of necrosis or cellular growth in the mammary gland. A more noticeable impact was noted in the rats subjected to a dosage of 4 mg/kg b.w of CVC-ZnO QDs, where the mammary tissue exhibited nearly normal architecture with an increased area of fibrosis representing residual tumor. Therefore, those data suggest that the CVC-ZnO QDs have the capability to provide a secure and efficient approach to treating cancer with significant anticancer activities.
5 Conclusion
Based on the research findings, pH-sensitive CVC-ZnO QDs have a significant anticancer effect against mammary carcinoma induced by DMBA in female SD rats. The results demonstrate that CVC-ZnO QDs significantly reduced tumor growth, improved the antioxidant status, decreased lipid peroxidation, and modulated the biotransformation enzyme activities in both plasma and mammary tissues. CVC-ZnO QDs also exhibited a positive impact on the lipid profile. Furthermore, the histological examination of the mammary and kidney tissue revealed that CVC-ZnO prevents DMBA-induced tissue damage. These findings suggest that CVC-ZnO QDs can be a safe and effective anticancer agent for treating mammary carcinoma. However, further studies are needed to explore the molecular mechanisms underlying the anticancer properties of CVC-ZnO QDs.
Acknowledgments
This project was supported by Researchers Supporting Project number (RSPD2023R712), King Saud University, Riyadh, Saudi Arabia and the RUSA 2.0 Project grant (RUSA-100-E-002), Annamalai University, Tamilnadu, India.
Data availability statement
Data will be provided upon request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Folate receptor-targeted multimodal polymersomes for delivery of quantum dots and doxorubicin to breast adenocarcinoma: in vitro and in vivo evaluation. International Journal of Pharmaceutics. 2016;500(1–2):162-178.
- [CrossRef] [Google Scholar]
- Prodigiosin/PU-H71 as a novel potential combined therapy for triple negative breast cancer (TNBC): preclinical insights. Scientific Reports. 2020;10(1):14706.
- [CrossRef] [Google Scholar]
- Role of quantum dots in pharmaceutical and biomedical analysis, and its application in drug delivery. TrAC Trends in Analytical Chemistry. 2020;131:116013
- [CrossRef] [Google Scholar]
- The effect of sodium nitrite on red cell GSH. Experientia. 1963;19(2):96-97.
- [CrossRef] [Google Scholar]
- Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chinese Medical Journal. 2021;134(07):783-791.
- [CrossRef] [Google Scholar]
- Carlberg, I. and Mannervik, B., 1985. [59] Glutathione reductase. In Methods in enzymology (Vol. 113, pp. 484-490). Academic press. https://doi.org/10.1016/S0076-6879(85)13062-4.
- Effect of Withania somnifera on DMBA induced carcinogenesis. Journal of Ethnopharmacology. 2001;75(2–3):165-168.
- [CrossRef] [Google Scholar]
- Chemomodulatory Effect of Capsaicin Encapsulated Chitosan Nanoparticles on Lipids, Lipoproteins and Glycoprotein Components in 7, 12-Dimethylbenz [a] anthracene (DMBA) Induced Mammary Carcinogenesis in Sprague-Dawley Rats. Eurasian Journal of Medicine and Oncology. 2021;5(4):350-357.
- [Google Scholar]
- Nanocarriers-mediated drug delivery systems for anticancer agents: an overview and perspectives. International Journal of Nanomedicine 2021:1313-1330.
- [CrossRef] [Google Scholar]
- An easy colorimetric micromethod for routine determination of free fatty acids in plasma. Clinica Chimica Acta. 1973;46(2):105-111.
- [CrossRef] [Google Scholar]
- Stable reagents for determination of serum triglycerides by a colorimetric Hantzsch condensation method. Clinical Chemistry. 1973;19(3):338-340.
- [Google Scholar]
- Clinical trials of antioxidants as cancer prevention agents: past, present, and future. Free Radical Biology and Medicine. 2011;51(5):1068-1084.
- [CrossRef] [Google Scholar]
- Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry. 1974;249(22):7130-7139.
- [CrossRef] [Google Scholar]
- Tumor targeting strategies of smart fluorescent nanoparticles and their applications in cancer diagnosis and treatment. Advanced Materials. 2019;31(40):1902409.
- [CrossRef] [Google Scholar]
- Oxidative stress and its role in cancer. Journal of cancer research and therapeutics. 2021;17(1):22-28.
- [CrossRef] [Google Scholar]
- Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Analytical Biochemistry. 1992;202(2):384-389.
- [CrossRef] [Google Scholar]
- The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorganic Chemistry and Applications. 2018;2018
- [CrossRef] [Google Scholar]
- Kakkar, P., Das, B. and Viswanathan, P.N., 1984. A modified spectrophotometric assay of superoxide dismutase.
- Dimethyl-Benz (a) anthracene: A mammary carcinogen and a neuroendocrine disruptor. Biochimie Open. 2016;3:49-55.
- [CrossRef] [Google Scholar]
- Evaluating the therapeutic potential of white button mushroom (Agaricus bisporus) against DMBA-induced breast cancer in Sprague Dawley rats. Journal of Food Biochemistry. 2021;45(12):e13979.
- [Google Scholar]
- Carvacrol affects breast cancer cells through TRPM7 mediated cell cycle regulation. Life Sciences. 2021;266:118894
- [CrossRef] [Google Scholar]
- Hesperidin protects renal and hepatic tissues against free radical-mediated oxidative stress during DMBA-induced experimental breast cancer. Journal of Environmental Pathology, Toxicology and Oncology. 2011;30(4)
- [CrossRef] [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351-358.
- [CrossRef] [Google Scholar]
- Omaye, S.T., Turnbull, J.D. and Sauberlich, H.E., 1979. [1] Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. In Methods in enzymology (Vol. 62, pp. 3-11). Academic press. https://doi.org/10.1016/0076-6879(79)62181-x.
- The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem. 1964;239(7):2370-2378.
- [Google Scholar]
- Carvacrol attenuates histopathogic and functional impairments induced by bilateral renal ischemia/reperfusion in rats. Biomedicine & Pharmacotherapy. 2018;98:656-661.
- [CrossRef] [Google Scholar]
- Palan, P.R., Mikhail, M.S., Basu, J. and Romney, S.L., 1991. Plasma levels of antioxidant β‐carotene and α‐tocopherol in uterine cervix dysplasias and cancer. https://doi.org/10.1080/01635589109514106.
- February. Horizons of nanotechnology applications in female specific cancers. In: Seminars in Cancer Biology. Vol Vol. 69. Academic Press; 2021. p. :376-390.
- [CrossRef] [Google Scholar]
- Early onset of lipoperoxidation in rat liver after carbon tetrachloride administration. Experimental and Molecular Pathology. 1968;9(2):271-278.
- [CrossRef] [Google Scholar]
- Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588-590.
- [CrossRef] [Google Scholar]
- Colorimetric assay of catalase. Analytical Biochemistry. 1972;47(2):389-394.
- [CrossRef] [Google Scholar]
- Chemopreventive effect of carvacrol on 1, 2-dimethylhydrazine induced experimental colon carcinogenesis. Journal of Cancer Research and Therapeutics. 2016;12(2):755-762.
- [CrossRef] [Google Scholar]
- Recent advances in folic acid engineered nanocarriers for treatment of breast cancer. Journal of Drug Delivery Science and Technology. 2020;56:101613
- [CrossRef] [Google Scholar]
- Plasma membrane lipids of normal and neoplastic tissues. Tumor Lipids R. Wood, editor. American Oil Chemists Soc. Champaign. 1973;111:126-138.
- [Google Scholar]
- Photothermal/NO combination therapy from plasmonic hybrid nanotherapeutics against breast cancer. Journal of Controlled Release. 2022;345:417-432.
- [CrossRef] [Google Scholar]
- Lipid peroxides and human diseases. Chemistry and Physics of Lipids. 1987;45(2–4):337-351.
- [CrossRef] [Google Scholar]
- Yao, Y., Zhou, Y., Liu, L., Xu, Y., Chen, Q., Wang, Y., Wu, S., Deng, Y., Zhang, J. and Shao, A., 2020. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Frontiers in molecular biosciences, 7, p.193. https://doi.org/10.3389%2Ffmolb.2020.00193.
- Microdetermination of plasma phospholipids by trichloroacetic acid precipitation. The Journal of Laboratory and Clinical Medicine. 1950;35(1):155-160.
- [Google Scholar]
- A new method for the direct determination of serum cholesterol. The Journal of Laboratory and Clinical Medicine. 1953;41(3):486-492.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.103029.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







