Translate this page into:
Anticancer and antioxidant activities of Nannochloropsis oculata and Chlorella sp. extracts in co-application with silver nanoparticle
⁎Corresponding author. azmuddin@umt.edu.my (Mohd Azmuddin Abdullah) joule1602@gmail.com (Mohd Azmuddin Abdullah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study examined the formulation of Microalgal Chloroform Extracts (MEs) and Silver nanoparticles (AgNPs) co-application for anticancer activity against MCF-7 and 4T1 cells, without affecting the non-cancerous Vero cell-lines. The concentration, ratios and duration of treatments were optimized, and the flow cytometric and cell cycle analyses were carried out. The metabolites based on Gas Chromatography-Mass Spectrometry (GC–MS) were determined and the antioxidant activities evaluated. The main compounds detected in Chlorella sp. and N. oculata, respectively, were hexanedioic acid, bis (2-ethylhexyl) ester (23.94, 36.47%), neophytadiene (16.82, 4.79%), eicosane (4.37, 15.04%), hexatriacontane (0, 12.77%), and 13-Docosenamide, (Z) (9.22, 0%). The AgNPs-N. oculata-CHL at (w/w) 1.5:1 and 2:1 ratios (w/w) exhibited cytotoxic IC50 of 10.47 and 17.78 µg/mL on MCF-7 cells; and 79.43 and 52.7 µg/mL against 4T1 cells after 72 h, respectively. The AgNPs-Chlorella sp.-CHL at 1:1 and 2:1 ratios exhibited IC50 of 19.05 and 14.45 µg/mL against MCF-7 cells; and 79.43 and 50.11 µg/mL on 4T1 cells after 72 h, respectively. There was no cytotoxicity against Vero cells at any of the treatments tested. The co-applications showed higher early and late apoptotic events and significant increase in sub G1 phase as compared to the single-applications. At the 2:1 ratio, the strongest anti-oxidant activities were shown by the AgNPs-Chlorella sp.-CHL (IC50 2.11 mg/mL) and N. oculata-CHL (IC50 2.98 mg/mL), as compared to the AgNPs-T. suecica-CHL (IC50 1.77 mg/mL). The AgNPs-MEs co-application exerted high anticancer and antioxidant activities, with no cytotoxic activity against Vero cells. The formulation could lead to the development of potent therapeutic agents against breast cancer with reduced or no side effect.
Keywords
Natural products
Microalgae
Silver nanoparticle
Co-application
Anticancer formulation
Antioxidant activity
- AgNPs
Silver nanoparticles
- NPs
Nanoparticles
- MEs
Microalgae extracts
- SPR
surface-plasmon resonance
- ROS
reactive oxygen species
- MET
Methanol
- CHL
Chloroform
- HEX
Hexane
- ETH
Ethanol
- W
Water
- PI
Propidium iodide
- XRD
X-ray Diffraction
- DMSO
Dimethyl sulfoxide
- MTT
3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide TMX, Tamoxifen
- SEM
Scanning Electron Microscopy
- GC–MS
Gas-chromatography mass spectrometry
Abbreviations
1 Introduction
Free-radical chain reactions can lead to many diseases such as diabetes, cancer, arthritis, and increased aging process. Effective free-radical scavenging activities is therefore important to quench the initiator radical (Saha et al., 2004). Biogenic compounds from plant and microalgal extracts are Generally Regarded As Safe (GRAS) to be developed as complementary anticancer, antitumor and antioxidant therapeutics, with little or no side effects (Abdullah et al., 2016, 2017; Martínez Andrade et al., 2018). Unicellular green algae Nannochloropsis oculata (Ochrophyta, Eustigmatophyceae) and Chlorella vulgaris Beijerinck (Chlorellaceae), have been explored for functional food, nutraceutical, pharmaceuticals, biochemicals, and animal feed applications (Abdullah et al., 2016, 2017; Shah and Abdullah, 2018).
Nanomaterials could revolutionize cancer diagnosis and therapy. The nanoparticles loaded with therapeutic drugs or agents have been utilized in the delivery systems to diseased sites (Abdullah et al., 2014; Gul-e-Saba and Abdullah, 2015; Supraja et al., 2016), via active or passive targeting to specific cells or tissues (Gurunathan et al., 2013). Silver nanoparticles (AgNPs) exhibit different levels of cytotoxicity on breast cancer cells (Hussein et al., 2020a), and free radical scavenging activity (Khan et al., 2015). As electron donors, AgNPs could react with the free radicals to reduce them into stable compound, and quench the radical chain reaction. The AgNPs could advance the theranostics application for diagnosis and therapeutics efficacy in the overall cancer treatment strategies (Wang, 2017).
Most of the reported work on the cytotoxicity on the cancer cells involves a single application of either Microalgal Extracts or AgNP. The Tetraselmis suecica chloroform extracts (CHL) and the AgNPs co-application at the 2:1 ratio, has been reported to attain the cytotoxic IC50 of 6.6 µg/mL on MCF-7, and 53.7 µg/mL on 4 T1 cells using the MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide). The single application of T. suecica-CHL exhibits the IC50 of 46.77 µg/mL on MCF-7, and 83.17 µg/mL on 4T1 cells. Both single and co-applications however do not exhibit any cytoxocity on the Vero cells (Hussein et al., 2020a). The GC–MS metabolite profiling of N. oculata, Chlorella and T. suecica hexane (HEX), methanol (MET), ethanol (ETH) and water (W) extracts, and the antimicrobial activities, including that of the CHL extracts, have been determined (Hussein et al., 2020b). The cytotoxicity of the microalgal extracts in co-application with elevated AgNP levels and the LC-MS profiling of CHL, HEX, MET, ETH and W extracts, have also been reported (Hussein et al., 2020c). To our knowledge, the cytotoxicity of the AgNPs-N. oculata and Chlorella sp. co-application against breast cancer cells (MCF-7 and 4T1) with reduced cytotoxicity on the non-cancerous Vero cells, together with their anti-oxidant activities, have not been established before.
The aims of this study were therefore to formulate the AgNPs and the Chlorella sp. and N. oculata CHL extracts as single or co-application, for anticancer activities against the MCF-7 and 4T1 cancer cell-lines, without affecting the Vero cell-lines. The ratios, duration, and concentrations of treatments were optimized. The evidence of necrotic or apoptotic cell death was evaluated by the flow cytometric and cell-cycle analyses. The antioxidant activities of Chlorella sp. and N. oculata MEs, in single and co-application with the AgNPs, were investigated, and the comparison was made with the T. suecica extracts as their cytotoxicities on the MCF-7, 4T1, and Vero cells are reported by Hussein et al. (2020a).
2 Materials and methods
2.1 Microalgal cultivation
The N. oculata, Chlorella sp., and T. suecica microalgal species used in the present study, were a gift from the Fisheries Research Institute of Malaysia, Kuala Muda, Kedah, Malaysia, and identified under the supervision of Dr. Mohd Fariduddin Othman. The microalgae was subcultured in the TMRL Enrichment medium (Table S1), as described previously (Shah, 2014; Shah and Abdullah, 2018; Hussein et al., 2020a). The cell number was determined by a Hemocytometer by harvesting every 2–3 days and the cell growth profile established.
2.2 Biosynthesis of AgNPs
The De Man Rogase and Sharp (MRS) media (Himedia, India) was inoculated with Lactobacillus plantarum culture. The bacteria was first isolated from the gastrointestinal tract of Oreochromis niloticus (Red tilapia) at the Institute of Tropical Aquaculture (AQUATROP), Universiti Malaysia Terengganu. The cultures were maintained according to the previous method (Jaffat et al., 2017). The L. plantarum cell-free supernatant was prepared as described before (Chaudhari et al., 2012). The biosynthesis of AgNPs was carried out by mixing silver nitrate (AgNO3) solution (1 mM) with the supernatant at 1:1 (v/v) ratio and incubated in the dark at 100 rpm, 35 °C. After 24 h, the color change from light yellow to reddish-brown was observed, indicating the silver ion reduction and the AgNPs formation.
2.3 Characterization of AgNPs
The characterization of AgNPs was according to Caroling et al. (2013). The UV–Vis spectrum to determine the Ag+ ions reduction was observed by UV–Visible Spectroscopy (UV-1800, Shimadzu, Japan), at the absorbance range of 200–800 nm. Deionized water was used as the blank. The shape and size of the AgNPs were measured by Scanning Electron Microscopy (SEM) (Japan, JSM-6610LV, JEOL). The X-ray Diffractometer (XRD) (Miniflex II, Rigaku, Japan) analyzed the crystal structure of the AgNPs. The ‘peak search’ and the ‘search match’ built-in program (PDXL, Rigaku, Japan) identified the XRD peak (Caroling et al., 2013).
2.4 Preparation of MEs
All solvents used were 100% solvent of analytical grade. The dried microalgae (0.5 g) were ground into fine powder and extracted for 24 h in 150 mL of methanol (MET), ethanol (ETH) and water (W) (polar), chloroform (CHL) (semi-polar), and hexane (HEX) (non-polar), separately, in a Soxhlet apparatus. The temperature used was dependent on the solvent boiling point (Fabrowska et al., 2018). For water, the extraction was carried out at 30 °C and sonicated for 10 min. The extracts were evaporated using a Rotary evaporator, weighed and stored at 4 °C for further analyses.
2.5 Gas Chromatography-Mass spectrometric (GC–MS) analyses
The MEs (1 mg) were dissolved in HEX (1 mL) and analysed by the GC–MS (QP2010 Ultra) with a capillary column (0.25 m film thickness, 30 m × 0.25 mm i.d), and connected to Shimadzu SH-Rxi-5Sil MS (Shimadzu, USA). The injector and MS interface temperatures were at 250 °C. The columns were run in a programmed-mode at 50 °C (first stage) for 2 min, with a linear ramp of 10 °C min−1, until 230 °C in the final stage. The split ratio mode 1:0 was used, and the carrier gas helium was at 4.6 mL min−1. The volume of the injected sample was 1 μL (Hussein et al., 2020a).
2.6 Preparation of AgNPs-MEs-CHL ratio
The preparation was carried out as shown in Table S2 according to Hussein et al. (2020a).
2.7 Determination of cytotoxic effects
2.7.1 Cancer and Vero cell-lines
The breast cancer cell lines were the MCF-7 (ATCC® HTB-22™ – the human breast cancer), and the 4T1 cells (ATCC® CRL-2539™ - the mammary carcinoma), and the non-cancerous ones were the Vero cells (ATCC® CCL-81™ - the African green monkey kidney epithelial cells). The procedure was carried out as reported by Hussein et al. (2020a).
2.7.2 Cytotoxic assay
The MCF-7, 4T1, and Vero cell-lines were grown in a 96-well plate at 5 × 104, 2 × 104, and 4 × 104 cells/mL, respectively, and incubated overnight. After 24 h, the cells were treated with single and co-applications of MEs and AgNPs at different concentrations (3.125–100 μg/mL) for 24, 48, and 72 h durations. Each well was subjected to MTT assay (Mosmann, 1983; Hussein et al., 2020a).
2.7.3 Flow cytometry and cell cycle analyses
The flow cytometric and cell cycle analyses were as described before by Hussein et al. (2020a).
2.8 Determination of antioxidant activities
The anti-oxidant activity was estimated by using the 2,2-diphenyl-2- picrylhydrazyl (DPPH) method with slight modification (Medhe et al., 2014). Quercetin (Sigma-Aldrich, USA) was used as the standard. Two hundred µL of fresh DPPH (Sigma-Aldrich, USA) solution (6 × 10−5 M in methanol) was added to a clear 96-well plate and mixed together with 20 µL of the samples or the standard, in two-fold serial dilutions of the extracts from 10 to 0 mg/mL The aluminum-covered plate was incubated for 30 min, at room temperature. The absorbance (Abs) was read at 517 nm and the antioxidant activity was determined as follows:
For the preparation of the AgNPs and MEs as single applications, various concentrations of AgNPs (stock of 10 mg/mL) and MEs (stock of 10 mg/mL) were prepared (0–10 mg/mL). For co-applications, each solution of AgNPs and MEs was mixed to form a final total concentration of 10 mg/mL at the ratios of 1:1, 1.5:1, and 2:1 (AgNPs:MEs (w/w)) as shown in Table S3. The anti-oxidant activities of the Chlorella and N. oculata MEs and AgNPs single and co-applications were compared with the T. suecica extracts, as the cytotoxic effects of the latter have been established (Hussein et al., 2020a).
2.9 Statistical analyses
All experiments were performed in triplicate and results as the means ± standard deviation (SD). The half-maximum inhibitory concentration (IC50) and the data were analyzed using GraphPad Prism (version 6, USA). The IC50 measures the compound efficiency in inhibiting the biological functions and biochemical processes.
3 Results
3.1 Characterization of microalgal biomass and GC–MS analyses
Fig. 1a shows the growth curve of N. oculata and Chlorella sp. cultivated in 250 mL shake flasks under continuous white light at 24–28 °C and shaken at 130 rpm. The highest cell density was achieved with Chlorella sp. at 60 × 106 cell/mL. The only essential macro/micronutrients present in the TMRL medium could have led to a reduced cell growth rate and density (Shah and Abdullah, 2018). The GC–MS detected 12 compounds in the CHL extracts (Table 1) that showed a high peak area (%) at different retention times (Rt). The major compounds in Chlorella sp. and N. oculata, respectively, were hexanedioic acid, bis(2-ethylhexyl) ester (23.94, 36.47%), neophytadiene (16.82, 4.79%), eicosane (4.37, 15.04%), hexatriacontane (0, 12.77%), and 13-Docosenamide, (Z) (9.22, 0%). Both Chlorella sp. and N. oculata registered hexanedioic acid, bis(2-ethylhexyl) ester as the major compound. Fatty acid Alkane Ester Terpene Amide group Vitamin NB: “–“ means “Not detected”.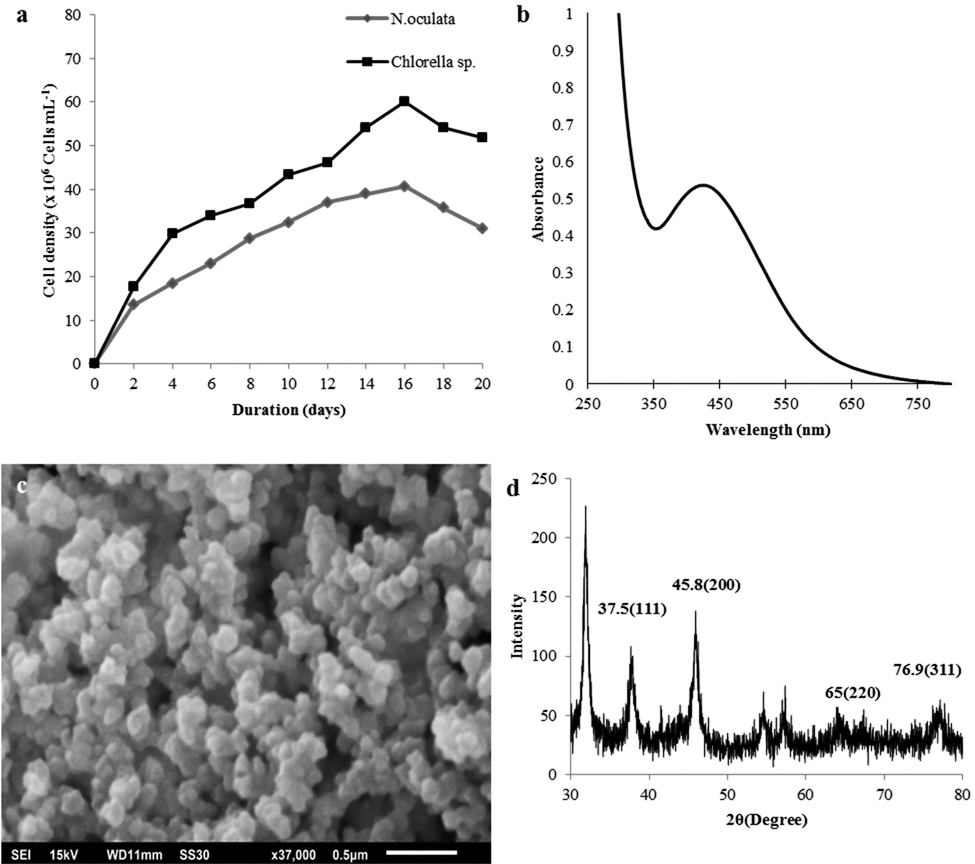
Characterization of microalgae and AgNPs: (a) Growth profile; (b) UV‐Vis spectra of AgNPs, (c) SEM of AgNPs (37000× magnification), (d) XRD of AgNPs.
Extracts/Compounds
Rt (min)
N. oculata
Chlorella sp.
(%)
(%)
Campesterol
54.7
–
5.09
Eicosane
43.4
15.04
4.37
Tetracontane
46.5
1.63
Hexatriacontane
49.5
12.77
Hexanedioic acid, bis (2-ethylhexyl) ester
41.5
36.47
23.94
9,12,15- Octadecatrienoic acid, methyl ester, (Z,Z,Z)
36.3
Diisooctyl phthalate
43.90
6.74
2.08
1 2-benzenedicarboxylic acid bis(8-methylnonyl) ester
49.3
5.99
2.42
Neophytadiene
30.9
4.79
16.82
Phytol
36.5
5.32
13-Docosenamide,(Z)-
47.7
–
9.22
Vitamin E
52.7
–
0.42
3.2 Characterization of AgNPs
Fig. 1b shows the UV–Vis spectroscopy where the AgNP formation was observed at the absorption peak of 424 nm, which suggests the Surface Plasmon Resonance (SPR) of the NPs. After the addition of 1 mM AgNO3 to the cell free supernatant, the AgNO3 was reduced to AgNPs, and after 24 h incubation at 35 °C, the reaction mixture exhibited the change of colour from light-yellow to reddish-brown (Jaffat et al., 2017). The SEM (Fig. 1c) shows that the AgNPs were mostly spheroid in shape, with the diameter ranging from 20 to 100 nm, and were slightly agglomerated, as similarly reported previously (Dakhil, 2017). The XRD analysis exhibited the peaks at 37.5°, 45.8°, 65°, and 76.9°, which are correlated to the (1 1 1), (2 0 0), (2 2 0), and (3 0 0) planes, respectively, that indicate the crystalline characteristic of silver (Fig. 1d).
3.3 Cytotoxic activities
3.3.1 Single application of MEs and AgNPs
The AgNPs showed the highest cytotoxicity against MCF-7 cells at IC50 of 23.98, 7.36, and 5.31 µg/mL, respectively, after 24, 48, and 72 h (Table 2). The Chlorella sp.-CHL exhibited the strongest cytotoxicity (IC50 39.81 µg/mL) after 72 h treatment, followed by N. oculata-CHL (IC50 42.65 µg/mL) and N. oculata-HEX (IC50 50.11 µg/mL). The AgNPs also showed significantly higher cytotoxicity against the 4T1 cell line at IC50 of 19.95, 19.05, and 17.78 µg/mL after 24, 48, and 72 h, respectively. None of the MEs showed any cytotoxicity against 4T1 cells except for the low cytotoxicity after 72 h with Chlorella sp.-CHL at IC50 83.17 µg/mL and N. oculata-MET (IC50 95.49 µg/mL). For the Vero cells, the AgNPs exhibited high cytotoxicity at IC50 35.48, 30.19, and 25.11 µg/mL after 24, 48, and 72 h, respectively, while the MEs did not show any cytotoxic effects (Table 2). The MEs-CHL and HEX may selectively inhibit the growth of specific cell types or tumor types, while the MEs-MET and W exhibited no significant cytotoxicity on any of the cell-lines. Therefore, the MEs-CHL were used for further study in co-application with the AgNPs.
Cell line/MEs
24 h
48 h
72 h
AgNPs
CHL
HEX
MET
W
CHL
HEX
MET
W
CHL
HEX
MET
W
24 h
48 h
72 h
1. MCF-7
N. oculata
57.54 ± 0.09
70.79 ± 0.07
70.79 ± 0.08
–
53.7 ± 0.17
56.2 ± 0.16
75.85 ± 0.05
91.2 ± 0.04
42.65 ± 0.18
50.11 ± 0.20
52.48 ± 0.10
91.2 ± 0.04
23.98 ± .12
7.36 ± 0.20
5.31 ± 0.20
Chlorella sp.
–
–
–
–
52.48 ± 0.12
100 ± 0.08
89.13 ± 0.10
70.8 ± 0.04
39.81 ± 0.15
79.43 ± 0.14
77.6 ± 0.12
63.1 ± 0.03
2. 4T1
N. oculata
–
–
–
–
–
–
–
–
–
–
95.49 ± 0.05
–
19.95 ± 0.17
19.05 ± 0.15
17.78 ± 0.10
Chlorella sp.
–
–
–
–
–
–
–
–
83.17 ± 0.07
–
–
–
3. Vero
N. oculata
–
–
–
–
–
–
–
–
–
–
–
–
35.48 ± 0.09
30.19 ± 0.10
25.11 ± 0.10
Chlorella sp.
–
–
–
–
–
–
–
–
–
–
–
–
3.3.2 AgNPs–MEs–CHL Co-application
The anticancer activity of the AgNPs-MEs-CHL against the MCF-7, 4T1, and Vero cell-lines are shown in Table 3. The AgNPs-N. oculata-CHL at the 1.5:1 and 2:1 ratios (w/w) after 72 h exhibited IC50 of 10.47 and 17.78 µg/mL against MCF-7 cells; and 79.43 and 52.7 µg/mL against 4T1 cells, respectively. The AgNPs-Chlorella sp.-CHL at the 1:1 and 2:1 ratios (w/w) after 72 h exhibited IC50 of 19.05 and 14.45 µg/mL against MCF-7 cells; and 79.43 and 50.11 µg/mL against 4T1 cells, respectively. These results against MCF-7 cells were comparable to the activity of the AgNPs in single application, suggesting that the higher AgNPs composition in the co-application enhanced the cytotoxicity. In general, the AgNPs-MEs-CHL treatment on the MCF-7 cells at the 1.5:1 and 2:1 ratios, achieved the cytotoxic IC50 of 19.95–41.86 µg/mL after 24 h; 15.13–37.15 µg/mL after 48 h; and 10.47–33.88 µg/mL after 72 h. With the 4T1 cell lines, the highest cytotoxicity was exhibited by the AgNPs-Chlorella sp.-CHL after 72 h at the IC50 of 48.97 µg/mL at the 1.5:1 ratio, and 50.11 µg/mL at the 2:1 ratio; followed by the AgNPs-N. oculata-CHL at the 2:1 ratio with the IC50 of 52.7 µg/mL after 72 h treatment, and 54.59 µg/mL after 48 h.
Co-application /Cell-lines
24 h
48 h
72 h
1:1
1.5:1
2:1
1.5:3
1:1
1.5:1
2:1
1.5:3
1:1
1.5:1
2:1
1.5:3
1. MCF-7
AgNPs-NC
30.19 ± 0.24
19.95 ± 0.20
28.18 ± 0.15
60.25 ± 0.10
25.7 ± 0.20
15.13 ± 0.20
20.41 ± 0.20
44.66 ± 0.23
20.89 ± 0.20
10.47 ± 0.30
17.78 ± 0.26
33.88 ± 0.20
AgNPs-CC
47.86 ± 0.10
41.86 ± 0.21
24.54 ± 0.25
52.48 ± 0.18
29.5 ± 0.10
37.15 ± 0.24
20.41 ± 0.24
50.11 ± 0.21
19.05 ± 0.20
33.88 ± 0.16
14.45 ± 0.20
47.86 ± 0.16
2. 4T1
AgNPs-NC
85.11 ± 0.03
85.11 ± 0.10
63.09 ± 0.10
–
75.85 ± 0.10
81.28 ± 0.04
54.59 ± 0.04
–
66.06 ± 0.08
79.43 ± 0.07
52.7 ± 0.15
–
AgNPs-CC
89.12 ± 0.09
58.88 ± 0.08
63.09 ± 0.08
–
87.09 ± 0.03
56.23 ± 0.10
60.25 ± 0.10
100 ± 0.08
79.43 ± 0.06
48.97 ± 0.06
50.11 ± 0.06
100 ± 0.03
3. Vero
AgNPs-NC
–
–
–
–
–
–
–
–
–
–
–
AgNPs-CC
–
–
–
–
–
–
–
–
–
–
–
–
The same dosage and ratio of AgNPs-MEs-CHL co-application that showed cytotoxicity on the MCF-7 cells could not produce the same cytotoxicity on the 4T1 cells. The higher MEs composition could mask the cytotoxicity of the AgNPs. Interestingly, all the AgNPs-MEs-CHL co-application showed no cytotoxic activity on the Vero cell-line for any of the treatment durations. Although the AgNPs in single application had shown cytotoxic effects, the presence of MEs at all the co-application ratios may have masked the AgNPs cytotoxicity against the Vero cell-lines (Table 2). Vero cells have been used as the in vitro normal cell control to evaluate cytotoxicity (Sombatsri et al., 2019). The Vero cell line is the most common mammalian continuous cell line used in research due to its sensitivity to different types of microbes, chemical compounds, and toxins, and thus, its suitability for use in the screening tests for natural products (Sit et al., 2018) and in cancer research (Siddiqui et al., 2019).
3.4 Flow cytometry and cell cycle analyses
Fig. 2a shows significant apoptotic activity in the MCF-7 cell-lines where the early apoptosis was the highest with the AgNPs (25.56%) and TMX (27.17%) treatments. The AgNPs-N. oculata-CHL achieved the highest early (17.88%) and late apoptosis (36.25%); followed by AgNPs-Chlorella sp.-CHL with early (17.42%) and late apoptosis (33.52%) within the cell population, after 24 h treatment. For the 4T1 cells (Fig. 2b), the highest early apoptosis was attained with the AgNPs at 16.33% of the cell population, while the TMX at 63.77% achieved the highest late apoptosis, followed by the AgNPs at 25.59% and AgNPs-Chlorella sp.-CHL (13.07%). For Vero cell-lines (Fig. 2c), the early and late apoptotic activities were higher after the treatment with TMX and AgNPs, but no IC50 value was recorded with any of the AgNPs–MEs-CHL treatment.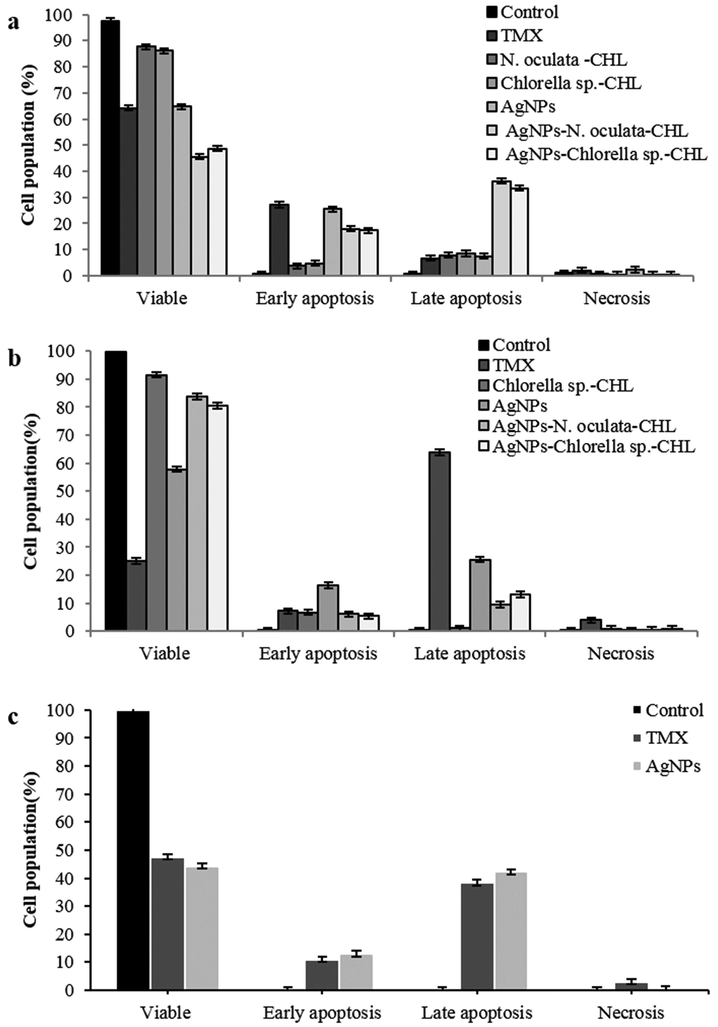
Flow cytometry of the live cells, early apoptotic, late apoptotic, and necrotic phase in treated and untreated (a) MCF-7, (b) 4T1, (c) Vero cell-lines (Values are percentages of each quadrant *p < 0.05).
Fig. 3a-c demonstrates the distribution of the events in the cell-cycle phases of the MCF-7, 4T1, and Vero cells, after 24 h. In the MCF-7 cells, a significant enhancement was observed in the Sub-G1 phase (the apoptotic event) in all the treated samples especially with the TMX (33.96%), AgNPs-N. oculata-CHL (32.24%), AgNPs-Chlorella sp.-CHL (30.86%), and AgNPs (29.1%). There was also a significant drop in the G1 phase to about 30% with TMX and AgNPs and 35–40% in the AgNPs-MEs-CHL, as compared to 49% in the control, and more than 50% in Chlorella sp.-CHL and N. oculata-CHL. In the S phase, there was a reduction from 26% (Control) to about 8.91, 9, 10.97, and 11.85% with the TMX, AgNPs, AgNPs-Chlorella sp.-CHL, and AgNPs-N. oculata-CHL treatments, respectively. In the G2/M phase, all the treated samples had a lower percentage than the control, thereby suggesting inhibited mitosis. For the 4T1 cells, a significant increase in the apoptotic events (Sub-G1 phase) was shown by the TMX (44.99%), followed by the AgNPs (20.93%), AgNPs-Chlorella sp.-CHL (18.43%), and AgNPs-N. oculata-CHL (17.28%). For the Vero cells, there was an increase from 10% (Control) to about 19.87 and 15.11% in the Sub-G1 phase and from 42% (Control) to 53% and 47% in the G0/G1 phase, with the TMX and AgNPs, respectively. However, the S phase decreased from 20% (Control) to about 9.97 and 13.8% with the TMX and AgNPs treatment, respectively. For the G2/M phase, the distribution at 16.47% with the AgNPs was comparable to the control (16.08%), but it was slightly decreased with the TMX (13.45%).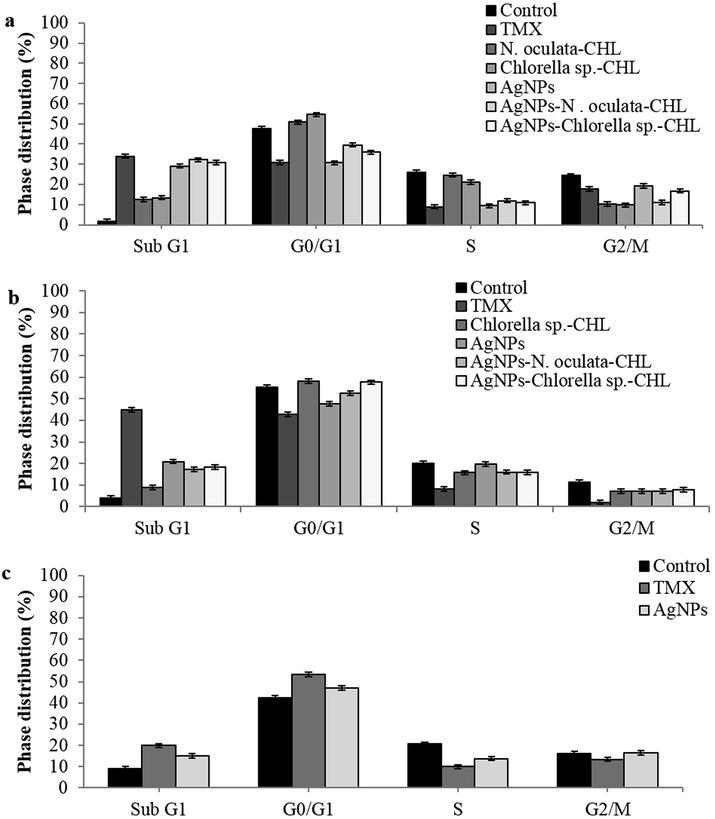
Cell-cycle analyses with the single and co-application of MEs-CHL and AgNPs, after 24 h treatment at the IC50 values against (a) MCF-7, (b) 4T1 and (c) Vero cell lines.
3.5 Antioxidant activities
The maximum antioxidant activity (IC50) of the single application at 10 mg/mL concentration was in the decreasing order of (mg/mL): N. oculata-CHL (2.23), AgNPs (2.66) and Chlorella sp.-CHL (3.16), in comparison to Chlorella sp.-MET (2.41), T. suecica-ETH (2.71), Chlorella sp.-ETH and N. oculata-HEX (2.98) and the standard Quercetin (0.39) (Table 4). For co-application, the strongest antioxidant activities exhibited by the AgNPs-Chlorella sp.-CHL (2:1) (2.11) and AgNPs-N. oculata-CHL (2.98) were lower than or comparable to the AgNPs-N. oculata-ETH (2:1 and 1.5:1) (1.88 and 2.07, respectively), AgNPs-T. suecica-CHL and ETH (2:1) (1.77), AgNPs-T. suecica-ETH (1.5:1) (2.19) and AgNPs-Chlorella sp.–ETH (2:1) (2.23). The AgNPs-MEs co-application however exhibited far superior antioxidant activities than the single application of either MEs or AgNPs.
Solvent/MEs
Single application IC50 (mg/mL)
Chloroform
Hexane
Methanol
Ethanol
Water
N. oculata
2.23 ± 0.02
2.98 ± 0.04
3.22 ± 0.02
5.84 ± 0.01
6.07 ± 0.01
Chlorella sp.
3.16 ± 0.01
7.07 ± 0.02
2.41 ± 0.08
2.98 ± 0.02
5.41 ± 0.01
T. suecica
3.54 ± 0.01
5.21 ± 0.02
3.16 ± 0.05
2.71 ± 0.01
5.84 ± 0.02
AgNPs
2.66 ± 0.004
Quercetin (Standard)
0.39 ± 0.04
Solvent/AgNPs:MEs
Co-application IC50 (mg/mL)
AgNPs-N. oculata
AgNPs-Chlorella sp.
AgNPs-T. suecica
1:1
1.5:1
2:1
1:1
1.5:1
2:1
1:1
1.5:1
2:1
Chloroform
5.01 ± 0.01
4.29 ± 0.003
2.98 ± 0.01
3.75 ± 0.001
2.51 ± 0.01
2.11 ± 0.01
7.49 ± 0.01
2.61 ± 0.01
1.77 ± 0.003
Hexane
5.01 ± 0.01
3.98 ± 0.002
2.81 ± 0.01
5.4 ± 0.02
3.48 ± 0.04
2.93 ± 0.01
4.13 ± 0.003
3.22 ± 0.003
2.93 ± 0.02
Methanol
4.21 ± 0.01
4.3 ± 0.01
3.34 ± 0.02
–
3.54 ± 0.01
3.04 ± 0.04
5.62 ± 0.01
3.54 ± 0.01
3.41 ± 0.03
Ethanol
3.04 ± 0.02
2.07 ± 0.01
1.88 ± 0.01
2.98 ± 0.01
2.64 ± 0.01
2.23 ± 0.02
2.51 ± 0.01
2.19 ± 0.01
1.77 ± 0.01
Water
4.54 ± 0.02
3.68 ± 0.02
3.16 ± 0.02
5.31 ± 0.01
4.73 ± 0.02
3.82 ± 0.02
4.38 ± 0.02
3.48 ± 0.03
3.22 ± 0.02
4 Discussion
The microalgal cell growth is influenced by the abiotic factors which include the environment and nutritional factors; and the biotic factors, such as the competition with the pathogens and other algae (Abdullah et al., 2016, 2017; Shah and Abdullah, 2018). In the case of AgNPs biosynthesis, proteins and enzymes, such as nitrate reductase, could play the role as regulatory agents (Jaffat et al., 2017). A specific and single SPR band suggests that the NPs have the shape of a spheroid while a remarkable peak broadening at around 350 nm to 480 nm indicates that the particles are polydispersed (Nephawe, 2015). The unassigned peaks can be attributed to the biomolecules (Supraja et al., 2016). In our study, the AgNPs single application showed cytotoxicity against MCF-7 cells, comparable to, if not better than, the IC50 of 7.19 μg/mL (Remya et al., 2015). Against the Vero cells, the cytotoxicity in our study was higher than the IC50 of 66.34 μg/mL (Remya et al., 2015) and 68.49 μg/mL (Priyadharshini et al., 2014). The anti-cancer mechanisms of the AgNPs against different cell lines may be associated with the oxidative stress, mitochondrial and DNA damage, and apoptotic induction (Rejeeth et al., 2014).
GC–MS is ideal for the study of mainly low polarity, thermally-stable volatile compounds and derivatizable. This however could lead to incomplete derivation and loss of metabolites and may create artefacts (Stringer et al., 2016). In comparison to Chlorella sp. and N. oculate-CHL which exhibited hexanedioic acid, bis(2-ethylhexyl) ester, neophytadiene and eicosane as compounds detected in both species, tetracontane (18.16%), eicosane (17.11%) and hexatriacontane (12.77%) are significantly detected in T. suecica-CHL, while hexanedioic acid, bis (2-ethylhexyl) ester (9.39%), and neophytadiene (7.14%) are moderately detected (Hussein et al., 2020a). Diisooctyl phthalate was detected in Chlorella sp.-CHL (2.08%) and N. oculata-CHL (6.74%) (Table 1), but none in T. suecica-CHL (Hussein et al., 2020a). This was therefore considered as not a major compound. Diethyl-phthalate and phthalic-acid derivatives such as Di-n-octyl-phthalate (DOP) are used as plasticizers and can be toxic to marine organisms (Chen and Sung, 2005). The origin of the compound is not clear but the concerns must be addressed in the future investigation.
Similarly, the GC–MS detects dibutyl phthalate and diamyl phthalate, and the phenolics including tetradecane, heptadecane, 2,6,10,14-tetramethyl, 1,2-benzenedicarboxylic acid, bis(2-methylpropyl)ester, and 1,2-benzenedicarboxylic acid, butyl 2-ethylhexylester, in the methanolic extracts of Chlorella vulgaris. The N. oculata extracts show trichloroacetic acid, pentadecyl ester, dodecane, 2,6,11-trimethyl, 1-decanol, 2-hexyl, dodecanoic acid, 1-methylethyl ester, heptadecane, eicosane, phytol, and hexadecanoic acid, methyl ester and. Both exhibit decane, 2,3,5,8-tetramethyl, pentadecane, and hexadecane (Bhuvana et al., 2019). The GC–MS analyses of Streptomyces sp. have shown the presence of diisooctyl phthalate, hexanedioic acid bis (2-ethylhexyl) ester, 2-pentanone, cyclohexasiloxane, 4-hydroxy-4-methyl, oxime-, dodecamethyl, cycloheptasiloxane, methoxy phenyl, and tetradecamethyl. These compounds exhibit anticancer, antioxidant, and antimicrobial activities (Phuong et al., 2018). The fatty acid constituents such as tetradecanoic acid, octadecanoic acid and hexadecanoic acid methyl esters, have been suggested to promote bioactivities (Musharraf et al., 2012). The pentadecanoic acid, 14-methyl-, methyl ester, 2- pentadecanone,6,10,14-trimethyl, phytol, benzeneacetic acid a, 3-bis(acetyloxy)-methyl ester, and 1,2- benzenediol,4-(2-aminopropyl), in Chlorococcum humicola methanol extracts, for example, exhibit anticancer and antioxidant activities (Balaji et al., 2017). The tetracontane and phytol detected in Plectranthus amboinicus extracts may have resulted in enhanced pharmacological activities (Swamy et al., 2017).
5 Conclusion
Chlorella sp. had a higher cell growth in TMRL medium, but both Chlorella sp. and N. oculata exhibited hexanedioic acid, bis(2-ethylhexyl) ester as the major compound. The green biosynthesis of AgNPs was also successfully characterized. The AgNPs-N. oculata-CHL (at the ratios of 1.5:1 and 2:1) showed the highest anticancer activities against the MCF-7, while the AgNPs-Chlorella sp.-CHL exhibited the highest cytotoxicity on the 4T1 cells. However, the AgNPs-MEs co-application had not shown any cytotoxicity on the Vero cells. The co-application furthermore induced the highest early and late apoptosis and sub G1 phase (apoptotic events) in the MCF-7 and 4T1 cells. The AgNPs-MEs co-application also exhibited superior antioxidant activities. The presence of AgNPs therefore enhanced the anticancer activities of the MEs, while the presence of MEs reduced the cytotoxic effects of the AgNPs against the non-cancerous Vero cells.
Funding
This research was funded by Fundamental Research Grant Scheme, Ministry of Higher Education Malaysia, under grant number FRGS/1/2015/SG05/UMT/02/4.
Acknowledgments
The authors thank the Science Officers at the Institute of Marine Biotechnology, Universiti Malaysia Terengganu for their assistance with the facilities for the experiments, and Mr. Syed Ahmad Tajudin and Mr. Haziq Hamid from the Laboratory of Animal Cell Culture in Universiti Sultan Zainal Abidin, Tembila Campus, Besut, Terengganu, for their assistance in the flow cytometric analyses.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cytotoxic effects of drug-loaded hyaluronan-glutaraldehyde cross-linked nanoparticles and the release kinetics modeling. J. Adv. Chem. Eng.. 2014;1(104):2.
- [Google Scholar]
- Integrated algal engineering for bioenergy generation, effluent remediation and production of high-value bioactive compounds. Biotechnol. Bioproc. Eng.. 2016;21:236-249.
- [Google Scholar]
- Integrated algal bioprocess engineering for enhanced productivity of lipid, carbohydrate and high-value bioactive compounds. Res. Rev. J. Microbiol. Biotechnol.. 2017;6:61-92.
- [Google Scholar]
- Anticancer, antioxidant activity and GC-MS analysis of selected micro algal members of chlorophyceae. Int. J. Pharm. Sci. Res.. 2017;8:3302-3314.
- [Google Scholar]
- Spectral characterization of bioactive compounds from microalgae: N. oculata and C. vulgaris. Biocatal. Agric. Biotechnol.. 2019;19:101094
- [Google Scholar]
- Biosynthesis of silver nanoparticles using aqueous Broccoli extract-characterization and study of antimicrobial, cytotoxic effects. Asian J. Pharm. Clin. Res.. 2013;6:165-172.
- [Google Scholar]
- Antimicrobial activity of extracellularly synthesized silver nanoparticles using Lactobacillus species obtained from VIZYLAC capsule. J. Appl. Pharm. Sci.. 2012;2:25-29.
- [Google Scholar]
- The toxic effects of phthalate esters on immune responses of giant freshwater prawn (Macrobrachium rosenbergii) via oral treatment. Aquat. Toxicol.. 2005;74:160-171.
- [Google Scholar]
- Biosynthesis of silver nanoparticle (AgNPs) using Lactobacillus and their effects on oxidative stress biomarkers in rats. J. King Saud Univ. - Sci.. 2017;29:462-467.
- [Google Scholar]
- Isolation of chlorophylls and carotenoids from freshwater algae using different extraction methods. Phycol. Res.. 2018;66:52-57.
- [Google Scholar]
- Gul-e-Saba, Abdullah, M.A., , 2015. Polymeric nanoparticle mediated targeted drug delivery to cancer cells. In: Thangadurai D, Sangeetha J. (Eds.), Biotechnology and Bioinformatics: Advances and Applications for Bioenergy, Bioremediation, and Biopharmaceutical Research. CRC Press/Apple Academic Press, New Jersey, USA, pp. 1–34.
- Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. Biomed. Res. Int.. 2013;2013:10.
- [Google Scholar]
- Cytotoxic effects of Tetraselmis suecica chloroform extracts with silver nanoparticle co-application on MCF-7, 4 T1, and Vero cell lines. J. Appl. Phycol.. 2020;32:127-143.
- [Google Scholar]
- Phytochemical screening, metabolite profiling and enhanced anti-microbial activities of microalgal crude extracts in co-application with silver nanoparticle. Bioresour. Bioproc.. 2020;7:39.
- [Google Scholar]
- Microalgal metabolites as anti-cancer/anti-oxidant agents reduce cytotoxicity of elevated silver nanoparticle levels against non-cancerous vero cells. Heliyon. 2020;6(10):e05263
- [Google Scholar]
- Antimicrobial activity of silver nano particles biosynthesized by Lactobacillus mixtures. Res. J. Pharm. Biol. Chem. Sci.. 2017;8:1911-1924.
- [Google Scholar]
- Electrochemical and antioxidant properties of biogenic silver nanoparticles. Int. J. Electrochem. Sci.. 2015;10:7905-7916.
- [Google Scholar]
- Enhanced antioxidant activity of gold nanoparticle embedded 3, 6-dihydroxyflavone: a combinational study. Appl. Nanosci.. 2014;4:153-161.
- [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods.. 1983;65:55-63.
- [Google Scholar]
- Biodiesel production from microalgal isolates of southern Pakistan and quantification of FAMEs by GC-MS/MS analysis. Chem. Cent. J.. 2012;6:149.
- [Google Scholar]
- Biosynthesis, characterization and antibacterial activity of silver and gold nanoparticles from the leaf and bark extracts of Zanthoxylum capense. M. Sc.Thesis: University of Johannesburg; 2015. p. :1-84.
- Bioactive compounds from marine Streptomyces sp. by Gas Chromatography-Mass Spectrometry. Pharm. Chem. J.. 2018;5:196-203.
- [Google Scholar]
- Microwave-mediated extracellular synthesis of metallic silver and zinc oxide nanoparticles using macro-algae (Gracilaria edulis) extracts and Its anticancer activity against human PC3 cell lines. Appl. Biochem. Biotechnol.. 2014;174:2777-2790.
- [Google Scholar]
- Rejeeth, C., Nataraj, B., Vivek, R., Sakthivel, M., 2014. Biosynthesis of silver nanoscale particles using Spirulina platensis induce growth-inhibitory effect on human breast cancer cell line MCF-7. Med. Aromat. Plant. 3(163), 2167-0412.
- An investigation on cytotoxic effect of bioactive AgNPs synthesized using Cassia fistula flower extract on breast cancer cell MCF-7. Biotechnol. Rep.. 2015;8:110-115.
- [Google Scholar]
- Evaluation of antioxidant and nitric oxide inhibitory activities of selected Malaysian medicinal plants. J. Ethnopharmacol.. 2004;92:263-267.
- [Google Scholar]
- Cell culture optimization and reactor studies of green and brown microalgae for enhanced lipid production. Seri Iskandar, Malaysia: Universiti Teknologi PETRONAS; 2014.
- Effects of macro/micronutrients on green and brown microalgal cell growth and fatty acids in photobioreactor and open-tank systems. Biocatal. Agric. Biotechnol.. 2018;14:10-17.
- [Google Scholar]
- Cytostatic and Anti-tumor Potential of Ajwa Date Pulp against Human Hepatocellular Carcinoma HepG2 Cells. Sci. Rep.. 2019;9(1):1-12.
- [Google Scholar]
- In vitro antidermatophytic activity and cytotoxicity of extracts derived from medicinal plants and marine algae. J. Mycol. Med.. 2018;28:561-567.
- [Google Scholar]
- Atalantums H-K from the peels of Atalantia monophylla and their cytotoxicity. Nat. Prod. Res. 2019:1-7.
- [Google Scholar]
- Metabolomics and its application to acute lung diseases. Front. Immunol.. 2016;7:44.
- [Google Scholar]
- Synthesis, characterization and dose dependent antimicrobial and anti-cancerous activity of phycogenic silver nanoparticles against human hepatic carcinoma (HepG2) cell line. AIMS Bioeng.. 2016;3:425-440.
- [Google Scholar]
- GC-MS based metabolite profiling, antioxidant and antimicrobial properties of different solvent extracts of Malaysian Plectranthus amboinicus leaves. Evidence-Based Complement. Altern. Med.. 2017;2017:10.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.10.011.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







