Translate this page into:
Antibacterial and toxicity studies of phytochemicals from Piper betle leaf extract
⁎Corresponding author. pakpimol.un@wu.ac.th (Pakpimol Ungcharoenwiwat)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The methanolic and ethanolic Piper betle L. (PB) extracts (PBM and PBE, respectively) yielded 14.14 % and 8.23 %, respectively. The phytochemicals in the PB extract were alkaloids, flavonoids, coumarins, tannins, terpenoids, cardiac glycosides, and saponins, whereas steroids and pholbatannins were obtained from Piper retrofractum (PR) and Glycosmis pentaphylla (GP) extracts, and anthaquinones were found only in the GP extract. Furthermore, only the PB extract exhibited antibacterial activities against Vibrio parahaemolyticus, Staphylococcus aureus, Escherichia coli, Bacillus cereus, and Pseudomonas aeruginosa with minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values ranging 0.24–3.91 mg/mL. The highest bactericidal activity was observed against V. parahaemolyticus. PBM and PBE extracts had total phenolic contents of 130 ± 4.46 and 147.69 ± 0.03 mg gallic acid equivalents (GAE)/g, respectively, with scavenging activity (IC50) of 0.03 mg/mL. The total phenolic contents were significantly decreased, whereas antibacterial activities remained stable (>50 % at 65 °C for 12 h). Toxicity evaluation showed that PBM and PBE caused hemolysis in a dose-dependent manner, with IC50 values of 0.24 and 0.44 mg/mL, respectively. Both extracts were moderately toxic to Artemia salina (LC50 = 0.58–0.61 mg/mL). Finally, the PB extract exhibited inhibitory activities against lipase, glucoamylase, and trypsin. Based on these findings, crude extracts of PB have the potential to be used as antibacterial, antidiabetic, anti-obesity, and dietary supplements.
Keywords
Antibacterial
Artemia salina
Digestive enzyme
Phenolic
Piper betle
1 Introduction
Medicinal plants are the potential source of bioactive ingredients in various traditional herbal medicines. Phytochemicals derived from medicinal plants have various health benefits including antimicrobial activity, antioxidant capacity, digestive enzyme inhibition, antithrombin activity (Subramaniam et al., 2020) and nutritional supports. Moreover, the intensive research reported the new findings in nephroprotective efficacy (Edo et al., 2023), hepatoprotective activity of Ricinodendron heudelotii (Baill.) on paracetamol-induced treatment (Edo et al., 2024a), blood sugar control of Cyperus esculentus L. (Edo et al., 2024b). Therefore, medicinal plants are a significant source of natural alternatives to chemical compounds for various products aimed at combating pathogenic organisms (microorganisms or pests). Approximately 25 % of all modern pharmaceuticals are of plant origin. Several useful medicinal plants are distributed in southern Thailand, including P. betle L. (PB), P. retrofractum Vahl. (PR), and G. pentaphylla (Retz.) (GP) DC.
PB or “Phlu”, in Thai, is a climber belonging to family called Piperaceae with a distinct scent. It was once used to treat black tooth stains by ancient Thais and as a broad-spectrum medication. PB is widely cultivated for local consumption and commercial purposes throughout South and Southeast Asia, particularly Thailand. Currently, PB leaves are also distributed in fresh markets to elderly individuals as wraps for areca nuts for chewing, religious ceremonies and traditional treatments. There are eight phytochemical groups: phenols, glycosides, alkaloids, flavonoids, steroids, saponins, tannins, and terpenoids. The primary bioactive compounds are β-caryophyllene, eugenol, and hydroxychavicol, estragole, 1,8‐cineol, α‐pinene, β‐pinene, caryophyllene, and hydroxycatechol (Biswas et al., 2022), which have antioxidant activity, antibacterial activity, anti-inflammatory activity (Parkpoom et al., 2016). PR or long pepper is commonly applied as a spice and condiment. It is also used in traditional medicine because of its antiflatulent, expectorant, and antitussive properties. The phytochemical profile of P. retrofractum comprises six primary compounds: quinones, sterols, glycosides, flavones, tannins, and alkaloids (Jadid et al., 2017). These compounds exhibit antifungal (Sari and Nugraheni, 2013) and antibacterial activities (Panphut et al., 2020). GP is commonly found in Thailand. Its leaves and stems are traditionally used to treat various ailments, including internal and external abscesses, fever, intestinal worms, and liver and skin problems (Bulbul and Jahan, 2016; Khandokar et al., 2021). These plant parts contain four primary phytochemical groups: alkaloids, phenolic compounds, terpenoids, and steroids (Aiyakool and Vajrodaya, 2014). The constituents of medicinal plants are microbially degradable and accumulate in the environment within a short time. However, the efficiency and safety of these plants require further investigations.
This study investigated the phytochemical composition of three medicinal plants: P. betle (PB), P. retrofractum (PR), and G. pentaphylla (GP). The research also evaluated their biological activities, including antibacterial and antioxidant properties, as well as the temperatures and storage time affecting on these activities for further applications. Finally, the toxicity and inhibition of digestive enzymes were studied to evaluate their efficacy as antibacterial agents and dietary supplements.
2 Materials and methods
2.1 Phytochemical extraction
GP stems and PB leaves from Mueang district, Phatthalung province, and PR fruits from Chulabhorn, Nakhon Si Thammarat province were collected from cultivated area. The voucher specimens of GP, PB and PR with herbarium accession number as 01579, 01582, 01,585 were deposited at herbarium of Walailak Botanic Park. The cleaned samples were placed in hot air oven at 40 °C for 3 days before being ground into a fine powder (mesh size of 0.25 mm). The phytochemicals were extracted from the powdered samples with 95 % methanol and 95 % ethanol using a modified maceration method (Ungcharoenwiwat et al. 2023). A stock solution of the crude extract (500 mg/mL) was prepared using 95 % methanol or ethanol. The crude extract solutions were kept at −20 °C until use.
2.2 Analysis of phytochemical groups from plant extracts
Ten phytoconstituents in the crude extracts were screened using a modified protocol described by Ayoola et al. (2008). The presence of compounds was qualitatively evaluated by observing the color change, sedimentation, and foam formation.
2.3 Evaluation of antibacterial activity
The inhibitory efficacy of all extracts was evaluated against bacterial strains, including B. cereus TISTR747, S. aureus TISTR2329, E. coli TISTR527, P. aeruginosa TISTR357 and V. parahaemolyticus TISTR1596. The single colony of all strains was re-streaked on nutrient agar (NA) at 35 ± 2 °C for 24 h (supplement with 3 % NaCl for V. parahaemolyticus). A direct suspension of bacterial strains (1 × 108 CFU/mL) was swabbed across three planes on Müller-Hinton agar (MHA) (with 3 % NaCl for V. parahaemolyticus). The crude extract solutions (50 µL) loaded in sterile discs (0.6 mm diameter) were placed on surface of agar in triplicate, and the kanamycin (500 µg/mL) and organic solvent were applied as a control. The activity was observed after incubation at 35 °C for 24 h. The clear zone of inhibition surrounding the paper disc was reported to be millimeters in diameter.
2.4 Evaluation of minimum inhibitory concentration (MIC) and minimum of bactericidal concentration (MBC)
The susceptibility of specific bacteria was studied as the MIC value using a broth microdilution assay modified from the Clinical and Laboratory Standards Institute (CLSI) (CLSI, 2009) and Ungcharoenwiwat et al. (2023). A two-fold serial dilution of the PB extract in a suitable solvent, ranging 500–0.24 mg/mL, was prepared into 96-well plates in triplicate and evaporated the solvent under vacuum at RT (28 ± 2 °C). An aliquot of bacterial suspension (10 µL), containing approximately 1 × 106 CFU/mL, was inoculated to each well containing MHB or 3 % NaCl MHB and then incubated at 35 °C. Kanamycin (500 µg/mL) and organic solvent were a control. After 20 h of incubation, the oxygen consumption of bacteria was qualitative determined using resazurin solution (0.18 %) for 4 h of incubation. The blue well at the lowest concentration of the crude extract was considered the MIC value. For MBC assay, a 10 µL from each well was dropped onto agar plates in triplicate, and the bacterial growth were observed after incubation at 35 °C for 24 h. The absence of bacterial colonies at the lowest concentration of the crude extract was recorded as the MBC value.
2.5 Determination of total phenolic content and antioxidant activity
Phenolic compounds are important constituents with various bioactivities. The total phenolic content (TPC) of all crude extracts was assayed using the Folin-Ciocalteu assay with UV–visible spectrophotometry (modified from Muhamad et al. (2019)). The TPC expressed as gallic acid equivalents (GAE) per gram of sample was calculated from calibration curve of gallic acid.
The antioxidant property acting as radical scavenger of the crude extracts was measured by 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity according to the protocols described by Muhamad et al. (2019). All analyses were carried out in triplicates. Free radical-scavenging activity (%) was calculated using the following equation:
2.6 Study of thermal stability and storage time
The lifetime of the phenolic contents and antibacterial stability of the crude PB extracts are of concern for quality control. The extracts were incubated at various temperatures at 28 (RT), 37 °C, and 65 °C for different durations (3, 6, and 12 h) and 121 °C for 15 min (autoclave condition). The temperature that resulted in the highest remaining total phenolic content was selected to study the effect of storage time (1, 7, 14, 30, and 90 days). All treatments were done in triplicate. The TPC, MIC, and MBC of the extracts were assessed as described above. The stability value of TPC (%) was reported using the following formular:
2.7 Analysis of phytochemical metabolites by LC-QTOF MS
Liquid Chromatograph-Quadrupole Time-of-Flight Mass Spectrometer (1290 Infinity II LC-6545 Quadrupole-TOF, Agilent Technologies, USA) was used for chemical components analysis. The substances from PBM were separated on Zorbax Eclipse Plus C18 Rapid Resolution HD (150 mm length x 2.1 mm inner-diameter, particle size 1.8 μm, Agilent, USA). The mobile phase A was 0.1 % Formic acid and 0.5 mM ammnonium formate in water, and B was 0.1 % Formic acid and 0.5 mM ammnonium formate in acetonitrile, at a flow rate of 200 µL/min. The program was specified as follows, 90 % B (15 min), 90 % B (24 min), 5 % B (26 min) and 5 % B (30 min). The UPLC condition was positive and negative electrospray mode for the MS analysis using data-dependent automatic MS/MS acquisition modes (AutoMSMS).
2.8 Hemolytic activity against human erythrocytes
Hemolytic activity refers to the ability of a phytochemicals in PB extracts to damage or lyse red blood cells, leading to the leakage of hemoglobin from the cells. This phenomenon can be used as an indicator of cellular toxicity, indicating the potential of a substance to harm the cells. This method was described by Madakka et al. (2021). Briefly, the PB extract was mixed with 10 % human erythrocytes at a ratio 1:2 and then incubated at 37 °C for 1 h (water bath). The final volume (1 mL) in each tube was adjusted by PBS solution, and the supernatant was separated at 1000 × g for 5 min. Triton X100 (1.0 %), PBS solution, and organic solvent were used as controls. Hemoglobin released into the supernatant was absorbed at 540 nm using a spectrophotometer, and hemolysis (%) was calculated using the following equation:
The hemolyticity was considered by comparing percentage of hemolyticity with negative control according to ASTM F756-00, if % hemolysis was 0 to 2.0 = non-hemolytic, 2.1 to 5.0 = slightly hemolytic, and more than 5.0 = hemolytic.
2.9 Determination of toxicity with Artemia salina model
The brine shrimp lethality assay, a modified method of Ramos et al. (2009) provides a preliminary evaluation of the toxicity of the crude extract. PB extract solution (2.4–153.6 mg/mL, 500 µL) were added to vials, and the organic solvent was evaporated with vacuum desiccator until dry. Five milliliters of 2.5 % artificial seawater were added to the vials to prepare a final concentration of 0.24–15.36 mg/mL PB extracts. Ten nauplii (24-h old) were transferred to each vial, and all vials were placed at room temperature (28 ± 2 °C) for 24 h. Evaporated solvent vials containing 2.5 % artificial seawater were used as negative controls. The toxicity was considered as LC50 < 100 µg/mL=high toxicity, 100 µg/mL>LC50 < 500 µg/mL=moderate toxicity, and LC50 > 1000 µg/mL=low toxicity.
2.10 Digestive enzyme inhibitory activity
Glucoamylase activity was determined by the DNS assay with 1 % soluble starch as the substrate. The crude extract solutions at 1.95 mg/mL were mixed with citrate buffer (pH 5.5) at ratio 1:1 to final volume at 500 µL. A glucoamylase solution from Aspergillus niger (5 U/mL in citrate buffer, pH 5.5, 500 µL) was mixed into test tube at 37 °C for 15 min. Enzyme activity was measured according to Sharma et al. (2014). The percentage inhibitory activity was calculated using the following equation:
Bovine pancreatic lipase activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate. The PB extract solutions at 1.95 mg/mL were mixed with Tris-HCl buffer (pH 8.5) (1:1 v/v) to final volume 200 µL. A lipase solution from the bovine pancreas (25 mg/mL, pH 8.5) was added to the test tubes and placed at 37 °C for 15 min. The enzyme activity was determined using method of Kanwar et al. (2005) and the inhibitory activity (%) was calculated using the above equation.
Bovine pancreatic trypsin activity was measured using azocasein as a substrate. The crude extract solutions at 1.95 mg/mL were mixed with Tris-HCl (pH 8.5) at ratio 1:1 to final volume 200 µL. Trypsin solution from bovine pancreas (1 U/mL, 50 µL) was mixed into each tube and incubated at 37 °C for 15 min. The enzyme reaction was analyzed using a method of Spelbrink et al. (2011) and the inhibitory activity (%) was calculated using a previously described equation.
2.11 Statistical analysis
All assays were conducted in triplicate and standard deviation, graph plots, and linear regression were generated using Microsoft Excel 2020. Statistical analyses were performed using one-way analysis of variance (ANOVA) and Tukey’s multiple tests using Minitab19. Statistical significance was set at p < 0.05.
3 Results and discussion
3.1 Phytochemical screening from medicinal plants
Plant extractions were performed for PB, PR and GP using 95 % methanol and ethanol. The extracts from GP yielded low amounts (3.1-4.2%) of brownish-orange paste. Crude PR extracts showed the highest yield of a brownish paste (PRE 15.23% and PRM 6.86%). For PB, the methanolic crude extract (PBM) had a higher yield (14.14%) than the ethanolic crude extract (PBE) (8.13%). Both the extracts appeared as dark greenish pastes. Ten phytochemical groups were screened in the crude extracts (Table 1). There were slightly different phytochemical groups in the two solvents except for GP. Detectable phytochemical groups were divided into five primary compounds, namely terpenoids, saponins, flavonoids, coumarins and tannins. Phenolic compounds are the large group and have high solubility in polar organic solvents, especially alcohols and often combine with numerous compounds in plant extracts. Remark +++ High detectable ++ Moderate detachable. + Low detachable *** High foam reaction . ** Moderate foam reaction * Low foam reaction ND Not detectable.
Compounds
P. retrofractum
P. betle
G. pentaphylla
Methanol
Ethanol
Methanol
Ethanol
Methanol
Ethanol
Alkaloid
+++
+++
+
+
ND
ND
Terpenoid
+++
+
+
+
+++
+++
Cardiac glycoside
ND
+
+
+
+
+
Saponin
**
*
*
*
***
**
Flavonoid
+
+
+++
+++
+
+
Coumarin
+
+++
+++
++
+++
+++
Pholbatannins
ND
ND
ND
ND
++
++
Tannin
++
+
+++
+++
+++
+++
Anthraquinones
ND
ND
ND
ND
+++
ND
Steroids
++
++
ND
ND
++
+++
4 Evaluation of antibacterial activity by disc diffusion method
The antibacterial potency of the six alcoholic plant extracts against the five bacteria was assessed using the disc diffusion method (Table 2). Inhibition zone of Gram-positive bacteria ranged 14.3–22.7 mm, whereas those of Gram-negative bacteria ranged of 9.3–23.2 mm. Only crude PB extracts (PBE and PBM) effectively inhibited all tested strains. PBE showed inhibition zones of 17.3– 23.2 mm, and PBM exhibited zones of 12.0–20.5 mm. S. aureus displayed the highest susceptibility (20.5 ± 2.1 mm), followed by E. coli (23.2 ± 0.2 mm), V. parahaemolyticus (19.3 ± 0.5 mm), B. cereus (17.3 ± 0.9 mm), and P. aeroginosa (13.3 ± 1.8 mm). Panphut et al. (2020) and Syahidah et al. (2017) reported similar findings, PB exhibited high inhibitory activity against Gram-positive bacteria, whereas PR exhibited low or no activity. However, the results of GP differed from those reported by Murugan et al. (2020), who reported high bacterial activity against S. aureus. Remark ND; Not detectable (No inhibition zone).
Samples
(50 mg/mL)
Inhibition zone (cm)
S. aureus
B. cereus
P. aeruginosa
E. coli
V. parahaemolyticus
GPM
ND
ND
ND
ND
ND
GPE
ND
ND
ND
ND
ND
PRM
ND
ND
ND
ND
ND
PRE
ND
ND
ND
ND
ND
PBM
20.5 ± 2.1
14.3 ± 1.9
12.0 ± 0.9
19.7 ± 0.9
19.3 ± 0.5
PBE
21.6 ± 0.4
17.3 ± 0.9
13.3 ± 1.9
23.2 ± 0.2
18.3 ± 2.8
Kanamycin
21.9 ± 0.5
22.7 ± 2.9
9.3 ± 1.2
20.0 ± 2.6
9.3 ± 0.9
4.1 Determination of MIC and MBC of plant extracts
Both alcoholic extracts of PB had potential inhibition against five pathogenic bacterial strains (Table 3). The MIC and MBC values of PBM and PBE ranged from 0.24 to 3.91 mg/mL and 0.98 to 3.91 mg/mL. The lowest MIC was found in V. parahaemolyticus (0.24 mg/mL, PBM), followed by B. cereus (0.98 mg/mL, PBE), P. aeruginosa and E. coli (1.95 mg/mL, PBM). For MBC values, there were one or two-folds increasing from MIC values that were found in treatments of B. cereus (3.91 mg/mL, PBM and PBE) and V. parahaemolyticus (1.95 mg/mL, PBE).
Bacterial species
PBM
(mg/mL)
PBE
(mg/mL)
Kanamycin (µg/mL)
Comparison
MIC
MBC
MIC
MBC
MIC
MBC
S. aureus
1.95
1.95
1.95
1.95
1.95
1.95
PBM=PBE
B. cereus
1.95
3.91
0.98
3.91
0.97
0.97
PBE
P. aeruginosa
1.95
1.95
3.91
3.91
7.81
7.81
PBM
E. coli
1.95
1.95
3.91
3.91
3.91
3.91
PBM
V. parahaemolyticus
0.24
0.24
0.98
1.95
31.25
31.25
PBM
These results were correspondence with the investigation of Chan et al. (2009), who reported that methanol-extracted bioactive substances, especially phenolic compounds, were more abundant than those extracted from ethanol because of their higher polarity. Phenolic compounds promote the cell membrane permeability in K+ and H+ transfer using carboxyl groups in aromatic hydrocarbons, which inhibit ATP synthesis by consuming a proton motive force (Nouri et al., 2014). Hydroxychavicol, eugenol, and chavibetol as main phenolic compounds in PB extracts, can precipitate DNA or penetrate bacterial cell walls (Jesonbabu et al., 2011).
4.2 Total phenolic content and antioxidant activity
The TPC analysis in extract was investigated to determine the redox properties acting as antioxidants. The methanolic crude extracts generally exhibited higher TPC than the ethanolic crude extracts, except for the crude PB extracts (Table 4). TPC values ranged 44.32–130.07 mg of GAE/g for methanolic extracts and 34.38–147.69 mg of GAE/g for ethanolic extracts. The highest TPC was observed in PBE at 147.69 ± 0.03 mg of GAE/g, followed by PBM at 130 ± 4.46 mg of GAE/g.
Crude extract
Total phenolic content (mg of GAE/g of extract)
Antioxidant activity IC50 (mg/mL)
GPM
44.32 ± 1.67
0.12
GPE
34.38 ± 1.30
0.90
PRM
46.48 ± 0.51
2.30
PRE
19.73 ± 6.63
3.40
PBM
130.07 ± 4.46
0.03
PBE
147.69 ± 1.82
0.03
DPPH scavenging activity, the activity increased in dose dependent manner, with IC50 values ranging 0.03–3.36 mg/mL. The highest IC50 values were observed for PRM and PRE, whereas the lowest IC50 values were observed in PBE and PBM. These suggested that the bioactive constituents possessing the antibacterial activity of PB extracts had also associated with their TPC and antioxidant activity. Moreover, the PB extract contained high amount of phenolic compound. This was supported by Altemimi et al. (2017), who reported that phenolic compounds transmitted electrons to other compounds, thereby exhibiting antioxidant activity. Antioxidant activity held significance for various applications, including reduction of inflammation, prevention of blood clots, and inhibition of enzymes.
4.3 Thermal stability and storage time
Temperature significantly affected the stability of both the TPC and the inhibitory potency of the PBM extracts, as shown in Fig. 1. TPC remained stable at temperatures between 28–35 °C and significantly decreased (p < 0.05) at higher temperatures. However, only 37.2 % of TPC remained after 12 h at 65 °C, and a similar decrease was observed under autoclave condition (33.21 %). The remaining antibacterial activity was stable at more than 50 % from MIC (1.95 mg/mL). A long-term storage study revealed a significant decrease in TPC (p < 0.05), with 85.9–22.3 % remaining after 7–90 days. Moreover, MBC decreased 2 folds of the MIC (3.81 mg/mL) for 90 days. According to Cheng et al. (2017), the temperature and storage time affected the structure of phenolic compounds by modifying their hydroxy groups. Moreover, the loss of TPC might be due to thermal degradation or heat-labile nature or degradative enzymes (Benjamin et al., 2022). On the other hand, microwave drying treatment could increase more the TPC than fresh sample (Benjamin et al., 2022), and high temperature showed the increasing of flavonoid content in ginger powder (Chan et al., 2009). The complex of polysaccharide and flavonoids had reported in thermostability improvement (Fernandes et al., 2020). These supported that crude PB extracts contained complex forms of phytochemical groups that might be released or change their forms leading to affect on thermal resistance and antibacterial activity. The stability of other active compounds and their functional group transformation should be intensive studied in the future.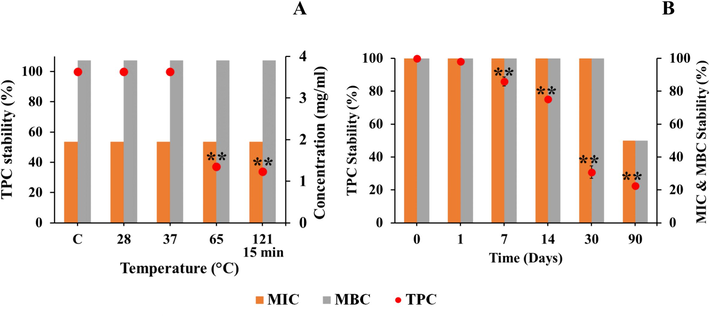
Stability of TPC and antibacterial activities against E. coli of PBM at different temperatures and autoclave condition (A) and at room temperature (28 °C) for 90 days (B).
4.4 Phytochemical metabolites by LC-QTOF/MS
The phytocomponents from PBM analyzed by LC-QTOF/MS with positive and negative electron spray ionization modes (ESI−/ESI+) revealed the retention time, mass, matching score and area sum (%) (Fig. 2 and Table 5.). The main components in extract from positive mode were steroids as mometasone furoate (12.3 %), alkaloids as erysopine (6.64 %) and quinazoline (4.31 %), quinones as heptaprenyl diphosphate (4.79 %), lipids as diglycerides (6.22 %), gingerglycolipid A (3.81 %) and stearidonic acid methyl ester (3.33 %). For the negative mode, the major components were flavonoids as demethoxyegonol (15.34 %), and phenolics that composed of 4-hydroxycinnamaldehyde (6.40 %), 2,5-dihydroxybenzal-dehyde (5.09 %), 1-(4-hydroxy-3-methoxyphenyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one (4.76 %) and isopulegone caffeate (2.72 %). Moreover, 3-hydroxychavicol 1-glucoside had also found in small area. The most of phenolics were detected by negative mode and other metabolites were determined by positive mode. The prevalent components in PBM extract had reported their biological properties including anti-bacteria of mometasone furoate (Neher et al., 2008), 2,5-dihydroxybenzaldehyde (Schabauer et al., 2018), 2,5-dihydroxybenzal-dehyde (Wong et al., 2008); anxiolytic activity of erysopine, antimicrobial and anti-complement activities of egonol and derivatives (Emirdağ-Öztürk et al., 2011); anti-inflammatory and antioxidant effects of 4-hydroxycinnamaldehyde (Lee et al., 2022). The chemical components of PBM varied based on several influences including the plant type, developmental phase, collecting time, habitat as well as extraction method.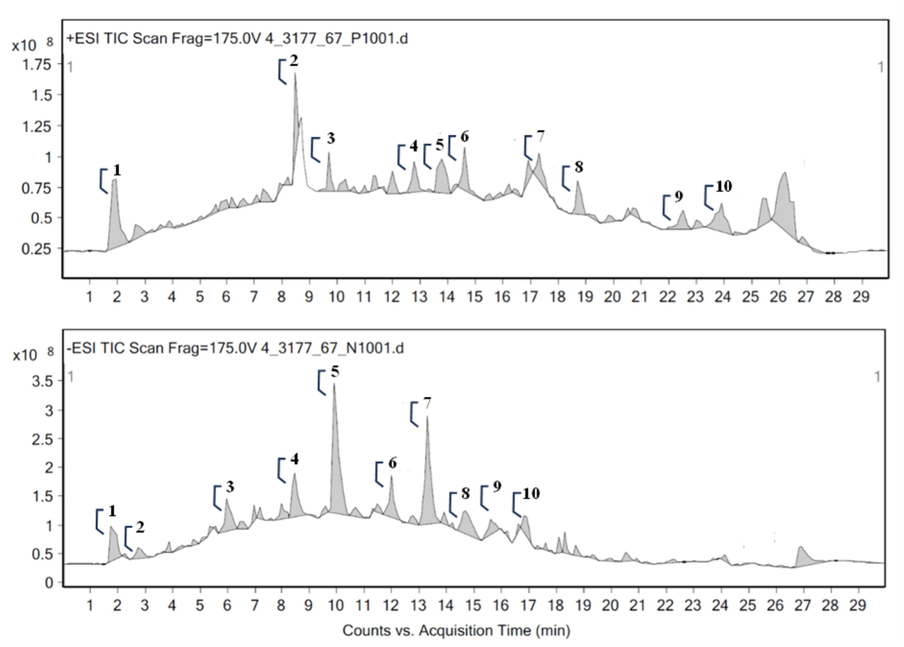
Total ion chromatogram of PBM analyzed by LC-MS/MS on positive and negative modes.
Peak
Rt
(Min)
Proposed compound
Formula
m/z
Mass
Score (DB)
Diff
(DB, ppm)
Area Sum
(%)
Positive mode
1
1.914
Mometasone Furoate
C27H30Cl2O6
543.1322
520.1431
65.99
−2.29
12.3
2
8.451
ZM 447,439
C21H36O10
536.2281
513.2389
96.07
−2.48
4.31
3
9.654
(9R,10S,12Z)-9,10-Dihydroxy-8-oxo-12-octadecenoic acid
C18H32O5
351.2142
328.2249
99.27
0.17
3.04
4
12.861
Gingerglycolipid A
C33H56O14
699.3561
676.3670
99.65
0.16
3.81
5
13.813
Erysopine
C17H19NO3
286.1442
285.1370
98.75
−1.72
6.64
6
14.540
all-trans-heptaprenyl diphosphate
C35H60O7P2
677.3721
654.3828
92.81
−2.17
4.79
7
17.346
Stearidonic acid methyl ester
C19H30O2
291.2323
290.2250
97.78
−1.44
3.33
8
18.874
Obtusilactone A
C19H32O3
309.2429
308.2356
98.57
−1.47
3.77
9
22.382
Azaspiracid 2
C48H73N O12
856.5237
855.5159
89.17
−3.07
3.26
10
23.985
DG(14:1(9Z)/22:6(4Z,7Z,
10Z,13Z,16Z,19Z)/0:0)C39H62O5
611.4672
610.4599
99.63
−0.28
6.22
Negative mode
1
1.896
D-Mannonate
C6H12O7
195.0515
196.0588
98.48
−2.36
5.63
2
2.811
Sulfamethoxypyrida-zine
C11H12N4O3S
279.0602
280.0675
−
−15.98
2.12
3
6.093
2,5-Dihydroxybenzal-dehyde
C7H6O3
137.0247
138.0320
87.16
−1.95
5.09
4
8.498
p-Hydroxycinnam-aldehyde
C9H8O2
147.0459
148.0531
−
−4.76
6.40
5
10.039
2-Fluoro-3-guanidino propionic acid
C4H8FN3O2
148.0533
149.0606
45.76
−3.8
19.65
6
11.930
1-(4-Hydroxy-3-methoxyphenyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one
C18H16O4
295.0980
296.1054
97.25
−1.93
4.76
7
13.221
Demethoxyegonol
C18H16O4
295.0982
296.1054
97.51
−1.85
15.34
8
14.724
N-Undecylbenzene sulfonic acid
C17H28O3S
311.1690
312.1763
96.82
−1.17
6.14
9
15.589
Isopulegone caffeate
C19H24O4
315.1604
316.1677
97.78
−0.8
2.72
10
16.942
4-Androsten-3,17-dione 19-aldehyde
C19H24O3
299.1655
300.1728
99.15
−0.84
3.06
4.5 Biological toxicity assay
4.5.1 Hemolytic activity
Hemolytic activity against erythrocytes is an important property to evaluate the potential safety of crude extracts for in vivo applications. In addition, the data can provide indirect information regarding the potential general toxicity. PBE and PBM exhibited hemolytic activity in a dose-dependent manner with IC50 values of 0.44 and 0.24 mg/mL, respectively (Fig. 3A). Potential mechanisms include the disruption of red blood cell membranes, ripping of the lipid bilayer and changes in the concentration of surrounding solution. According to Zohra and Fawzia (2014), the terpenoids and glycosides in betle leaf extracts could adhere to the erythrocytic membrane, eventually leading to disintegration.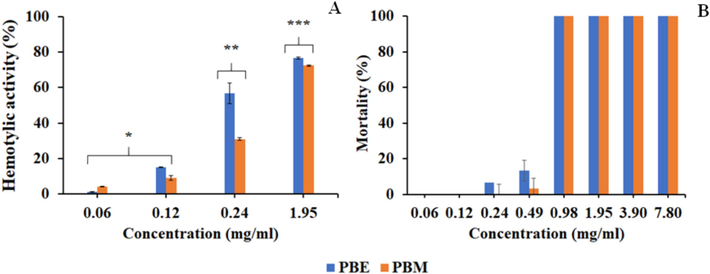
Hemolytic activity against human erythrocyte (A) and toxicity evaluation using A. salina larvae (B) by crude PB extracts.
4.5.2 Determination of toxicity against a. Salina
A. salina larvae lethality assay was investigated to assess the in vivo toxicity of PBM and PBE extracts (0.06–7.80 mg/mL). The morphology of A. salina larvae treated with all concentrations of extracts remained unchanged compared to that of the control group for 24 h. Abnormalities in swimming behavior and body size were observed (Table 6). The swimming or body size compared to the control group showed no significant difference (p > 0.05). At low concentrations (0.06–0.24 mg/mL), the survival rate exceeded 90 % between treatments (Fig. 3B).
Crude extracts
Concentration
(mg/mL)
Abnormality characters
Swimming (%)
Body sizes (mm)
Control
0
0
3.00 ± 0.17
PBM
0.06
76.67 ± 5.77
2.93 ± 0.11
0.12
93.33 ± 11.54
2.93 ± 0.05
0.24
80.00 ± 10.00
2.93 ± 0.00
0.49
84.72 ± 6.05
3.00 ± 0.17
PBE
0.06
90.00 ± 0.00
3.00 ± 0.17
0.12
80.00 ± 10.00
3.00 ± 0.30
0.24
83.33 ± 5.77
3.00 ± 0.10
0.49
86.67 ± 11.54
2.90 ± 0.10
Larval characteristics are shown in Fig. 4. After 24 h, the LC50 value (lethal concentration for 50 % of the population) of A. salina treated with PBM and PBE ranged 0.49–0.98 mg/mL. Complete mortality (100 %) was exhibited at 0.98–7.80 mg/mL. Therefore, the LC50 of PBM and PBE against A. salina were estimated as LC50 at 0.58 and 0.61 mg/mL that could be classified as moderate toxic. The toxicity of the crude PB extracts was evaluated to be low to moderate in human erythrocyte and organism. Therefore, it may not be suitable for internal use in organisms and can potentially be applied as a disinfecting agent at specific concentrations.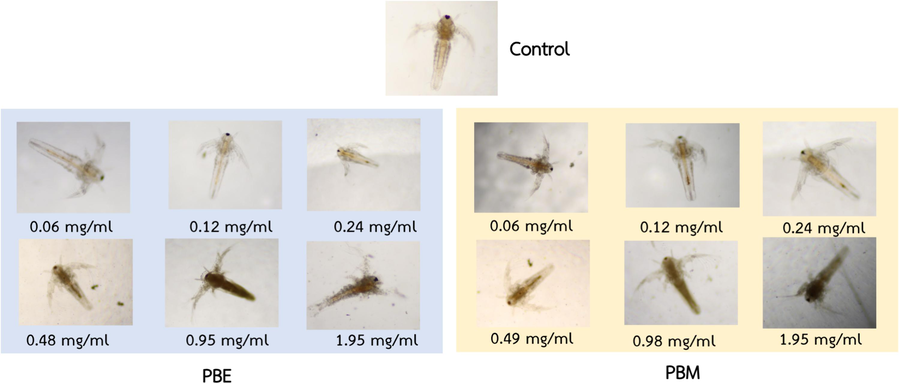
Morphology of A. salina treated with crude PB extracts at 24 h for toxicity evaluation.
4.6 Determination of digestive enzyme inhibition
Enzyme inhibition by plant extracts has potential as an alternative healthcare supplements. The results showed that PBM exhibited a low degree of alpha-glucoamylase inhibition as 10.39 %, whereas PBE activated the enzyme activity (−25.62 % inhibition). PBM and PBE showed lipase inhibition rates of 50.04 and 64.52 %, respectively. Conversely, PBM and PBE exhibited minimal inhibition of trypsin activity (5.86 and 10.03 %). Polyphenol, flavonoids, and glycoside groups in plant extracts are likely to contribute to this effect (Alias et al., 2017). Ado et al. (2013) reported that some plant extracts promoted enzyme activity by more than 20 %; moreover, phytochemical substances such as eugenol or flavonoids could delay the onset of enzyme reaction (Boskabady and Ramazani-Assari, 2001). Active inhibitors were mainly found in the phenolic and alkaloids groups that can directly bind to the catalytic or active sites of enzyme especially hydroxy groups (Ado et al., 2013). These findings suggest that PB extract has potential for applications as an alternative supplement in healthcare.
5 Conclusions
The present study revealed that alcoholic solvents (methanol and ethanol) were effective in extracting numerous phytochemical compounds from GP, PR, and PB. Only crude PB extracts exhibited antibacterial properties against five bacteria, ranging from 0.24-3.91 mg/mL of MIC and MBC values. The highest bactericidal activity was observed against V. parahaemolyticus (MBC=0.24 mg/mL). Furthermore, crude PB extracts were considered moderately toxic to A. salina with LC50 at 0.58–0.61 mg/mL. The total phenolic content significantly decreased at 65 °C after 12 h of incubation, whereas the antibacterial potential remained stable with 50 % of its initial activity. Moreover, crude PB extracts showed high inhibitory activity against lipase. Based on these results, the crude PB extracts have potential for use as antibacterial agents, and dietary supplements.
6 Institutional review board statement
The study of biological toxicity was approved by Ethics Committee in Human Research and the Institutional Animal Care and Use Committee of Walailak University, Thailand (Approval no.: WUEC20-171–01 and WU-AICUC-63–022), and the microbiological study was approved by Institutional Biosafety and Biosecurity of Walailak University (Approval no.: WU-IBC-63–020).
7 Disclosure of funding
This work was partially supported by Development and Promotion of Science and Technology Talents Project (DPST) Scholarship from Royal Government of Thailand, Plant Genetic Conservation Project Under the Royal Initiative of Her Royal Highness Princess Maha Chakri Sirindhorn (RSPG) conducted by RSPG-Walailak University coordinating center, and The Center for Scientific and Technological Equipment, Walailak University, Thailand.
CRediT authorship contribution statement
Thiti Sonphakdi: Writing – original draft, Validation, Investigation. Akio Tani: Writing – review & editing, Visualization. Apirak Payaka: Methodology, Conceptualization. Pakpimol Ungcharoenwiwat: Writing – original draft, Validation, Project administration, Methodology, Investigation, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anti- and pro-lipase activity of selected medicinal, herbal and aquatic plants, and structure elucidation of an anti-lipase compound. Molecules.. 2013;18:14651-14669.
- [CrossRef] [Google Scholar]
- Aiyakool, W., Vajrodaya, S., 2014. Comparative phytochemistry of Glycosmis pentaphylla (Retz.) DC (Rutaceae) in Thailand by thin layer chromatographic technique, in: Agricultural Sciences: Leading Thailand to World Class Standards. Presented at the The 52nd Kasetsart University Annual Conference, 4-7 February 2014, Kasetsart University, Thailand. Vol. 4: Science, Natural Resources and Environment., Kasetsart University, Bangkok, Thailand., pp. 52–59.
- Anti-obesity potential of selected tropical plants via pancreatic lipase inhibition. AOWMC. 2017;6:1-11.
- [Google Scholar]
- Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants (basel).. 2017;6:42.
- [CrossRef] [Google Scholar]
- Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop. J. Pharm. Res.. 2008;7:1019-1024.
- [CrossRef] [Google Scholar]
- The Effects of drying techniques on phytochemical contents and biological activities on selected bamboo leaves. Molecules. 2022;27:6458.
- [CrossRef] [Google Scholar]
- Betelvine (Piper betle L.): A comprehensive insight into its ethnopharmacology, phytochemistry, and pharmacological, biomedical and therapeutic attributes. J Cell Mol Med. 2022;26:3083-3119.
- [CrossRef] [Google Scholar]
- Relaxant effect of Pimpinella anisum on isolated guinea pig tracheal chains and its possible mechanism(s) J. Ethnopharmacol. 2001;74:83-88.
- [CrossRef] [Google Scholar]
- Study on antioxidant and antimicrobial activities of methanolic leaf extract of Glycosmis pentaphylla against various microbial strains. J Pharmacogn Phytochem. 2016;5:53-57.
- [Google Scholar]
- Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem.. 2009;113:166-172.
- [CrossRef] [Google Scholar]
- Effects of storage time and temperature on polyphenolic content and qualitative characteristics of freeze-dried and spray-dried bayberry powder. LWT - Food Sci. Technol.. 2017;78:235-240.
- [CrossRef] [Google Scholar]
- CLSI, 2009. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically; approved standard-Eight edition. CLSI documents M07-A8, in: Clinical and Laboratory Standards Institute (CLSI). Clinical and Laboratory Standard Institute, Wayne, PA.
- Edo, G.I., Ugbune, U., Onoharigho, F.O., Ezekiel, G.O., Agbo, J.J., 2023. Antioxidant activities of Reissantia indica willd. (mopane paddle-pod) and nephroprotective effect on paracetamol-induced nephrotoxicity in male wistar rats. Nutrire 48, 26. https://doi.org/10.1186/s41110-023-00214-x.
- The ameliorative effect of methanol extract of Ricinodendron heudelotii (Baill.) leaves on paracetamol-induced hepatotoxicity in Wistar rats. Drug and Chemical Toxicology 2024:1-10.
- [CrossRef] [Google Scholar]
- Edo, G.I., Samuel, P.O., Nwachukwu, S.C., Ikpekoro, V.O., Promise, O., Oghenegueke, O., Ongulu, J., Otunuya, C.F., Rapheal, O.A., Ajokpaoghene, M.O., Okolie, M.C., Ajakaye, R.S., 2024b. A review on the biological and bioactive components of Cyperus esculentus L.: insight on food, health and nutrition. Journal of the Science of Food and Agriculture. https://doi.org/10.1002/jsfa.13570.
- Synthesis of egonol derivatives and their antimicrobial activities. Bioorganic & Medicinal Chemistry, Imaging Probes. 2011;19:1179-1188.
- [CrossRef] [Google Scholar]
- Impact of grape pectic polysaccharides on anthocyanins thermostability. Carbohydrate Polymers. 2020;239:116240
- [CrossRef] [Google Scholar]
- Jadid, N., Hidayati, D., Hartanti, S.R., Arraniry, B.A., Rachman, R.Y., Wikanta, W., 2017. Antioxidant activities of different solvent extracts of Piper retrofractum Vahl. using DPPH assay. AIP Conference Proceedings 1854, 020019. https://doi.org/10.1063/1.4985410.
- The potential activity of hydroxychavicol against pathogenic bacteria. J. Bacteriol. Parasitol.. 2011;2:1-4.
- [CrossRef] [Google Scholar]
- Methods for inhibition of residual lipase activity in colorimetric assay: a comparative study. IJBB. 2005;42:233-237.
- [Google Scholar]
- Khandokar, L., Bari, Md.S., Seidel, V., Haque, Md.A., 2021. Ethnomedicinal uses, phytochemistry, pharmacological activities and toxicological profile of Glycosmis pentaphylla (Retz.) DC.: A review. J. Ethnopharmacol. 278, 114313. https://doi.org/10.1016/j.jep.2021.114313.
- Molecular mechanism of Cinnamomum cassia against gastric damage and identification of active compounds. Biomolecules. 2022;12:525.
- [CrossRef] [Google Scholar]
- Evaluating the antimicrobial activity and antitumor screening of green synthesized silver nanoparticles compounds, using Syzygium jambolanum, towards MCF7 cell line (Breast cancer cell line) J. Photochem. Photobiol.. 2021;6:100028
- [CrossRef] [Google Scholar]
- Boiling increase antioxidant activity, total phenolic content and total flavonoid content in Plectranthus amboinicus leaves. GSCBPS. 2019;6:024-030.
- [Google Scholar]
- Glycosmis pentaphylla (Rutaceae): a natural candidate for the isolation of potential bioactive arborine and skimmianine compounds for controlling multidrug-resistant Staphylococcus aureus. Front. Public Health.. 2020;8:176.
- [CrossRef] [Google Scholar]
- Antibacterial activity of mometasone furoate. Arch. Otolaryngol. Head. Neck. Surg.. 2008;134:519-521.
- [CrossRef] [Google Scholar]
- Phytochemical, antioxidant, antibacterial, and α-amylase inhibitory properties of different extracts from betel leaves. Ind. Crops Prod.. 2014;62:47-52.
- [CrossRef] [Google Scholar]
- In vitro antimicrobial activity of Piper retrofractum fruit extracts against microbial pathogens causing infections in human and animals. Int. J. Microbiol.. 2020;2020:e5638961.
- [Google Scholar]
- Antimicrobial and antioxidant capacity of leaf extract of Piper betle Linn. for development to cosmetics. SDU Res. J.. 2016;9:1-20.
- [Google Scholar]
- Antibacterial and cytotoxic properties of some plant crude extracts used in Northeastern folk medicine. Rev. Bras. Farmacogn.. 2009;19:376-381.
- [CrossRef] [Google Scholar]
- Antifungal activity test of Piper retrofractum leaf ethanol extract on Candida albicans growth. Asian J. Nat. Prod. Biochem.. 2013;11:36-42.
- [Google Scholar]
- Gentisaldehyde and its derivative 2,3-dihydroxybenzaldehyde show antimicrobial activities against bovine mastitis Staphylococcus aureus. Front. Vet. Sci.. 2018;5:148.
- [CrossRef] [Google Scholar]
- Optimization of critical growth parameters for enhancing extracellular lipase production by alkalophilic Bacillus sp. Biocatalysis and Agricultural Biotechnology. 2014;3:205-211.
- [CrossRef] [Google Scholar]
- Quantitative determination of trypsin inhibitory activity in complex matrices. The Open Food Science Journal. 2011;42–46
- [CrossRef] [Google Scholar]
- Antidiabetic, antithrombin and cytotoxic bioactive compounds in five cultivars of Piper betle L. Environ. Technol. Innov.. 2020;20:101140
- [CrossRef] [Google Scholar]
- Phytochemical analysis, identification and quantification of antibacterial active compounds in betel leaves, Piper betle methanolic extract. PJBS. 2017;20:70-81.
- [CrossRef] [Google Scholar]
- Ungcharoenwiwat, P., Thaweesuwanasak, M., Kanzaki, H., Nitoda, T., 2023. Antibacterial and antioxidant activities, lethality assay and chemical profile in crude extract of Biancaea sappan (L.) Tod. for anti-Vibrio agent. Journal of King Saud University - Science 35, 102594. https://doi.org/10.1016/j.jksus.2023.102594.
- Antibacterial activities of naturally occurring compounds against Mycobacterium avium subsp. paratuberculosis. Appl. Environ. Microbiol.. 2008;74:5986-5990.
- [CrossRef] [Google Scholar]
- Hemolytic activity of different herbal extracts used in Algeria. IJPSR. 2014;5:495-500.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103430.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







