Translate this page into:
Antibacterial and antioxidant activities, lethality assay and chemical profile in crude extract of Biancaea sappan (L.) Tod. for anti-Vibrio agent
⁎Corresponding author at: Department of Biology, School of Science, Walailak University, Nakhon Si Thammarat 80160, Thailand. pakpimol.un@wu.ac.th (Pakpimol Ungcharoenwiwat) pakpimol.un@mail.wu.ac.th (Pakpimol Ungcharoenwiwat)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Background and objectives
The bacterial infections are still an important cause of death for organisms leading to interesting finding the new antibacterial agent from natural source. The antimicrobial activity of Biancaea sappan extract (BSE) has been reported with a few mentions of anti-Vibrio efficiency. In this study, the heartwood extract from B. sappan was investigated for antibacterial activity, stability, toxicity, and anti-Vibrio in seawater.
Methods
The BSE was tested for antimicrobial activity to determine the minimum inhibitory concentration and minimum bactericidal concentration (MIC&MBC). The crude extract was evaluated an antioxidant activity through 2,2–diphenyl–1–picrylhydrazyl (DPPH) radical scavenging capacity and content of total phenolics according to Folin–Ciocalteu colorimetric method. Next, the BSE was determined thermal, diluent stabilities and Artemia salina toxicity before the time–kill study for anti-Vibrio in seawater. The major components in extract were analyzed using LC-Q-TOF-MS/MS.
Results
BSE was yielded at 9.56 %. The lowest MIC and MBC were 0.49 mg/mL for V. parahaemolyticus TISTR1596. The TPC in BSE was 31.46 GAE/g, and antioxidant activity with a 50 % inhibitory concentration (IC50) of 0.288 mg/mL. TPC in BSE was stable at high temperatures, and distilled water (>90 %).
It was non-acute lethality toward A. salina for 24 h (LC50 = 7.374 mg/mL). At 3MIC and 4MIC of BSE were effective for killing V. parahaemolyticus within 2 h.
Conclusions
The BSE showed an effective inhibitory against V. parahaemolyticus with the lowest MIC and MBC. The TPC was stable with non-acute toxic effects on A. salina. It showed high potential anti-Vibrio activity in seawater that may be shown the usefulness in controlling Vibrio infection.
Keywords
Anti-bacterial
Anti-Vibrio
Biancaea sappan
Phenolic
Lethality
1 Introduction
Thailand is one of the five leading exporters of seafood products in the world which has increased Thai farmers’ earnings. In 2012, shrimp farmers faced a disease outbreak called as early mortality syndrome (EMS) or acute hepatopancreatic necrosis disease (AHPND) caused by Vibrio parahaemolyticus (Nguyen et al., 2018). Shrimp suffering from AHPND exhibited severe atrophy of hepatopancreas (HP), growth reduction, whitening of the hepatopancreas, reduction in size of hepatopancreas that led to high mortality rate and affected on shrimp exports and economic losses. For shrimp farming, some chemicals as formalin and antibiotics are applied before shrimp laying eggs for disease control. Using antibiotics can lead to antibiotic-resistant pathogens (Komolafe, 2003). Alternative natural substances from medicinal plants possess antibacterial, antioxidant and anti-inflammatory activities are increasingly focused for applications (Nikmaram et al., 2018).
Bioactive compounds including beneficial phytochemicals are greatly found in medicinal plants. Several studies show that many plants are a rich source of antioxidants (Altemimi et al., 2017). For example, phenolic compounds including flavonoids, lignins and tannins in many parts of plants present various antimicrobial agents depending on types and number of phytochemical contents (Bouarab-Chibane et al., 2019). The medicinal plant extracts exhibit potential alternative antimicrobial agents (Altemimi et al., 2017).
Biancaea sappan (L.) Tod. (synonymous with Caesalpinia sappan L.) known as Brazil or sappan wood is a medicinal plant in family Fabaceae and widely found throughout Southeast Asia. Red dye is commonly extracted from the plant heartwood and important component in Thai traditional medicine to treat an anemia, diarrhea, dysentery, skin infections, and tuberculosis. Major substances in sappan wood were investigated, and various phenolic components such as brazilin, brazilein, chalcones, coumarin, flavones, homoisoflavonoids and xanthone were isolated. Brazilin and brazilein are bioactive compounds used as dying agent and has been reported in various activities including anti-acne, anti-allergic, anti-inflammatory, antibacterial, anti-photoaging, antioxidant, antihyperglycemic and vasorelaxant properties (Nirmal et al., 2015). Brazilein, sappanchalcone, protosappanin C-E had been reported anti-inflammatory effects in lipopolysaccharide (LPS)-induced macrophages (J774.1) (Washiyama et al., 2009). The alcoholic sappan extract also showed a hepatoprotective property to isolated hepatocyte and Wistar strain albino rat model that might be due to brazilin, flavonoids and phenolic compounds (Srilakshmi et al., 2010). The related patent had reported the use of the sappan extract for treatment of angiogenesis associated diseases consisting of lung cancer, liver cancer, pancreatic cancer, etc (Yang et al., 2007). Moreover, oral administration of sappan extract in rats showed no gross apparent change of internal organs with comparison to control (Sireeratawong et al., 2010).
The overall aims of this study were investigation of the antibacterial activity of crude extract from B. sappan heartwood against the following bacteria: Bacillus cereus, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and V. parahaemolyticus. Additionally, stability and toxicity aspects of the phytochemicals were determined, and the preliminary application as an anti-Vibrio substance in seawater was evaluated for further its application.
2 Materials and methods
2.1 Extraction of plant heartwood
Heartwood of B. sappan was collected from medicinal plants shops in Tha Sala sub-district, Nakhon Si Thammarat province. Heartwood powder (50 g) was soaked in 300 mL (1:6 w/v) of 95 % ethanol with shaking at 150 rpm for 5 days (Hemthanon and Ungcharoenwiwat, 2022), and the crude extract was separated by a double layer of white cloth and filter paper. The extract was concentrated using a rotary evaporator at 50 °C and stored in glass bottle covered with aluminum foil at −20 °C until use. The yield (%) was calculated by formula: Extract yield (%) = (crude extract (g) / plant powder (g)) × 100. The B. sappan extract (BSE) (1,250 mg) was prepared in DMSO (1 mL) and diluted with distilled water to 125 mg/mL (10 % DMSO) before filtering with a 0.22 μm membrane. The BSE stock (125 mg/mL) was stored at −20 °C until next study.
2.2 Bacterial strains and chemicals
The pathogenic bacteria causing foodborne illness, wound infection, and other general diseases including two Gram-positive bacteria (B. cereus TISTR747 and S. aureus TISTR2329) and three Gram-negative bacteria (E. coli TISTR527, P. aeruginosa TISTR357 and V. parahaemolyticus TISTR1596) were used for antibacterial activity assay. All bacterial strains were provided by TISTR Culture Collection (Bangkok MIRCEN, Bangkok, Thailand). Each strain was cultured on nutrient agar (NA) at 35 °C for 18–24 h. All chemicals were of analytical grade.
2.3 Antibacterial activity assay
2.3.1 Bacterial preparation
Among the five bacterial strains, B. cereus TISTR747, E. coli TISTR527, P. aeruginosa TISTR357 and S. aureus TISTR2329 were cultured in 5 mL of nutrient broth (NB), and V. parahaemolyticus TISTR1596 was cultured in 5 mL of NB containing 3 % NaCl (NB + 3 % NaCl) at 35 ± 2 °C for 24 h. All bacterial cultures were streaked on the same medium to obtain single colonies. The bacterial colony suspensions were prepared to a turbidity equivalent to 0.5 McFarland turbidity standard (∼108 CFU/mL) using a spectrophotometer at 625 nm (0.08–0.10). The bacterial strains were diluted in Mueller Hinton Broth (MHB) (only V. parahaemolyticus was diluted in MHB + 3 % NaCl) at a ratio of 1:100 to yield approximately 1.5 × 106 CFU/mL.
2.3.2 Determination of minimum inhibitory and minimum bactericidal concentrations
The minimum inhibitory concentration (MIC) of BSE can be measured using the broth microdilution assay, as described in previous research, in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines. The BSE solution (100 μL) was prepared by twofold serial dilution with MHB in a 96-well microtiter plate ranging from 62.5 to 0.49 mg/mL, and DMSO (10 %) and kanamycin (1,250 µg/mL) were used as control treatment. The adjusted bacterial suspension (10 μL) was applied into each well (105 CFU/mL). After 20 h of incubation, the bacterial growth was detected using resazurin solution with continuous incubation for 2–4 h. The lowest concentration of BSE showing a blue color, and an unchanged color indicated bacterial growth inhibitory.
The mixture in blue wells for each bacterial test set (10 μL) was placed onto NA (NA + 3 % NaCl for V. parahaemolyticus) that were then incubated at 35 °C for 24 h. The lowest concentration without bacterial growth was observed to be the MBC. The determination was done in triplicate.
2.4 Determination of total phenolic content
The BSE was qualitatively determined total phenolic content (TPC) by the Folin–Ciocalteu assay modified from Wong-Paz et al. (Wong-Paz et al., 2014). Briefly, 40 μL of BSE and 40 μL of Folin–Ciocalteu reagent were mixed at room temperature (RT, 28 ± 2 °C) for 5 min, and 40 μL of 0.01 M sodium carbonate was then added. The mixture was allowed to stand for 5 min in the dark, and then diluted with DI water. All tubes were measured the absorbance at 790 nm, and distilled water was used as a blank. The TPC was expressed as milligrams of gallic acid equivalents per gram of dried extract (mg GAE/g) in accordance with calibration curve of gallic acid.
2.5 Determination an antioxidant activity
BSE antioxidant activity was evaluated using 2,2–diphenyl–1–picrylhydrazyl (DPPH) radical scavenging activity with modified from Farasat et al. (Farasat et al., 2014). Briefly, BSE stock solutions were made to obtain concentrations ranging from 0.03 to 31.25 mg/mL. The samples (100 μL) were mixed with 1 mL of 100 µM DPPH in MeOH, and reactions were performed at RT in the dark for 30 min. The absorbance was measured at 515 nm, and 95 % ethanol and 95 % ethanol + DPPH solutions were used as controls, and all assays were tested in triplicate. The percentage of radical scavenging activity was calculated using the formula:
DPPH scavenging activity was recorded and plotted on a graph, and the half-maximal inhibitory concentration (IC50) value was then calculated.
2.6 Thermal and solvent stabilities
2.6.1 Temperature
The solution of crude extract dissolved in was autoclaved (121 °C, 15 min), pasteurized (63 °C, 20 min), or allowed to stand at room temperature (28 ± 2 °C).
2.6.2 Distilled water and seawater
Crude extracts were dissolved in distilled water or seawater (450 µL) and incubated at room temperature for 0, 6, or 12 h.
The TPC was determined for all treatments using the Folin–Ciocalteu method, and the MIC and MBC were determined using the broth microdilution assay. A stability assessment for BSE was evaluated as the percentage of TPC (%TPC), and a remaining TPC > 90 % was considered to show the substance’s stability.
2.7 Brine shrimp lethality bioassay
BSE toxicity was evaluated on the basis of its ability to kill brine shrimp (A. salina) as proposed by Meyer et al. (Meyer et al., 1982), with some modifications by Ramos et al (Ramos et al., 2009). For the experiment, 400 μL of BSE was prepared in 4.6 mL of 2.5 % artificial seawater to obtain various concentrations (0.12, 0.24, 0.49, 0.98, 1.96, 3.91, 7.81, 15.62, and 31.25 mg/mL) in vials that contained ten live brine shrimp nauplii. All treatments and controls were let to stand at RT for 24 h. The experiment was performed in triplicate. After incubation, the surviving nauplii in each vial were counted and calculated as % mortality. An acute toxicity was determined using the median lethal concentration test (LC50), which was the concentration of BSE that killed brine shrimp (50 %) at an exposure time.
The toxicity evaluation with the LC50 value was performed using Clarkson’s toxicity index (Clarkson et al., 2004) as follows: non-toxic (LC50 > 1,000 µg/mL), low toxicity (LC50 500–1,000 µg/mL), medium toxicity (LC50 100–500 µg/mL), and high toxicity (LC50 0–100 µg/mL).
2.8 Chemical profiling of BSE
The chemical profiling of BSE was determined by LC-Q-TOF-MS/MS. The analysis was performed on an Agilent 1290 Infinity II LC system coupled to a G6545 quadrupole time-of-flight (Q-TOF) mass spectrometer (Agilent Technologies, USA). The analysis was carried out with Zorbax Eclipse Plus C18 Rapid Resolution HD (150 mm length × 2.1 mm inner diameter, particle size 1.8 μm) (Agilent Technologies, USA) maintained at 25 °C. The mobile phase composed of 1 % acetic acid in water (solvent A) and acetonitrile (solvent B) applying the following gradient: 0–5 min (A: 95 %, B: 5 %), 5–10 min (A: 80 %, B: 20 %), 10–15 min (A: 70 %, B: 30 %), 15–20 min (A: 65 %, B: 35 %), 20–25 min (A: 55 %, B: 45 %), 25–30 min (A: 25 %, B: 75 %), 30–32 min (A: 5 %, B: 95 %) system with a flow rate 0.2 mL/min. The sample volume was 2 μL, and mass spectra were acquired in the negative ion mode with a mass range of 100–1,500 m/z for MS and MSMS in automatic mode. Mass spectral analysis was carried out on a MassHunter WorkStation-Qualitative Analysis, and Agilent personal Compound Database and Library (PCDL) version B.08.00 build 92 was employed to create the custom database.
2.9 Anti-Vibrio activity in seawater
The time–kill behavior at MIC and MBC values of BSE against V. parahaemolyticus TISTR1596 was determined. The bacterial strain was cultured and prepared to yield approximately 108 CFU/mL. The BSE stock solution (125 mg/mL) was diluted to make 1MIC, 2MIC, 3MIC, and 4MIC in a test tube containing 10 mL of seawater, and 10 % DMSO was used as a control treatment. The tests were performed in triplicate. The bacterial suspension (100 µL) was added to each tube (106 CFU/mL), and all treatments were incubated at 35 ± 2 °C for 0, 3, 6 and 12 h. Ten microliters of each treatment was then placed onto TCBS agar and incubated at 35 ± 2 °C for 24 h. The bacterial growth at each time point was determined and calculated in CFU/mL.
2.10 Statistical analysis
The results were expressed as the mean ± standard deviation (SD). All experiments were conducted in triplicate and analyzed using a one-way analysis of variance. Data were analyzed using SPSS (IBM Corp., Armonk, NY, USA). Values were considered statistically significant at p < 0.05.
3 Results
3.1 Plant extraction yield
The ethanolic BSE was obtained using the maceration method with 95 % ethanol. The BSE had a red–brown color, a sticky paste texture, and a distinctive smell. Fifty grams of leaf powder was used, and 4.78 g of the crude extract was obtained, which was a BSE yield of 9.56 % (w/w). Most previous studies reported that the sappan heartwood contained a red dye component that contained brazilin. Brazilin was the major active ingredient in group of flavonoids that is a phenolic compound. It can be dissolved in high polarity solvents because of the hydroxyl group in its structure (Rondão et al., 2013; Nirmal et al., 2015). It was extracted along with the phytochemical substances in sappan heartwood, and the crude extract was a shiny brown powder with a 17.60 % yield (Bukke et al., 2015). Therefore, important factors affecting the extraction yield may be the type, amount, and form of phytochemical substances that could dissolve in the extraction solvents.
3.2 Determination of the minimum inhibitory concentration and minimum bactericidal concentration
Antibacterial activity against the five pathogenic bacteria was determined. The results are shown in Table 1. BSE showed highly efficient inhibitory effects on Gram-positive and Gram-negative bacteria, with a MIC range of 0.49–3.91 mg/mL. It had a low MIC value toward V. parahaemolyticus TISTR1596 (0.49 mg/mL), S. aureus TISTR2329, B. cereus TISTR747 (0.98 mg/mL), and E. coli TISTR527 (3.91 mg/mL). As a result, the sappan methanolic extract which inhibited both groups of bacteria and showed the highest inhibitory to E. coli MTCC443 and B. subtilis MTCC10619 (MIC = 2 mg/mL) (Bukke et al., 2015). In the same way, the MBC values of BSE were 0.49, 15.62, 31.25, and 62.50 mg/mL against V. parahaemolyticus, S. aureus, B. cereus TISTR747, and E. coli, respectively. Therefore, the BSE had the highest bioactivity against V. parahaemolyticus, which might play a role in the specificity of the main compounds in crude extract to the bacterial cell wall. The main compounds in the BSE were phenolic compounds that could interact with the peptidoglycans of Gram-positive bacteria and the outer membrane of Gram-negative bacteria. There are many modes of action in the BSE such as membrane disruption, enzyme inactivation, and adhesin binding (Tiwari et al., 2011) that could affect the inhibitory and bactericidal activity toward two groups of pathogenic bacteria (Gram-positive and Gram-negative bacteria). Additionally, the stability and toxicity of both crude extracts were then determined, and preliminary evaluated as anti-Vibrio substance in seawater for its application in shrimp farming.
Microorganisms
B. sappan extract
MIC (mg/mL)
MBC (mg/mL)
S. aureus TISTR2329
0.98
15.62
B. cereus TISTR747
0.98
31.25
E. coli TISTR527
3.91
62.50
P. aeruginosa TISTR357
> 62.50
> 62.50
V. parahaemolyticus TISTR1596
0.49
0.49
3.3 Total phenolic content and antioxidant property
The TPC of the BSE was measured using the Folin–Ciocalteu assay. BSE had a high TPC (31.46 mg GAE/g). However, the aqueous extract of B. sappan in Thailand had been reported a TPC of 147.02 ± 0.63 mg GAE/g (Taokaenchan et al., 2017). The amount of TPC in plants depends on the source and type of plant as well as the type of extraction solvent. The antioxidant activity in the BSE was measured using the DPPH assay. The percentage of inhibition of BSE at different concentrations is shown in Table 2. The BSE exhibited the highest scavenging activity on the DPPH radical at 83.05 % for 3.91 mg/mL and had an IC50 value of 0.288 mg/mL. The antioxidant effects accorded to the amount of the TPC resulted from antioxidants containing an aromatic ring and a hydroxyl group as free radical terminators (Abdel-Hameed, 2009), which can bind with any free radical. The BSE had effective DPPH radical scavenging activity and exhibited remarkable antioxidant activity at a low concentration of 0.98 mg/mL (81.67 %). Furthermore, Butkrup et al. (Butkhup and Samappito, 2011) found that the scavenging effect of BSE on the DPPH radical was 82.93 % at 2.00 mg/mL of crude extract.
Crude extract
Solvent
Concentration (mg/mL)
DPPH radical scavenging activity (%)
B. sappan
95 % ethanol
0.03
10.81 ± 0.66
0.06
14.69 ± 2.04
0.12
21.79 ± 3.22
0.24
36.39 ± 1.65
0.49
66.90 ± 3.51
0.98
81.67 ± 0.16
1.96
83.01 ± 1.10
3.91
83.05 ± 0.45
7.81
82.00 ± 0.60
15.62
81.96 ± 1.63
31.25
80.28 + 0.57
3.4 Stability studies of the total phenolic content and antibacterial substances under various conditions
The phytochemical substances in BSE were determined at different temperatures (room temperature, pasteurization, and autoclave) and with different diluents (distilled water and seawater). The thermal stability of TPC is shown in Fig. 1. The TPC increased to over 100 % under both pasteurization and sterilization conditions (116.27 and 121.01 %), which indicates that it is resistant to heat degradation because of temperature kinetics that increased the TPC (Molaveisi et al., 2019). Generally, the temperature affected the amount of phenolic content, broke the phenolic structure, and caused instability. However, some phenolic compounds were heat tolerant such as quercetin in the Leguminosae family, which showed an increasing phenolic compound content at 120 °C (Sharma et al., 2015). Furthermore, Ghafoor et al. (Ghafoor et al., 2019) found that the difference in the TPC may be due to the temperature and plant structure. For the effect of diluent on TPC for 24 h, the remaining TPC in distilled water was over 90 %, while that in seawater was approximately 40 %–50 % at 12–24 h (Fig. 1B). Therefore, BSE had the stability of phytochemical substances in distilled water. The crude extracts were not stable in seawater, which may have been caused by the interaction between the ions in seawater and the hydroxyl group in the phenolic compounds (Atkins, 1922), which could lead to a decrease in the TPC. However, the BSE had low stability in seawater, but it still had high anti-Vibrio activity with antibacterial substances that were similarly active, and it showed the same lowest of MIC and MBC against this strain (Table 3).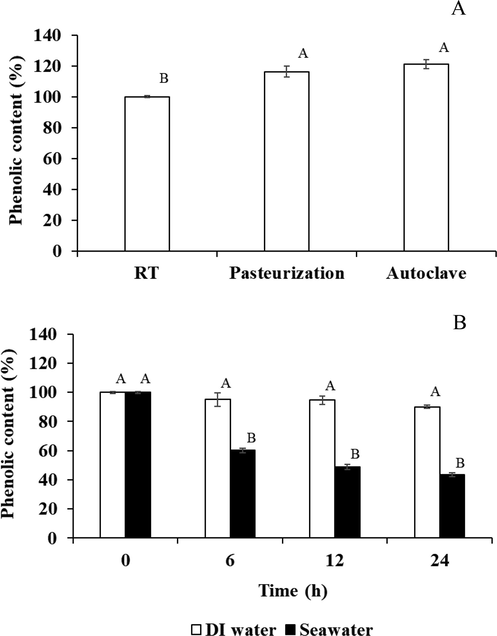
Thermal stability of the TPC in BSE at room temperature, pasteurization (63 °C, 20 min) and autoclave (121 °C, 15 min) conditions (A). Stability of the TPC in BSE dissolved in distilled water and seawater at 0, 6, 12, and 24 h (B).
Condition
Condition
BSE against V. parahaemolyticus
MIC (mg/mL)
MBC (mg/mL)
Room temperature
28 °C
0.49
0.49
Pasteurization
63 °C, 20 min
0.49
0.49
Autoclave
121 °C, 15 min
0.49
0.49
DI water
0
0.49
0.49
6
0.49
0.49
12
0.49
0.49
24
0.49
0.49
Seawater
0
0.49
0.49
6
0.49
0.49
12
0.49
0.49
24
0.49
0.49
3.5 Toxicity test using A. salina model
The BSE was tested using A. salina to evaluate the median lethal concentration (LC50). A. salina larvae mortality in crude extracts after 24 h is shown in Table 4. A. salina mortality ranged from 0.12 to 31.25 mg/mL of crude extract, and the acute toxicity test of crude extracts within 24 h showed a LC50 value > 1,000 µg/mL, which indicated a non-toxic substance (Clarkson et al., 2004). A LC50 value at 7.374 mg/mL of BSE killed up to 50 % of the A. salina population. Fikriah et al. (Fikriah and Lestari, 2011) studied the acute toxicity test using brine shrimp and found that the ethanolic extracts from bark and stem of Sopang were toxic, with a LC50 value below 1,000 µg/mL at 204.644 and 239.883 µg/mL. The main active component affecting A. salina larvae mortality was a flavonoid. The flavonoid mode of action was binding of the OH-group to integral cell membrane proteins, which interferes with active transport of Na+ and K+ ions. The active substance–integral protein complex disrupted Na+ ion transport into the cell, and the resulting high accumulation caused the cell membrane to rupture (Nurhayati et al., 2006). However, heartwood extract of B. sappan (250–5000 mg/kg) was reported to be safe with no acute or subacute toxicity in rats (Sireeratawong et al., 2010; Nirmal et al., 2015). Remark: Molting +++: High ++: Moderate +: Low -: Normal. Swimming +++: Normal ++: Slow +: Stay −: Non-swimming. Body size ++: Long +: Normal −: Short.
Crude extract
Concentration (mg/mL)
Mortality (%)
Growth of A. salina
Molting
Swimming
Body size
B. sappan
0.12
0.00
−
+++
+
0.24
0.00
−
+++
+
0.49
0.00
+
+++
+
0.98
3.33
++
++
−
1.95
6.67
++
++
−
3.91
33.33
++
+
−
7.81
53.33
+++
+
−
15.62
86.67
+++
+
−
31.25
100.00
+++
−
−
3.6 Chemical profiling of BSE
The total ion chromatograms (TIC) of BSE in the negative mode from LC-Q-TOF-MS/MS are shown in Fig. 2. Eighty-four retention times of peak were detected, and 45 proposed compounds were identified from fragmentation patterns in tandem MS compared with libraries and fragmentation profile. From TIC, 10 major peaks were demonstrated at Rt 2.275–14.534 min (Table 5). The highest area sum (24.47 %) at Rt 7.994 min was identified as 4,7-Dihydroxy-2H-1-benzopyran-2-one or 4,7 dihydroxycoumarin that belonged to the class of 7-hydroxycoumarins. It has not been reported in B.sappan, and several studies have reported its derivatives to antiproliferative activity against four different cancer cell lines (Govindaiah et al., 2019). One member, umbelliferone (UMB), showed B. cereus inhibition and antioxidant activity with non-toxic in acute or chronic oral gavage administration to laboratory animals (Cruz et al., 2020). Next, the peak of 7.772 min (9.45 % area sum) was assigned as Methyl 7-desoxypurpurogallin-7-carboxylate trimethyl ether that was cyclic ketone phenolic compound and methylation on purpurogallin carboxylic acid exhibiting many bioactivities (Jhoo, 2008). Brazilein was then found at Rt 10.410 min (8.38 % area sum) that was reported anti-inflammatory, anticancer, antibacterial and antioxidant properties (Liang et al., 2013; Krihariyani et al., 2020). Biochanin A, an O-methylated isoflavone in the class of 7-hydroxyisoflavone, was detected at Rt 13.174 (8.41 % area sum). It had been reported the high inhibitory against pathogenic bacteria as Xanthomonas axonopodis pv. glycines that DNA synthesis and flagella formation were altered as well as the composition of the bacterial membrane (Kai-Xuan et al., 2020). It had also been indicated their anti-inflammatory, anticancer, neuroprotective, antioxidant, anti-microbial, and hepatoprotective properties. Next, (2S,2″S,3S,3″R,4S)-3,4′,5,7-Tetrahydroxyflavan (2->7,4->8)-3,4′,5,7-tetra hydroxyflavan or geranin A (4.13 %) was an A-type proanthocyanin that was found anti-protozoa and antioxidant activities (Calzada et al., 2001; Maldonado et al., 2005). Finally, heliannone C (3.83 %) was identified with 285.07 m/z that had few reports of this bioactive flavonoid. As result and their activities, there is no report available on the synergistic activity of each substance, but the crude extract normally contained various active substances that was the combination form leading to be a possible synergism of biological activities.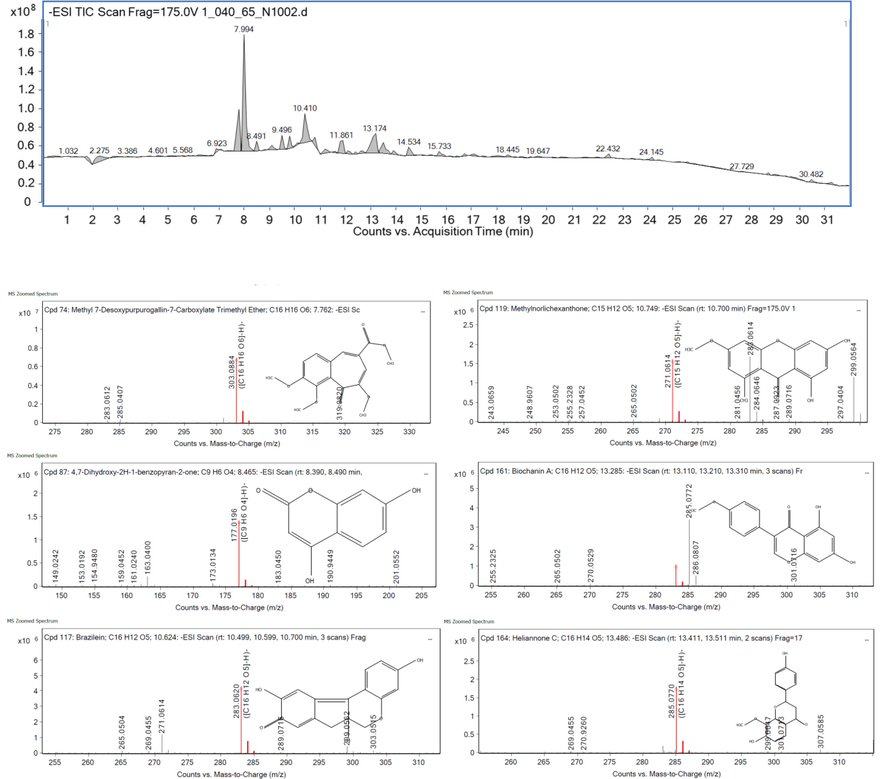
LC-MS/MS chromatograms of ethanolic extract of B. sappan (BSE) in negative mode and MS zoomed spectrums of six major components.
No.
Rt (Min)
Proposed compound
Formula
m/z
Mass
Score (DB)
Diff
(DB, ppm)Area sum %
1
2.275
Sucrose
C12H22O11
341.1089
342.1161
99.83
0.27
3.84
2
7.772
Methyl 7-Desoxypurpurogallin-7-Carboxylate Trimethyl Ether
C16H16O6
303.0884
304.0955
96.44
−2.78
9.45
3
7.994
4,7-Dihydroxy-2H-1-benzopyran-2-one
C9H6 O4
177.0196
178.0268
99.39
−1.23
24.47
4
9.496
Demethylsulochrin
C16H14O7
317.0669
318.0743
96.27
−1.21
2.61
5
9.801
Machaerol C
C18H20O7
347.1138
348.1210
99.70
−0.40
2.27
6
10.41
Brazilein
C16H12O5
283.0620
284.0692
96.01
−2.43
8.38
7
11.861
(2S,2″S,3S,3″R,4S)-3,4′,5,7-Tetrahydroxyflavan (2->7,4->8)-3,4′,5,7-tetrahydroxyflavan
C30H24O10
603.1509
544.1369
99.71
0.03
4.13
8
13.174
Biochanin A
C16H12O5
283.0615
284.0687
84.62
−0.86
8.41
9
13.504
Heliannone C
C16H14O5
285.0770
286.0843
99.37
−0.54
3.83
10
14.534
Duartin (-)
C18H20O6
331.1191
332.1263
99.28
−0.89
2.32
3.7 Determination of anti-Vibrio activity in seawater
The anti-Vibrio activity was tested in seawater at 1MIC–4MIC of crude extract for 0–12 h. The colony surviving in TCBS agar was measured by calculating the colony forming units (CFU/mL), and survival trends are shown in Fig. 3.
Morphology of brine shrimp (A. salina) at various BSE concentrations after 24 h.
The control condition was tested in DMSO, and the bacterial number was 105 to 106 CFU/mL at a dilution of 10-6 to 10-1, which showed a slight increase from 0 h to 12 h. The bactericidal activity of BSE increased in a concentration-dependent manner. The 1MIC, 2MIC, and 3MIC values showed a gradual decreasing trend until there was less than 50 % of bacteria at 1 h. All BSE concentrations showed a high efficiency for killing V. parahaemolyticus within 6 h. The 3MIC and 4MIC values killed V. parahaemolyticus within the shortest time (2 h) followed by 2MIC (3 h) and 1MIC (6 h). The 4MIC value had the highest effective inhibition and killed V. parahaemolyticus within 1 h. The anti-Vibrio activity might be affected by the various types of phenolic compounds found in B. sappan heartwood including brazilin. Brazilin was a major active compound and had a variety of actions (Nirmal et al., 2015) including interfering with Na+ ions, enzyme inactivation, interfering with energy metabolism, and membrane disruption (Fikriah and Lestari, 2011; Tiwari et al., 2011). Cell membrane rupture may have caused cell death. The BSE showed a strong inhibitory effect and can be used in shrimp farming or other aquatic fields where V. parahaemolyticus is problematic (see Fig. 4).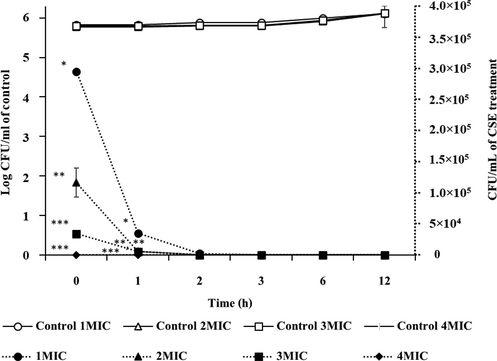
Time–kill curve for V. parahaemolyticus in B. sappan extract at 1, 2, 3, and 4MIC for 12 h.
4 Conclusion
The ethanolic heartwood extract of B. sappan yielded 9.56 % (w/w), and it showed effective antibacterial activity against four pathogenic bacteria including B. cereus, E. coli, S. aureus and V. parahaemolyticus. It showed the lowest MIC and MBC value for V. parahaemolyticus (0.49 mg/mL) and had scavenging activity on the DPPH radical at an IC50 of 0.288 mg/mL. TPC remained high under pasteurization and sterilization conditions (116 %–121 %), and it was more stable in DI water (>90 %) than seawater (approximately 44 %) for 24 h without reduction of antibacterial activity. The BSE contained major components including 4,7-Dihydroxy-2H-1-benzopyran-2-one, Methyl-7-desoxypurpurogallin-7-carboxylate trimethylether, Brazilein and biochanin A with no acute toxicity to A. salina. This crude extract at > 2MIC showed a strong anti-Vibrio effect in seawater that had a time–kill of less than 2 h.
Ethics approval
The experimental procedure of lethality test by A. salina model was approved by Walailak university institutional animal care and use committee (WU-IACUC) (Approval number: U1-08835-2563).
Acknowledgements
The authors express their gratitude to the Plant Genetic Conservation Project Under the Royal Initiation of Her Royal Highness Princess Maha Chakri Sirindhorn (RSPG), Development and Promotion of Science and Technology Talents Project (DPST) and chemistry of bioactive compound laboratory, Faculty of Agriculture, Okayama University for support this research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6:42.
- [CrossRef] [Google Scholar]
- The hydrogen ion concentration of sea water in its biological relations. J. Mar. Biol. Assoc. U. K.. 1922;12:717-771.
- [CrossRef] [Google Scholar]
- Antibacterial properties of polyphenols: characterization and QSAR (Quantitative Structure-Activity Relationship) models. Front. Microbiol.. 2019;10:829.
- [CrossRef] [Google Scholar]
- Comparative study of in vitro antibacterial activity of leaves, bark, heart wood and seed extracts of Caesalpinia sappan L. Asian Pac. J. Trop. Dis.. 2015;5:903-907.
- [CrossRef] [Google Scholar]
- In vitro free radical scavenging and antimicrobial activity of some selected Thai medicinal plants. Res. J. Med. Plants. 2011;5:254-265.
- [CrossRef] [Google Scholar]
- Geranins C and D, additional new antiprotozoal A-type proanthocyanidins from Geranium niveum. Planta Med.. 2001;67:677-680.
- [CrossRef] [Google Scholar]
- In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol.. 2004;92:177-191.
- [CrossRef] [Google Scholar]
- Umbelliferone (7-hydroxycoumarin): A non-toxic antidiarrheal and antiulcerogenic coumarin. Biomed. Pharmacother.. 2020;129:110432
- [CrossRef] [Google Scholar]
- Antioxidant activity, total phenolics and flavonoid contents of some edible green seaweeds from Northern Coasts of the Persian Gulf, Iran. J. Pharm. Res. IJPR. 2014;13:163-170.
- [Google Scholar]
- Acute toxicity test of bark and stem ethanol extract of Sopang (Caesalpinia sappan Linn) by brine shrimp lethality test. Folia Medica Indones.. 2011;47:58.
- [Google Scholar]
- The effect of heating temperature on total phenolic content, antioxidant activity, and phenolic compounds of plum and mahaleb fruits. Int. J. Food Eng.. 2019;15
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of novel 4,7-dihydroxycoumarin derivatives as anticancer agents. Bioorg. Med. Chem. Lett.. 2019;29:1819-1824.
- [CrossRef] [Google Scholar]
- Antibacterial activity, stability, and hemolytic activity of heartwood extract from Caesalpinia sappan for application on nonwoven fabric. Electron. J. Biotechnol.. 2022;55:9-17.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effects of purpurogallin carboxylic acid, an oxidation product of gallic acid in fermented tea. Korean J. Food Sci. Technol. 2008
- [Google Scholar]
- Antibacterial mechanism of Biochanin A and its efficacy for the control of Xanthomonas axonopodis pv. glycines in soybean. Pest Manag. Sci.. 2020;77
- [CrossRef] [Google Scholar]
- Antibiotic resistance in bacteria - an emerging public health problem. Malawi Med. J. J. Med. Assoc. Malawi. 2003;15:63-67.
- [CrossRef] [Google Scholar]
- In silico study on antibacterial activity and brazilein ADME of sappan wood (Caesalpinia sappan L.) against Escherichia coli (strain K12) Syst. Rev. Pharm.. 2020;11:290-296.
- [Google Scholar]
- Brazilein from Caesalpinia sappan L. antioxidant inhibits adipocyte differentiation and induces apoptosis through caspase-3 activity and anthelmintic activities against Hymenolepis nana and Anisakis simplex. Evid. Based Complement. Alternat. Med.. 2013;2013:e864892.
- [CrossRef] [Google Scholar]
- Antioxidant activity of A-type proanthocyanidins from Geranium niveum (Geraniaceae) J. Agric. Food Chem.. 2005;53:1996-2001.
- [CrossRef] [Google Scholar]
- Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med.. 1982;45:31-34.
- [CrossRef] [Google Scholar]
- Kinetics of temperature effect on antioxidant activity, phenolic compounds and color of Iranian jujube honey. Heliyon. 2019;5:e01129.
- [Google Scholar]
- Screening antibacterial effects of Vietnamese plant extracts against pathogens caused acute hepatopancreatic necrosis disease in shrimps. Asian J. Pharm Clin. Res.. 2018;77
- [CrossRef] [Google Scholar]
- Application of plant extracts to improve the shelf-life, nutritional and health-related properties of ready-to-eat meat products. Meat Sci.. 2018;145:245-255.
- [CrossRef] [Google Scholar]
- Brazilin from Caesalpinia sappan heartwood and its pharmacological activities: A review. Asian Pac. J. Trop. Med.. 2015;8:421-430.
- [CrossRef] [Google Scholar]
- Uji Toksisitas Ekstrak Eucheuma Alvarezii terhadap Artemia salina sebagai Studi Pendahuluan Potensi Antikanker. Akta Kim.. 2006;2:41-46.
- [Google Scholar]
- Antibacterial and cytotoxic properties of some plant crude extracts used in Northeastern folk medicine. Rev. Bras. Farmacogn.. 2009;19:376-381.
- [CrossRef] [Google Scholar]
- Brazilwood reds: the (Photo) chemistry of brazilin and brazilein. J. Phys. Chem. A. 2013;117:10650-10660.
- [CrossRef] [Google Scholar]
- Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J. Food Drug Anal.. 2015;23:243-252.
- [CrossRef] [Google Scholar]
- Toxicity evaluation of sappan wood extract in rats. J. Med. Assoc. Thail. Chotmaihet Thangphaet. 2010;93(Suppl 7):S50-S57.
- [Google Scholar]
- Hepatoprotective properties of Caesalpinia sappan Linn. heartwood on carbon tetrachloride induced toxicity. Indian J. Exp. Biol.. 2010;48:905-910.
- [Google Scholar]
- Taokaenchan, N., Areesrisom, P., Tangtragoon, T., Kongbuntad, W., Tarachai, Y., Kawaree, R., 2017. Effect of dying temperature to antioxidant property and nutritivevalue of Caesalpinia sappan L. tea. Presented at the 8th Academically Conference of Plant Genetic Conservation Project, Saraburi: Thailand, pp. 165–170.
- Anti-inflammatory constituents of sappan lignum. Biol. Pharm. Bull.. 2009;32:941-944.
- [Google Scholar]
- Microplate quantification of total phenolic content from plant extracts obtained by conventional and ultrasound methods. Phytochem. Anal.. 2014;25:439-444.
- [CrossRef] [Google Scholar]
- Yang, H.O., Kim, M.Y., Kwon, Y.-G., Choi, Y.H., 2007. Use of the extract of Caesalpinia sappan l. and compounds therefrom. WO2007066928A1.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102594.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







