Translate this page into:
Antibacterial activity of the Saudi Red Sea sponges against Gram-positive pathogens
⁎Corresponding author at: Department of Biology, Faculty of Science, Taibah University, Medina, Saudi Arabia. rafifi2001@hotmail.com (Rafat Afifi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Marine sponges are on focus for their ability to produce compounds with antimicrobial activity. In this study, extracts from nine Red Sea sponges obtained from Saudi coasts (Farasan Islands, Al Lith, Al-Qunfudhah and Yanbu) were screened against clinical and marine bacterial isolates. Results showed that the extracts of Callyspongia crassa and Aplysina fulva were potent against the Gram-positive Bacillus subtilis pathogens (inhibition zone: 9–15 and 16–25 respectively; LC50 = 18.2 and 21.7 µg/mL respectively). Extracts of Callyspongia crassa and Aplysina fulva showed also activity against Staphylococcus aureus (inhibition zone: 5–8 and 16–25 respectively; LC50 = 215.2 and 302.6 µg/mL respectively). However, extracts of Callyspongia siphonella, Phyllospongia lamellose, Iotrocbota purpurea and Clathria spp. showed no activity against all tested strains including the Gram-negative E. coli. With respect to marine bacterial strains, extracts of Piona vastifica and Callyspongia crassa showed high activity (LC50 = 19–22 μg/mL) whereas the rest showed low activity (LC50 = 270–350 µg/mL).
These findings provide evidence that marine sponges from the Red Sea have the potential to be good candidates in the search for new effective drugs against Gram-positive pathogenic bacteria.
Keywords
The Red Sea sponges
Antibacterial
Human pathogenic bacteria
Marine bacteria
1 Introduction
Oceans cover more than 70% of the surface of the earth, and is still to a great extent unexplored. The development of new technologies that facilitate sub-sea sampling and harvesting has widened the accessibility to areas below sea level (Synnes, 2007). This resulted in the isolation of bioactive compounds from marine organisms against viral, bacterial, parasitic and fungal diseases (Abdelmohsen et al., 2017; Andersen, 2017; Anjum et al., 2016). Marine natural products are exceptional in their structural/chemical features and consequently in their pharmacological properties than the terrestrial natural products (Ibrahim and Mohamed, 2016; Kiuru et al., 2014).
Marine sponges, belong to the phylum Porifera (Andersen, 2017; Anjum et al., 2016), are gaining more attention by researchers and industrial sectors from all over the world due to their ability to produce a variety of bioactive secondary metabolites that have many applications including drug discovery (Garcia-Vilas et al., 2015; Kobayashi, 2016; Mioso et al., 2017). About 5000 compounds to date were isolated from sponges worldwide, accounting for about 30% of all compounds obtained from the marine environment so far. Approximately, more than two hundred newly discovered bioactive products derived from sponges are reported yearly since the last decade (Hu et al., 2011). It is hypothesized that sponges have used different metabolic pathways to produce such diverse and unique bioactive compounds to support the sponges’ survival in the sea. This includes defense against microbial infection and as agents used in the competition for space in the crowded marine reef environment (Mehbub et al., 2014; Roue et al., 2012). Several medications derived from sponges are available in the market and others are in clinical trials including Eribulin Mesylate, Cytarabine, and Vidarabine (Martins et al., 2014).
The exceptional geographical location of the Red sea in the desert coupled with its unique physical (such as depth and relatively high surface temperature) and chemical (high salinity) characteristics offer great biological diversity and endemism not presented elsewhere (Behzad et al., 2016). These characteristics of the Red Sea provide the opportunity for bioprospecting of natural product compounds with medicinal and nutritional values. For example, a previous study that was conducted on extracts obtained from five sponge species has shown promising antimicrobial activity against a spectrum of pathogenic bacterial and fungal strains (Perveen et al., 2002). Metabolites: sipholenol A and 2,10-dibromo-3-chloro-7-chamigrene that was isolated from Red Sea Siphonochalina siphonella extracts showed potent antifouling effects (Al-Lihaibi et al., 2015). Moreover, chemical compounds isolated from Petrosia sp.; Hyrtios erectus, Siphonochalina sp. and Haliclona sp. were found to be cytotoxic against several cancer cell lines including HepG2, MCF-7 WI-38, and Vero (Abdel-Lateff et al., 2016, 2014; Alarif et al., 2013, 2016). In this study, extracts from nine Red Sea sponges obtained from Saudi coasts (Farasan Islands, Al Lith, Al-Qunfudhah and Yanbu) were screened against clinical and marine bacterial strains.
2 Material and methods
2.1 Study area
The Red Sea Saudi coast is about 1700 km extended from Gulf of Aqaba to Yemen. In this study, sponges were collected from 4 regions along the Saudi Red Sea coast. These regions are Yanbu, Al Lith, Al Qunfudhah, and Farasan Islands (Fig. 1). Samples were collected from both inshore and offshore sites.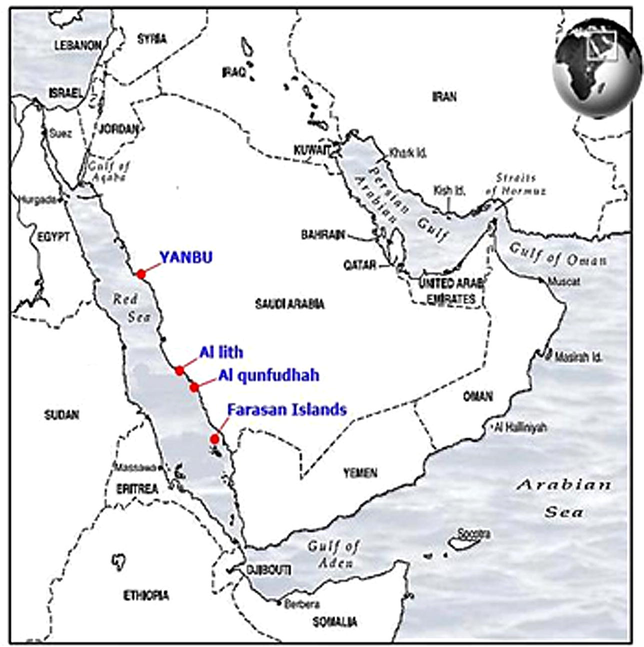
Sampling locations. The map shows the collection sites for sponges species collected from the Saudi Red Sea coasts.
2.2 Collection of sponges
Approvals to collect the samples were obtained from local Saudi authorities. Samples were collected from sites that offered a combination of high biodiversity and density to avoid any adverse effects to the sampled sites during the period from November 2015 to May 2016. Samples were collected from the indicated coasts at depths 1–20 m using skin and Scuba diving techniques. Sites were carefully photographed for documentation so that the specimens could be collected from the site in the future. The following species were collected from each region: Iotrocbota purpurea, Xestospogia spp., Clathria spp., Phyllospongia lamellose, Stylissa carteri, Piona vastifica, Aplysina fulva, Callyspongia siphonella and Callyspongia crassa. Samples of the same species obtained from the different coasts were processed separately. The collected samples were washed with freshwater and then frozen immediately and maintained at −20 °C before starting the extraction. Identification of sponges' species was carried out based on skeletal slides and dissociated spicule mounts. Identification of sponges species were kindly confirmed by Dr. Tarek A. Temraz, Marine Science Department, Suez Canal University, Ismailia, Egypt. Specimens of the collected species were deposited in the Porifera collections of the Zoological Museum, Biology Department, Taibah University.
2.3 Extraction
The frozen samples were left to defrost for one hour at room temperature. Defrosted samples (20 g) were then dissected using scissors into small pieces and overnight extracted several times with sufficient amount (600 mL) of Methylene chloride/Methanol (1:1) as previously described (Ines et al., 2007). The extract was then filtered through Whatman No. 1 filter paper (Rotavapor R-200, BUCHI) at 40 °C and the residue was transferred into small vials and labeled. Extracts (yield between 0.4 and 1.0 g) were stored at −80 °C until used.
2.4 Organisms and culture conditions
All media and solutions used for culture were sterilized by autoclaving (121 °C, 15 min). Pathogenic isolates of 2 Gram-positive standard strains (Staphylococcus aureus [ATCC: 29213] and Bacillus subtilis [ATCC: 9372]) and one Gram-negative (E. coli [ATCC 25922]) were used in the study. Pathogens were stored on nutrient agar plates (OXOID, CM3) and were propagated in nutrient broth (OXOID, CM1) medium at 37 °C.
Marine bacterial strains were isolated from the water surrounding the sponges. Following the technique of Biswa and Doble (2013). In brief, standard serial dilution and plating techniques were performed on marine agar (18 g Difco marine broth, 9 g NaCl and 18 g Difco agar, per 1 L of deionized H2O), and kept at 24–25 °C. The isolated bacteria were categorized into Gram-positive or negative bacteria using Gram stain technique. Antibacterial activities were evaluated by the disk diffusion and the microbroth dilution methods as recommended by the Clinical and Laboratory Standards Institute (CLSI).
2.5 Well diffusion method
The well diffusion method was conducted according to Magaldi et al. (2004). Bacterial broth culture of each bacterial strain was adjusted to 0.5 McFarland standards. One mL of the bacterial suspension was then added to melted agar (50–55 °C). The inoculated agar was then plated (9 cm diameter) and left until the medium had solidified. After that, wells (4 mm diameter) were cut out of the agar, and 20 µL of the sponge extracts dissolved in Dimethyl Sulphoxide (DMSO) were placed into each well. The vehicle (20 µL) alone without extracts was placed into the control wells. Assay plates were kept at 37 °C for overnight. The endpoint of the assay was detected by visualizing the clear zone of inhibition of growth around the wells. Each experiment was repeated twice.
2.6 Microtiter diffusion method
The microdilution assay test was performed in 96-well microtiter plates as previously described (Ghuman et al., 2016). In brief, activity was determined by comparison of growth in the presence of extract to cells in media only as a control. Extracts were diluted in DMSO to produce a gradient of final concentrations that ranged from 500 µg/mL to 1 µg/mL. Bacteria growth was assayed after 24 h by measuring the optical density at 630 nm using a microtiter plate reader (MRX, DYNEX Technologies, Inc, Chantilly, VA, USA). Extracts were regarded as inactive when the optical density was more than 90% of the control assayed after 24 h. Absorbance values were converted into growth percentage and the medium lethal concentration (LC50) was obtained graphically from the dose response curves. The experiment was repeated 4 times and the mean value of LC50 was reported.
3 Results
3.1 Antibacterial activity of sponges extract against human pathogenic bacteria
Table 1 summarizes the results of antibacterial activities of the 9 extracts of sponges (at concentrations of 250–500 µg/mL) against Bacillus subtilis, Staphylococcus aureus and E. coli bacterial strains tested using the agar well diffusion method. − no activity; + weak activity (1–4 mm); ++ moderate activity (5–8 mm); +++ good activity (9–15 mm); ++++ highly active (16–25 mm).
Sponge
Bacillus subtilis (inhibition zone mm)
Staphylococcus aureus (inhibition zone mm)
E. coli (inhibition zone mm)
500 µg/mL
250 µg/mL
500 µg/mL
250 µg/mL
500 µg/mL
250 µg/mL
Iotrocbota purpurea
−
−
−
−
−
−
Xestospogia spp.
++
++
−
−
−
−
Clathria spp.
−
−
−
−
−
−
Phyllospongia lamellosa
+++
++
−
−
−
−
Stylissa carteri
−
−
−
−
−
−
Piona vastifica
+++
−
+++
−
−
−
Aplysina fulva
+++
+++
++
++
−
−
Callyspongia siphonella
++
++
−
−
−
−
Callyspongia crassa
++++
+++
++++
++++
−
−
Strong activity against Bacillus subtilis was detected by Callyspongia crassa (inhibition zone: 15–20 mm) whereas moderate-good activity was detected for Xestospogia spp., Phyllospongia lamellose, Piona vastifica, Aplysina fulva, Callyspongia siphonella with inhibition zones ranged between 8 and 11 mm at 500 µg/mL concentration. With respect to Staphylococcus aureus pathogen, strong inhibition was also detected for Callyspongia crassa (19–24 mm) whereas moderate-good activity was detected for Piona vastifica and Aplysina fulva with inhibition zones ranged from 8 to 9 mm at 500 µg/mL concentration. All tested extracts of marine sponges showed no activity against E. coli (Table 1).
3.2 Antibacterial activity testing against marine bacteria
The extracts of sponges were assayed for their activity against three Marine bacterial isolates (Bacillus spp. and Staphylococcus spp., Gram-positive, Table 2) at a concentration of 500 µg/mL.
Sponge species
Inhibition zone (mm diameter)
Isolate 1*
Isolate 2#
Isolate 3$
Iotrocbota purpurea
10
9
−ve
Xestospogia spp.
9
8
7
Clathria spp.
9
8
7
Phyllospongia lamellosa
6
6
−ve
Stylissa carteri
8
8
7
Piona vastifica
9
6
8
Aplysina fulva
9
8
10
Callyspongia siphonella
11
8
9
Callyspongia crassa
11
11
12
The results showed that the extract from all examined sponge species showed moderate to good activity against tested marine bacterial species (isolated from the environment surrounding the sponges). The highest activity against marine bacteria was detected for Callyspongia crassa whereas the lowest activity was detected for Phyllospongia lamellosa (inhibition zones 11–12 mm and 6 mm respectively).
3.3 Half Maximal Inhibitory concentration (LC50)
In order to determine the Half Maximal Inhibitory Concentration (IC50) of sponge extracts, the microdilution assay was used and the extracts were tested over a range of concentrations (5–500 µg/mL; Table 3). Four replicates for each concentration were used. According to LC50, extracts of Callyspongia crassa, Piona vastifica, Aplysina fulva and Callyspongia siphonella were the most active against Bacillus subtilis with LC50 of 18.2, 20.3, 21.7 and 22.5 µg/mL respectively. The extract of Xestospogia spp. showed the least activity against Bacillus subtilis (LC50 = 224.6 µg/mL). Extracts of Callyspongia siphonella had a considerable effect in the inhibition of Staphylococcus aureus (184.4 µg/mL).
Sponge
Bacillus subtilis
LC50 (µg/mL)
Staphylococcus aureus
LC50 (µg/mL)
Xestospogia spp.
224.6 ± 32.0
249.5 ± 33.5
Phyllospongia lamellosa
115.4 ± 26.2
214.7 ± 26.1
Piona vastifica
20.3 ± 2.25
302.6 ± 39.4
Aplysina fulva
21.7 ± 2.61
215.2 ± 43.1
Callyspongia siphonella
22.5 ± 5.31
184.4 ± 17.03
Callyspongia crassa
18.2 ± 3.56
326.2 ± 32.9
With respect to marine bacterial strains and LC50, extracts Piona vastifica and Callyspongia crassa were the most active with LC50 ranged between 19 and 22 µg/mL (Table 4). Extracts obtained from other sponge extracts were less active (LC50 = 103–350 µg/mL; Table 4).
Sponge
Isolate 1*
LC50 (µg/mL)Isolate 2#
LC50 (µg/mL)Isolate 3$
LC50 (µg/mL)
Xestospogia spp.
183.2 ± 30.9
201.5 ± 25.3
245.4 ± 28.8
Phyllospongia lamellosa
103.5 ± 7.21
155.1 ± 21.8
270.4 ± 37.6
Piona vastifica
22.6 ± 5.46
22.8 ± 2.58
19.4 ± 2.53
Aplysina fulva
109.5 ± 19.6
186.5 ± 24.6
238.2 ± 20.1
Callyspongia siphonella
155.1 ± 36.3
120.3 ± 29.3
349.6 ± 33.7
Callyspongia crassa
22.3 ± 2.29
18.6 ± 2.06
22.2 ± 4.96
4 Discussion
The aim of the current study was to screen extracts derived from nine Red Sea Saudi sponges for their activity against selected bacterial pathogens. Extracts derived from Callyspongia crassa and Aplysina fulva were found potent against tested Gram-positive human pathogens. These data are of importance, especially with the increasing threat of bacterial antibiotic resistant in both hospital settings and the food industry (Antelmann et al., 2008; Chang et al., 2015).
The antimicrobial metabolic defenses in sponges are well established (Gochfeld et al., 2012; Rohde et al., 2015). In the current study, the antibacterial activity against the Gram-positive bacteria was detected in the majority (about 60%) of the sponges with ranges from moderate to potent activities. This result is similar to most studies that examined the antimicrobial activity of the Red Sea sponges (Perveen et al., 2002) and sponges obtained from other locations (Beesoo et al., 2017; Gopi et al., 2012; Ines et al., 2007). In addition, Gram-positive pathogens were more sensitive than Gram-negative ones as no activity was detected in all examined sponge extracts against the E. coli bacteria. This is in concordance with several previous investigations that showed susceptibility of Gram-positive pathogens to sponge bioactive compounds whereas Gram-negative ones were resistant (Gupta et al., 2012; Mora et al., 2008; Nazemi et al., 2014). This could reflect the marine environment that surrounded the sponges and is rich in Gram-positive bacteria.
The results showed that most of the examined sponges showed antimicrobial activity against bacterial isolates from the sponge's natural marine environment. Many bioactive metabolites and compounds isolated from sponges displayed potential bioactivities such as antimicrobial, anti-inflammatory and cytotoxic activities (Agrawal et al., 2016; Andersen, 2017; Malve, 2016). Therefore, the antimicrobial defenses among the Red Sea sponges are common and the chemical defenses of the sponges may play a key role in protecting them against the microbial pathogens. In fact, sponges have been shown to form a wide spectrum of interactions (symbiotic or competitive) with microorganisms such as bacteria, fungus, and others. These interactions are suggested to play an important role in the sponge's survival through the production of bioactive substances including antimicrobial compounds (Laport et al., 2009; Selvin et al., 2010).
Several mechanisms were suggested to account for the diversity of secondary metabolites produced by sponges. One mechanism is related to the fact that sponges are usually associated with microorganisms that account for more than one-third of the sponge volume and contribute to a good extent to its metabolism (Indraningrat et al., 2016; Selvin et al., 2010). Another mechanism is the use of such metabolites as chemical means to protect themselves against predation (Gomes Filho et al., 2014). Another possible ecological role of these antibacterial metabolites is to act as antifouling agents (Muller et al., 2013).
One of the limitations of the current study is that the antibacterial activity of the Red Sea sponges was examined on only three pathogens. Expanding the study to include more pathogens is a matter of future research. In addition, fractionation of the crude extract to identify bioactive compounds in the sponges that showed a potent effect against Staphylococcus aureus and Bacillus subtilis is strongly recommended. Moreover, to better judge the magnitude of the antibacterial, including positive controls is also recommended.
5 Conclusion
Sponges collected from some the Saudi Red Sea coasts (i.e. Yanbu, Al Lith, Al Qunfudhah and Farasan Islands) had the potentiality to inhibit the growth of certain reference and marine bacteria.
Acknowledgments
This work is supported by Dean of Scientific Research, Taibah University – Saudi Arabia. Many thanks for Mr. Derham Sanad and Mr. Motaz Berry for their help in collecting the samples. The authors would like also to thank Dr. Wael Samir for his help in categorization of marine bacteria and Dr. Tarek A. Temraz for Identification of sponge species.
References
- Cytotoxic effects of three new metabolites from Red Sea marine sponge, Petrosia sp. Environ. Toxicol. Pharmacol.. 2014;37:928-935.
- [Google Scholar]
- Antiproliferative effects of triterpenoidal derivatives, obtained from the marine sponge Siphonochalina sp., on human hepatic and colorectal cancer cells. Z. Naturforsch. [C]. 2016;71:29-35.
- [Google Scholar]
- Potential of marine natural products against drug-resistant fungal, viral, and parasitic infections. Lancet Infect. Dis.. 2017;17:e30-e41.
- [Google Scholar]
- The pharmacological potential of non-ribosomal peptides from marine sponge and tunicates. Front. Pharmacol.. 2016;7:333.
- [Google Scholar]
- A new cytotoxic brominated acetylenic hydrocarbon from the marine sponge Haliclona sp. with a selective effect against human breast cancer. Zeitschrift fur Naturforschung. [C]. 2013;68:70-75.
- [Google Scholar]
- Cytotoxic scalarane-type sesterterpenes from the Saudi Red Sea sponge Hyrtios erectus. J. Asian Nat. Prod. Res.. 2016;18:611-617.
- [Google Scholar]
- Proteomic signatures uncover thiol-specific electrophile resistance mechanisms in Bacillus subtilis. Expert Rev. Proteomics. 2008;5:77-90.
- [Google Scholar]
- Antibacterial and antibiotic potentiating activities of tropical marine sponge extracts. Comp. Biochem. Physiol. C: Toxicol. Pharmacol.. 2017;196:81-90.
- [Google Scholar]
- Production of acylated homoserine lactone by gram-positive bacteria isolated from marine water. FEMS Microbiol. Lett.. 2013;343:34-41.
- [Google Scholar]
- Antibiotic resistance in the treatment of Staphylococcus aureus Keratitis: a 20-year review. Cornea. 2015;34:698-703.
- [Google Scholar]
- Aeroplysinin-1, a sponge-derived multi-targeted bioactive marine drug. Mar. Drugs. 2015;14:1.
- [Google Scholar]
- Antimicrobial activity, phenolic content, and cytotoxicity of medicinal plant extracts used for treating dermatological diseases and wound healing in KwaZulu-Natal, South Africa. Front. Pharmacol.. 2016;7:320.
- [Google Scholar]
- Trade-offs in defensive metabolite production but not ecological function in healthy and diseased sponges. J. Chem. Ecol.. 2012;38:451-462.
- [Google Scholar]
- Marine sponge lectins: actual status on properties and biological activities. Molecules. 2014;20:348-357.
- [Google Scholar]
- Antibacterial potential of sponge endosymbiont marine Enterobacter sp at Kavaratti Island, Lakshadweep archipelago. Asian Pac. J. Trop. Med.. 2012;5:142-146.
- [Google Scholar]
- Bicyclic C21 terpenoids from the marine sponge Clathria compressa. J. Nat. Prod.. 2012;75:1223-1227.
- [Google Scholar]
- Statistical research on marine natural products based on data obtained between 1985 and 2008. Mar. Drugs. 2011;9:514-525.
- [Google Scholar]
- Marine pyridoacridine alkaloids: biosynthesis and biological activities. Chem. Biodivers.. 2016;13:37-47.
- [Google Scholar]
- Bioprospecting sponge-associated microbes for antimicrobial compounds. Mar. Drugs. 2016;14
- [Google Scholar]
- Screening of antimicrobial activity of marine sponge extracts collected from Tunisian coast. Proc. West. Pharmacol. Soc.. 2007;50:152-155.
- [Google Scholar]
- Exploring marine resources for bioactive compounds. Planta Med.. 2014;80:1234-1246.
- [Google Scholar]
- Search for new bioactive marine natural products and application to drug development. Chem. Pharm. Bull. (Tokyo). 2016;64:1079-1083.
- [Google Scholar]
- Marine sponges: potential sources of new antimicrobial drugs. Curr. Pharm. Biotechnol.. 2009;10:86-105.
- [Google Scholar]
- Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis.. 2004;8:39-45.
- [Google Scholar]
- Exploring the ocean for new drug developments: marine pharmacology. J. Pharm. Bioallied Sci.. 2016;8:83-91.
- [Google Scholar]
- Marketed marine natural products in the pharmaceutical and cosmeceutical industries: tips for success. Mar. Drugs. 2014;12:35.
- [Google Scholar]
- Marine sponge derived natural products between 2001 and 2010: trends and opportunities for discovery of bioactives. Mar. Drugs. 2014;12:38.
- [Google Scholar]
- Cytotoxic compounds derived from marine sponges. A review (2010-2012) Molecules. 2017;22
- [Google Scholar]
- Evaluation of marine sponge extracts as new sources of antimicrobial substances. Rev. Esp. Quimioter.. 2008;21:174-179.
- [Google Scholar]
- Principles of biofouling protection in marine sponges: a model for the design of novel biomimetic and bio-inspired coatings in the marine environment? Mar. Biotechnol. (NY). 2013;15:375-398.
- [Google Scholar]
- Antifungal and antibacterial activity of Haliclona sp. from the Persian Gulf, Iran. J. Mycol. Med.. 2014;24:220-224.
- [Google Scholar]
- Preliminary investigations of antimicrobial screening of crude extracts of sponges and gorgonians species from Saudi Red Sea Coast. Pak. J. Pharmacol.. 2002;19:7.
- [Google Scholar]
- Prevalence and mechanisms of dynamic chemical defenses in tropical sponges. PLoS ONE. 2015;10:e0132236.
- [Google Scholar]
- Assessing calcareous sponges and their associated bacteria for the discovery of new bioactive natural products. Nat. Prod. Rep.. 2012;29:739-751.
- [Google Scholar]
- Sponge-microbial interactions: ecological implications and bioprospecting avenues. Crit. Rev. Microbiol.. 2010;36:82-90.
- [Google Scholar]
- Bioprospecting of organisms from the deep sea: scientific and environmental aspects. Clean Technol. Environ. Policy. 2007;9:7.
- [Google Scholar]







