Translate this page into:
An overview on therapeutic efficacy and challenges of nanoparticles in blood cancer therapy
⁎Corresponding author. cherryjiaping88@163.com (Jiaping Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cancer is a disease with limited therapeutic options, despite tremendous improvements in medical research and technology. Cancer metastasis and recurrence are major causes of disability and death. Traditional cancer treatments have many limitations, prompting nanotechnology innovation for more precise and less harmful cancer treatment, also known as cancer nanomedicine. In recent years, novel biomolecule-stabilized nanomaterials have emerged as prominent next-generation materials. Nanoparticles, facilitated by advances in nanotechnology, provide possibilities for effective blood cancer therapy. Nanoparticles are amenable to modification. They can be designed to target and control the dose of drug that enter the target region. As a result, nanoparticles may enhance drug effectiveness while reducing negative effects. Several experimental difficulties must be overcome to get nanoparticle treatment to the bedside. Finally it is vital to develop effective formulations that can address the aforementioned concerns while also offering precision targeting of tumour areas without affecting healthy tissue survival. This review primarily focuses on the highlights of current research progress using nanoparticles to deliver various blood cancer therapeutic medicines to illustrate nanoparticles' promise in leukemia, myeloma, and lymphoma therapy. Finally, we explore prospects and possible initiatives for potential therapeutic nanomedicine research.
Keywords
Nanoparticle
Nanomaterial
Cancer
Leukemia
Myeloma
Lymphoma
Therapy
- GQDs
-
Gold quantum dots
- InP
-
Indium phosphate
- CdSe
-
Cadmium selenium
- InAs
-
Indium Arsenate
- CdTe
-
Cadmium tellurium
Abbreviations
1 Introduction
Cancer is foreseen cause of illness and death in every region of the world during the next several decades. Based on the survey, 60,650 new cases of leukemia were reported by the Globocan in 2021, and a total of 23,660 persons died because of the illness in the United States. Leukemia, lymphoma, myeloma, and plasma cell disorders are the most prevailing blood cancers. Leukemia is a group of malignant diseases that originate from hematopoietic stem cells. Different kinds of leukemia exhibit commonly known signs and symptoms, including anemia, leukopenia, exhaustion, weakness, and susceptibility to numerous infections (Rafiq et al., 2018). Multiple myeloma is a malignant blood disease that damages plasma cells that make antibodies. Multiple myeloma is a rare malignancy that affects people aged 65–70. Multiple myeloma occurrences have increased in recent years due to a rise in the number of elderly people.
Cancer is without a doubt one of the most pressing problems for scientists to address. To cure or remove malignancies, traditional therapeutic approaches such as chemotherapy, radiation, surgery, and combinational treatment are generally acknowledged. Even though chemotherapy is still a highly effective cancer treatment, it is still linked with significant and severe side effects. Recurrence is always a possibility, and these malignancies might develop resistance to chemotherapy and radiation therapies (Abu Rakhey et al., 2022). As a result, finding a novel therapeutic alternative for treating tumors and preventing cancer spread is critical. Therefore researcher’s directions were now into rapidly developing field known as Nanotechnology. Nanoparticles (NPs) (1–100 nm) are important in nanoscience and nanotechnology. NPs in particular have unique properties that make it possible to find potential biomarkers with more specificity and sensitivity. Nanoparticles improve the effectiveness of anticancer therapy in living organisms by a large amount compared to traditional chemotherapy. They also change the regularity and distribution of cancer cells, which helps fight drug resistance. Most nanomedicine research is done to treat hard tumours because of something called enhanced permeability and retention (EPR), which makes it easier for nanomedicines to get into tissues. (Mitchell, et al., 2021). Nanomaterials for leukemia and lymphoma, which do not have an EPR effect, are treated differently from those for solid malignancies (Vinhas, 2017). It is well-established that maintaining optimal molecular ratios between the administered medications at the site of action is essential for the effectiveness of cancer treatment. The delayed release of the payload from the NPs carriers ensures that the injected medications are available in the correct proportion. However, it is anticipated that the NPS systems would have unique qualities that will significantly enhance the therapy of blood cancer. NPs, on the other hand, have pioneered non-invasive liquid cancer diagnosis and therapy. In this review, we explore at the most recent advances in nanomedicine in the treatment of blood cancer, from preclinical research to clinical trials. Additionally, this review paper highlights and explores the benefits of nanoplatforms that strongly influences, targeted therapies. The purpose of this review will make researchers assess the effectiveness of employing nanocomposite for blood cancer-related targeted treatment and diagnosis.
2 Methodology
In January 2022, we ran a literature search on the subject with key word blood cancer nanoparticle, nanoparticle therapy, nanocomposite, using the Thomson Reuters, Web of Science research site and Science direct to understand the significance of this research in present era. The results were narrowed for the last 2 decades by specifying a time range ranging from 2000 to 2024. We searched for cancer and molecular processes, cancer and therapy, nanoparticle, nanoparticle therapy, and nanocomposite in research articles.
Papers matching these word constraints were then thoroughly scrutinized and their results were documented. The research findings were limited to 2000–2022 over the last two decades. The findings revealed 125,224 document results records that were represented using the pie chart style, with a result count of 28 documents by subject area. The result (Figs. 1a-1b) demonstrates how diverse and transversal the literature relevant to this sort of study is, as well as how scientific output is increasingly focused on nanotechnology, pharmacology, and chemistry.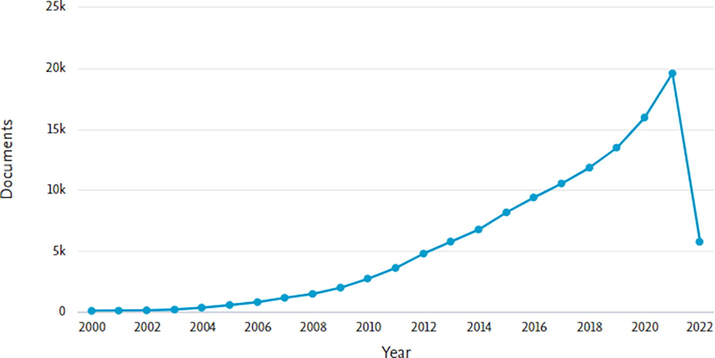
Represents document by year.
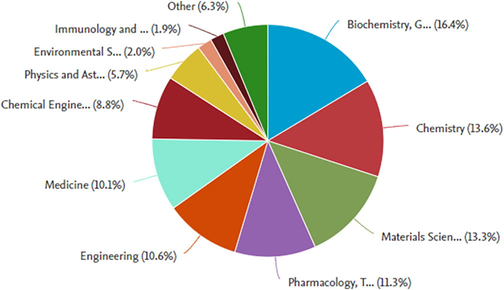
Represents document by subject area.
2.1 Blood cancer an overview
Human blood, which makes up around 8 % of a healthy adult's weight, is vital to the body's operation. Each organ in your body receives oxygen and nutrients from the blood as it moves through the bloodstream. The plasma is a dense solution that contains about 90 % water. Plasma includes vital components in addition to water, salt, and enzymes. Antibodies, coagulation factors, and the proteins albumin and fibrinogen are among them. Serum albumin is the most common plasma protein. It is a tiny molecule whose main purpose is to retain water in circulation via its osmotic impact (Schlegel et al. 2013). White blood cell abnormality and overproduction are the most common causes of blood cancer (Fig. 2). About 10 % of all malignancies diagnosed in the United States each year are blood cancers. Men are more likely than women to get malignancies of the blood (such as leukemia, lymphoma, and myeloma). About a quarter of all malignancies in children are caused by childhood leukemia. As with other types of cancer, treatments for blood malignancies range from no treatment to typical cancer therapies including immunotherapies, chemotherapies, and targeted drugs. Before deciding on therapy, a correct diagnosis is critical since there are over 100 distinct forms of blood malignancies (Thakur et al., 2021).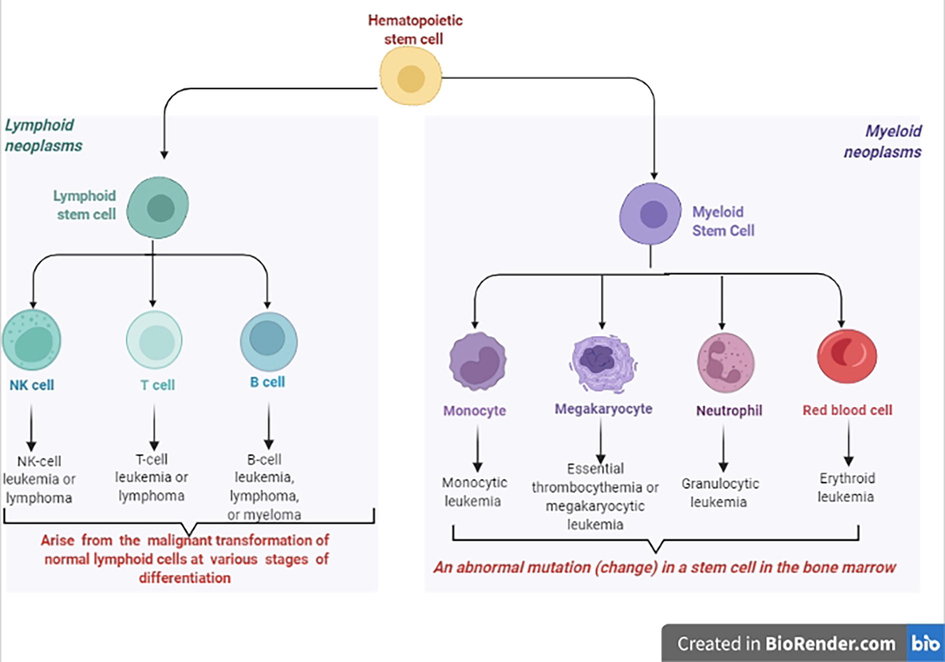
Hematopoietic stem cells in leukemia and lymphoma.
2.2 Types of blood cancer
The majority of blood cancers, also known as hematologic malignancies, initiate in the bone marrow, where blood is formed. Blood cancers develop when aberrant blood cells begin to proliferate uncontrollably, interfering with the function of regular blood cells, which fight infection and make new blood cells (Fig. 3).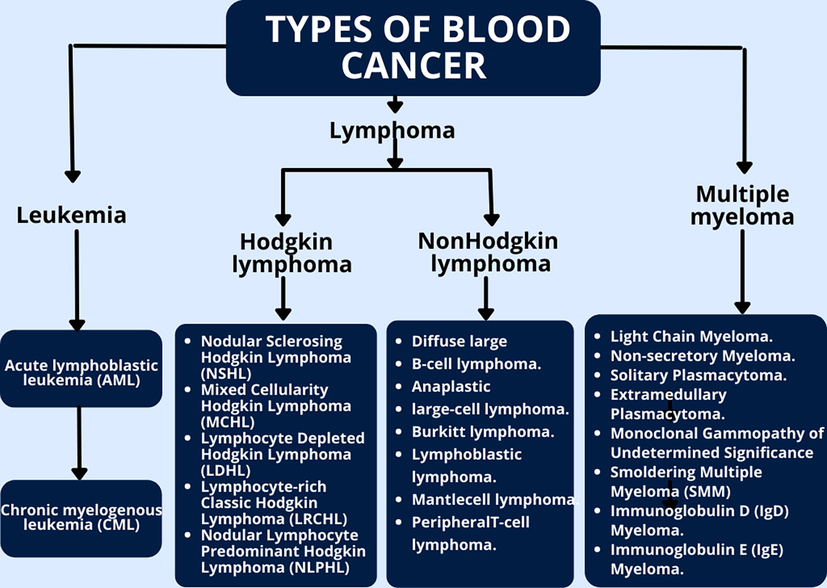
Three main categories of blood cancer.
2.3 Leukemia
Leukemia is a malignancy of the white blood cells or cells that are destined to be white blood cells. Leukemia-affected white blood cells can't fight infections. Lymphocytic leukemia (acute lymphocytic leukemia) and other types of immune cells may be affected by leukemia, which can be acute or chronic in nature (myeloid leukemia). In children under 15, it is the most frequent form of blood cancer. Leukemia is classified into several subtypes. Leukemia is classified primarily into acute and chronic forms based on the rate of development. Leukemia is classified primarily into lymphoid and myeloid leukemia based on the cell types involved (Shen et al., 2020; Zoulikha and He, 2022; Zeng et al., 2021). Leukemia is divided into four types based on clinical symptoms: In addition to Acute Myeloid Leukemia (AML), Acute lymphoblastic leukemia (ALL) and chronic myelogenous leukemia (CML) are the most prevalent leukemia in children, estimating about 80 % of childhood leukemia cases, and CLL accounting for 90 % of all adult cases.
2.4 Lymphoma
As part of the immune system, lymphoma is a malignancy of the lymphatic system, especially the nodes. It is a kind of white blood cell that affects lymphocytes. The most common kind of lymphoma is Hodgkin's lymphoma. Non-Hodgkin lymphoma, on the other hand, refers to all other types of lymphoma. Lymphoma comes in more than 70 varieties. Both slow-growing and aggressive species may be found here. Hodgkin lymphoma is the widespread lymphoma, whereas non-Hodgkin lymphoma is the second most common form. Adults and children are susceptible to both Hodgkin and non-Hodgkin lymphoma. White blood cells are known as lymphocytes, or B and T lymphocytes, account for the majority of lymphomas (T cells). As the disease spreads, the malignant cells might spread to other regions of the body through lymphatic vessels. It is possible for them to create tumors in this way (Vinhas, 2017). The kind of lymphoma and stage determine the therapy and chances of a cure. In terms of Lymphoma, there are two main categories.
-
Non-Hodgkin lymphoma is a cancer of the lymphatic system.
When it comes to lymphoma, the most frequent kind is non-Hodgkins lymphoma (NHL). It is more common among the elderly. For non-Hodgkin lymphoma treatment options include chemotherapy, radiotherapy, immunotherapy, targeted therapy, and stem cell transplantation, among others (Sapkota 2022).
-
Hodgkin's lymphoma
Hodgkin's lymphoma is a cancer of the immune system that affects the lymphatic system. If discovered and treated early, Hodgkin's disease is one of the most treatable kinds of cancer. Hodgkin lymphoma may be treated with chemotherapy, immunotherapy, and stem cell transplantation (Shanbhag, 2018).
2.5 Myeloma
Myeloma is a cancer of the plasma cells, which are lymphocytes that create antibodies to protect against infections. As a result of myeloma's impact on the immune system, the body becomes more prone to infection. Myeloma arises from a mutation in the plasma cell's genome. As the cell's instructions, DNA serves as the blueprint. When the bone marrow produces new plasma cells, the DNA is modified. The aberrant plasma cell reproduces. This is myeloma. Myeloma cells produce an aberrant version of an antibody. Neither myeloma nor lymphoma grows. The aberrant plasma cells in the bone marrow and the para-protein in the body produce most of the issues caused by this condition. Idiopathic myeloma damages active bone marrow. It comprises arms, legs, shoulders, spine, skull, pelvis, and rib cage (Cowan, 2022).
2.6 Blood cancer and nanomedicines: prospects for therapy
Nanoparticles with a diameter of less than 100 nm exhibit characteristics that are not seen in bulk samples of the same material. The overall shape of 0D, 1D, 2D, and 3D nanoparticles differentiates them. The surface layer, the shell layer, and the core, which is the NP's most important central element and is sometimes referred to as the NP itself (Boisseau, 2011). The unique qualities of these materials, such as their high surface-to-volume ratio, dissimilarity, sub-micron size, and improved targeting mechanism, have made them very beneficial in a variety of industries. NPs penetrate deep into the tissue, increasing permeability and retention (EPR). Surface characteristics change bioavailability and half-life by effectively bridging epithelial fenestration (Shin et al., 2016). Hydrophilic polymer-coated NPs, for example, decrease opsonization and immune system clearance. The features of particle polymers may also be employed to boost pharmaceutical or active moiety release rates. The therapeutic effectiveness of NPs in blood cancer management and treatment is governed by their specific properties. (Fig. 4 summarizes the application of nanoparticles as a drug delivery system. Further specific advantages on like increased stability and biocompatibility, greater permeability and retention, and accurate targeting were highlighted).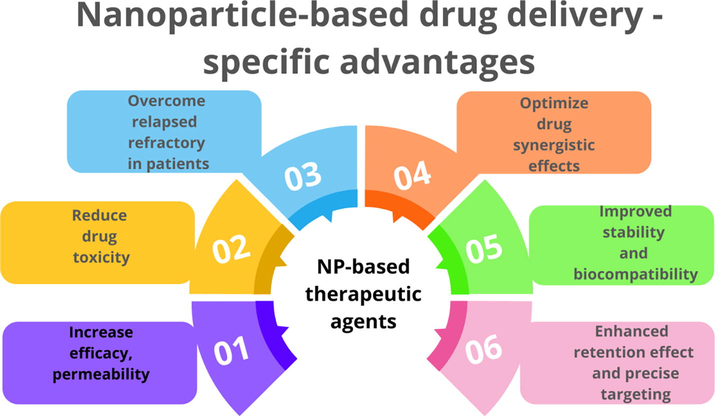
Nanoparticle-based drug delivery- specific advantages.
2.7 Types of nanoparticles
Organic and inorganic nanoparticles are two of the most prevalent types. Micelles, dendrimers, liposomes, hybrid, and compact polymeric nanoparticles make up the first category. Fullerenes, quantum dots, silica, and metal nanoparticles are members of the second category. Nanoparticles may also be classified according to their shape, size, and chemical characteristics.
2.8 Nanoparticles from organic materials
The most often reported organic nanoparticles by researchers include polymeric nanoparticles (PNPs), liposomes, and extracellular vehicles (EVs). PNPs may be synthesized from biological or manmade polymers, and since they are biocompatible and biodegradable, they are among the best popular natural ways to resolve several nanoparticle-based challenges (El-Say et al., 2017). They may obtain via nanoprecipitation, filtration, supercritical technologies, two-step emulsification, and many more technologies. Their sizes and stability may be altered during production.
Liposomes are spherical vesicles consisting of a fatty lipid bilayer. Cholesterol, phospholipids, surfactants, and proteins may be used to form nanoparticles (Alavi et al., 2017). Many different ways can be used to make liposomes, such as sonication, extruding, or using the Mozafari method (Mozafari, 2005). As they can hold both water- and water-based drugs, they can be thought of as delivery systems that can also hold specific biomolecules and other nanomaterials (Mauricio et al., 2018; Dumontel et al., 2017; Cauda et al., 2010).
Exosomes (70–150 nm) are one of the most often employed extracellular vehicles (EVs) in research. They are released by all types of cells and assist in the regulation of cell metabolism and pathophysiology (Mathieu et al., 2019). Exosome-like nanoparticles are the most powerful, bio-compatible, therapeutics available at present. They can be made naturally or synthetically. For nano theranostics research and applications, the ME-HAD (European Network on Microvesicles and Exosomes in Health and Disease) clearly demonstrates nano-sized exosomes and microvesicles adaptability in a variety of ways (Fais et al., 2016).
2.9 Nanoparticles from inorganic materials
There are three types of inorganic nanoparticles: quantum dots, metallic nanoparticles, and nanoparticles made of metal oxides, or MNPs. In particular, peptides, proteins, polysaccharides, and other macromolecules may be used to reduce hazardous heavy metal leaks while increasing overall NP stability. (Cauda V 2010) GQDs, InP, CdSe, InAs, and CdTe are all commonly used nanoparticles in bioimaging and biosensing. (Mauricio et al., 2018). MNPs are made up of magnetic and precious metals, like Palladium (PdNPs), silver (AgNPs), gold (AuNPs), and copper (CuNPs). MNPs like these can be used as theranostics agents, and magnetic ones can be used as contrast imaging and biosensors (Vallabani et al., 2018). Iron oxide (Fe3O4), zirconia (ZrO2), mesoporous silica nanoparticles (MSNs), zinc oxide, Ceria (CeO2), and titania (TiO2) are all biocompatible MONPs. They have high chemical stability, antioxidant, catalytic abilities, and can be used for implants and cell imaging (Garino et al., 2019).
2.10 Diagnosis using nanoparticles
Recent breakthroughs in nanotechnology have fostered the development of a variety of nanoparticle improvisations for diagnostic and therapeutic purposes. Diagnostic nanoparticles are designed to visualize abnormalities and increase knowledge of key (patho-) physiological principles underlying different illnesses and disease therapies. However, owing to the complicated demands on their pharmacokinetic characteristics and elimination, nanodiagnostics are only helpful in a restricted number of clinical settings. As a result, the vast majority of nanoparticle formulations now employed in clinics are used for therapeutic reasons. Therapeutic nanoparticles are designed to promote the accumulation and release of pharmacologically active drugs at the diseased site, boost therapeutic effectiveness, and minimise the frequency and severity of adverse effects by lowering their location in healthy tissues.
Anti-CD20 antibodies, such as Rituximab, target the CD20 antigen and are combined with QDs or magnetic nanoparticles to target lymphoma cells. QDs coupled with rituximab solely bind to tumor cells that enable flow cytometry identification of non-Hodgkin lymphoma cells. Comparatively, the results are more sensitive and specific than immunohistochemistry (Shariatifar et al., 2019). For early detection of leukemia, QDs coupled with Sgc8c aptamer may be used to image tumor cells in culture or in vivo. Researchers used anti-CD20 antibody-linked magnetic nanoparticles to efficiently separate lymphoma cells from bodily fluids (Yu et al., 2016). Simultaneously, they grabbed cancer cells from plasma samples after being functionalized with hyaluronic acids. This method detects tumor cells and provides feedback on their state and therapeutic response by measuring changes in mass loading (Sahoo et al., 2017).
2.11 Passive targeting benefits of nanomedicines
Passive targeting is based on the mechanism for delivering drugs to specialized tumors or tissue sites, through leaking blood vessels that mostly need a framework for delivering, because of their own shape, form, interfacial cell viability, and many characteristics, so that it can reach the end organ or tumor. This means that the number of drugs in the bone marrow is linked to the number of reticuloendothelial cells in the vessels that carry them. The particle size is pivotal because the trans-cellular channel travels between endothelial cells in the bone marrow. According to research, the endothelium wall fenestrae is less than 150 nm in diameter. Nanoparticles smaller than 60 nm can be used to enter and spread into the interstitial space of the bone marrow through reticuloendothelial sinusoidal blood capillaries. A prolonged circulation time in blood vessels is also necessary for nanomedicine to be effective in bone marrow drug delivery. Liposomes having a diameter of less than 100 nm flow freely and have less interaction with plasma proteins. Nevertheless, nanoparticle with a tiny size has a constraint, since drug encapsulation effectiveness is limited by nanoparticles smaller than 50 nm. Surface energy has an important impact on bone marrow absorption, in addition to diameter size in nanomedicine. Negatively charged liposomes have been shown to boost the efficacy of bone marrow absorption by macrophages. As a result, nanoparticles for blood malignancies should be between 50 and 100 nm in size (Jiang et al., 2022). The great stability and variable blood circulation duration of PLGA NPs make them suitable for passive targeting (Wicki et al., 2015).
2.12 Nanomedicines for blood cancer
Drug delivery systems using different types of nanoparticles for the treatment of various blood cancers have shown significant effectiveness in recent decades and are currently being employed in clinical studies. In contrast to other types of cancer, most of the research has concentrated on solid tumors, with just a handful attempting to develop drug delivery techniques for blood cancers. We have reported the most up-to-date information about nanomedicine-based therapy for the treatment of blood cancers.
2.13 Nanomedicines in the treatment of leukemia
Acute myeloid leukemia (AML) is another common blood cancer. A large number of aberrant myoblasts in the bone marrow induce the disease. The best treatment for AML is currently chemotherapy. However, the overall survival rate for people with AML who only get single chemotherapy is still very low (Arber et al., 2016). AML therapy often involves the use of a combination of two or more anticancer drugs to enhance the result, but there can be a number of negative effects (Dombret and Gardin, 2016). Therefore, different medication delivery approaches were studied to enhance the anti-AML impact. Recently lipid-drug conjugate encapsulated cytarabine was used in a stage III clinical study. Pluronic block copolymers sensitise drug-resistant tumours. SP1049C, a Pluronic-based micellar formulation of doxorubicin (Dox), completed a Phase II clinical investigation. Cytarabine liposomes might assist leukemia cells to remain in the bone marrow and make the treatment more effective in a xenograft model of AML (Tardi et al., 2016).
CPX-351- Vyxeos was used to treat newly diagnosed treatment-related AML and AML with myelodysplastic alterations that were also authorized by the FDA and EMA for therapy. CPX-351 was first made and tested with leukemia cell lines in both in vitro and animal studies (Bahreyni et al., 2017). The findings suggest that the liposomal-encased cytarabine and daunorubicin might have the best synergic activity and the least amount of antagonism at a ratio of 5:1. This meant that more people responded, the remissions lasted longer, and the bone marrows were healthier for longer than if they took a free medication combination including the maximal tolerable dosages of cytarabine and daunorubicin (Chen et al., 2018). Researchers examined the evidence from clinical trials and found that CPX-351 was superior to conventional treatment in high-risk AML patients, elderly sAML patients, and those with low-risk AML (Krauss et al., 2019).
Myelomonocytic leukemia has been treated using a novel kind of graphene termed “many-layered graphene” (FLG) (Deb and Vimala, 2018). Graphene sheets combined with aptamers enable the accurate identification of cancer cells in the blood. In contrast to antibodies, aptamers are tiny, synthetic single-stranded oligonucleotides. They may be combined with nanoparticles to enable the identification of certain types of cancer, such as carbon QDs coated with zinc oxide nanospheres or chitosan. AuNPs combined with Sgc8c aptamer or Sgc8c and ATP aptamers directly functionalize graphene sheets, allowing them to interact with PTK7, which is overexpressed in ALL T cells. To collect and immobilize leukemia cells on a quartz crystal microbalance sensor surface for critical measurement of resonant frequency variations, the Sgc8c aptamer, and fluorescent mesoporous silica nanoparticles may be used synergistically (Khoshfetrat and Mehrgardi, 2017).
Surface-enhanced Raman scattering (SERS) was used to create a portable sensor for AML research and detection. To enhance malignant cells' weak Raman signal, hollow-core photonic crystal fibers are coupled with silver nanoparticles. Additionally, SERS AuNPs were used to identify leukemia and surface proteins of lymphoma cell such as CD45, CD19, and CD20 (Khetani et al., 2015) (Fig. 5).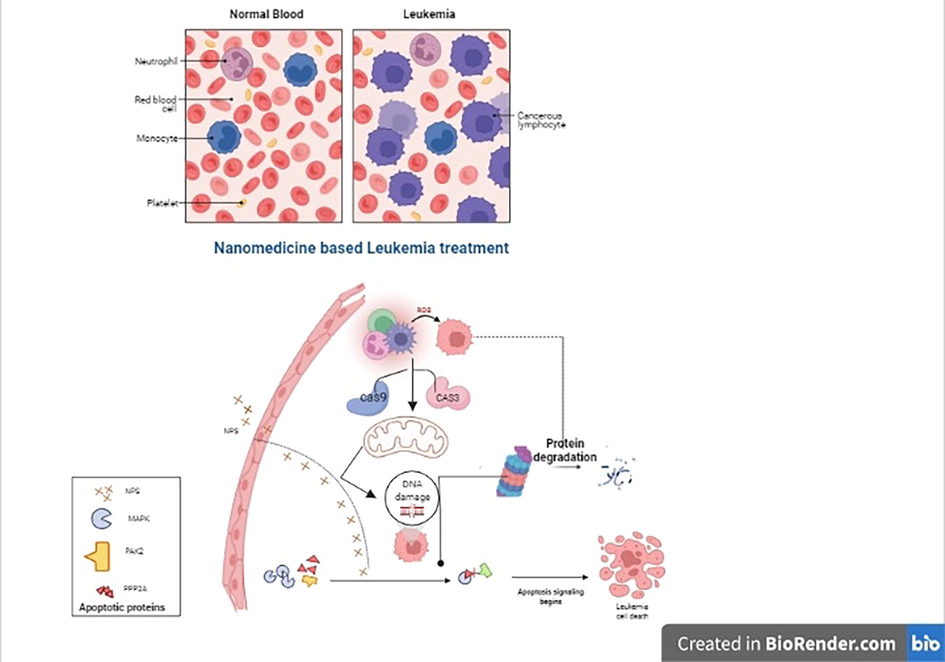
The illustration depicts a nanoparticle-mediated delivery treatment that demonstrates enhanced medication effectiveness in bridging the cascade pathway and therapeutic efficacy against Leukemia.
AZD2811 polymer nanoparticles are packed with an inhibitor for aurora kinase B. Animal models of acute myeloid leukemia were used to investigate AZD2811 (AML). It has been found to be more effective than a free aurora kinase B inhibitor in reducing tumor growth and causing apoptosis (AZD1152). Furthermore, this formulation has shown a temporary cellular decrease in bone marrow, suggesting that it might be used to target residual illness. AZD2811 is now being evaluated in two clinical studies for its safety, pharmacokinetics, and tolerability (Lancet et al., 2018) (Table 1).
Type of NP
Tumor
Target
Approach
Reference
Niosome (NS)-
AML in vitro and in vivo
DNR-CD123-PEG-NS
CD123-targeted vesicle
Liu et al., 2017
PPI-G4-M3 dendrimers
Chronic lymphocytic leukemia (CLL)
NFκB pathway
Modulate the expression of pro- and antiapoptotic genes and proteins
Franiak-Pietryga et al., 2017; Franiak-Pietryga et al., 2018
Sphingomyelin/cholesterol liposome loaded with vincristine
Philadelphia chromosome-negative acute lymphoblastic leukemia
Diffuse large B cell lymphoma (DLBCL)
Encapsulated in the aqueous interior of liposomes
Boehlke and Winter, 2006; Wang et al., 2015.
liposome co-loaded with daunorubicin and cytarabine
tAML, AML-MRC
Anthracycline topoisomerase inhibitor, Nucleoside metabolic inhibitor
Cellular internalization, encapsulation
Blair, 2018
BAT1-liposome
CLL peripheral blood mononuclear cells (PBMCs)
Prevent the binding of the chemokine CXCL12 to CXCR4 in CLL
Disrupt the lymphocyte interactions
McCallion et al., 2019
2.14 Nanomedicines in the treatment of multiple myeloma
Multiple myeloma still can't be cured because it's hard to get the tumor cells out of the bone marrow and out of the body. Bortezomib nanoparticles encased in PEG-PLGA slowed the growth of myeloma in mouse models (Swami et al., 2014). Carfilzomib-loaded liposome nanoparticles were able to reach myeloma cells (Ashley et al., 2014). Doxorubicin liposomes combined with bortezomib have received FDA approval to be used in clinical trials. A phase III clinical study found that liposomal doxorubicin was better than bortezomib alone (Orlowski et al., 2016).
During the last few years, protease inhibitors have been used a lot to treat multiple myeloma (Du and Zhuang, 2021). Bortezomib, a class I protease inhibitor, was encapsulated into pH-sensitive covalent bonds combining phenylboronic acid and catechol (Lee et al., 2018). This made the nanoparticles injectable. At pH 7.4, approximately 7 % of the bortezomib in the composite was released, while in an acidic environment, roughly 85 % was set to release over 216 h. In experiments on mice, the bortezomib-loaded micelle/hydrogel composite slowed down the growth of tumors in a xenograft mouse model. This shows that hydrogel can be used for subcutaneous administration and long-term drug delivery. A system that uses antibodies to deliver myeloma drugs has also been looked at. Nanoparticles with monoclonal anti-CD38 antibodies that were encased in S3I-1757, which is a STAT3 inhibitor, were effective in multiple myeloma therapy. A nanoparticle delivery method with or without an anti-CD38 antibody was proposed by many researchers (Jiang et al., 2022). During the in vitro drug release study, two formulations were found to have a similar amount of drug release with approximately 68 %. Nanoparticles with an anti-CD38 antibody on them increased drug uptake in two different types of Multiple myeloma cells. Anti-CD38 antibody-coated nanoparticles were found to be more effective at reducing tumor size in a xenograft mouse model than uncoated nanoparticles after 12 days of drug administration. This means that nanoparticles loaded with STAT3 inhibitors can be even more effective at treating multiple myeloma if they have the anti-CD38 antibody on them (Jiang et al., 2022).
Patients with multiple sclerosis (MM) who exhibit VLA-4 (Very Late Antigen-4, commonly referred to as 4–1 integrin) or LPAM-1 (Leukocyte Peyer's Patch Adhesion Molecule-1, often referred to as 4–7 integrin) have a poor prognosis (Neri et al., 2011). VLA-4 and LPAM-1 are extensively expressed in normal tissue, focusing on both receptors may improve cellular target and specificity. This strategy improved an ineffective ligand. They also showed a low affinity for cells with just one or no VLA-4 or LPAM-1 receptor, allowing them to discriminate among myeloma cells as well as normal cells. This method observed the value of dual receptor targeting in enhancing therapy selectivity and efficacy. BCMA is a member of the TNF receptor superfamily, along with BAFF. BCMA stimulates the spread of cancer cells and adaptability to chemotherapy. Thus, monoclonal antibodies, immune toxins, specific T-cell engagers, and adoptive immunotherapy target BCMA. PLGA and lipid-based nanoparticles were examined for their capacity to activate myeloma-specific CD8 + cytotoxic T cells. These formulae effectively transport BCMA peptides to dendritic cells. PLGA/peptide-loaded cell took 18 h, while liposome/peptide-loaded cell took 30 min. Degranulation-dependent BCMA cytotoxicity and Th1-type cytokine release were seen with the PLGA/peptide. To induce cytotoxicity, “gradual” antigen absorption and BCMA7280 [YLMFLLRKI] peptide injection may be more effective. It is possible to improve glucocorticoid composition to improve pharmacokinetics, reduce adverse effects, and promote tumor accumulation (Stefanick et al., 2019). Researchers used fluorescently labeled liposomes carrying dexamethasone in a human-mouse MM model. Unbound dexamethasone had no influence on tumor formation in human bone cancer scaffolds at the same dose (Bae et al., 2020). (Fig. 6). Carfilzomib (CFZ) was marketed as alternate chemotherapy for refractory MM. A6-polymeric micelles (A6-PMs) were designed to release CFZ in human MM cell lines LP-1 overexpressing CD44-receptors. A6 has a longer half-life, seems to be much more stable, and has less systemic toxicity than other CFZ-sulfobutylether-cyclodextrins (Table 1).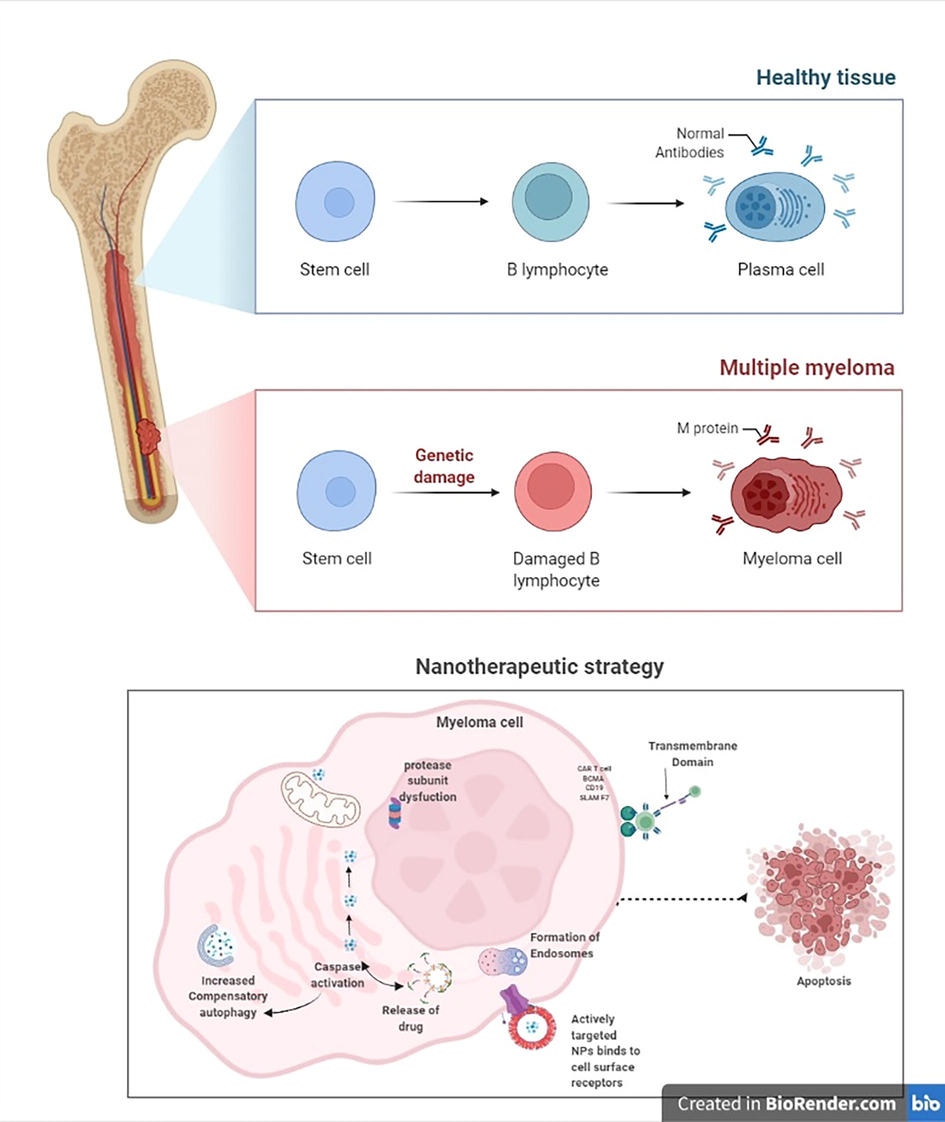
A figure depicts normal and myeloma cells, as well as a schematic diagram of the nanotherapeutic strategy: i) the process of targeted nanoparticle cellular internalization via non-receptor mediated passive entry, ii) followed by endocytosis and cascade stabilization, targeting nuclear localization signals, transcription, and transgene expression.
2.15 Nanomedicines in the treatment of lymphoma
One of the most common cancers, lymphoma, occurs in the lymph nodes. B cell lymphomas, including Hodgkin's lymphomas and the vast majority of non-Hodgkin lymphomas, account for the vast majority of all lymphomas (Novo et al., 2019). B cell lymphomas are often treated with a combination of chemotherapy and stem cell transplantation, although recurrence is common (Deshantri et al., 2018). In the treatment of B cell lymphomas, antibody conjugates opened up new therapeutic possibilities. Ibritumomab tiuxetan (Zevalin®) and Brentuximab vedotin (Adcetris®) are two FDA-approved antibody-drug conjugates for Hodgkin lymphoma and non-Hodgkin lymphoma. They are both commercially available in both Seattle and Irvine. Furthermore, modern technology allows anticancer drugs to be delivered specifically to malignant cells without harming good cells or causing systemic toxicity, enabling them to reach lymph nodes. The treatment of CD20 + B-cell lymphoma was greatly improved by a nano-antibody targeted chemotherapeutic delivery system using a small modification of current cancer medications (Nevala et al., 2017). The siRNA targeting Bcl2-loaded diatomite nanoparticles showed considerable gains in biological efficiency for the tailored therapy of lymphomas (Martucci et al., 2016). Layer-by-layer nanoparticles were designed to make a siRNA nano-formulation that could be used to treat non-Hodgkin lymphoma. The nano-formulation was made by targeting two different types of siRNA. In the circulation, the LbL-NP shields siRNA from nucleases by encasing it in polyelectrolyte layers. Hyaluronic acid (a CD44 ligand) is covalently linked to CD20 antibodies in the outermost layer. LbL-NP is taken up by blood cancer cells through receptor-mediated endocytosis of the CD20/CD44 dual-targeting outer layer. With the use of the dual-targeting strategy for lymphoma and leukaemia cell internalization, BCL-2 siRNA expression is considerably reduced. Both cell culture and animal models of orthotopic non-lymphoma Hodgkin's were treated with siRNA-loaded nanoparticles that triggered apoptosis and slowed the growth of blood cancer cells (Choi et al., 2019).
The surface of lymphoma cells contains a variety of non-glycosylated antigens. The CD20 antigen is required for the activation and proliferation of B lymphocytes. This antigen on tumoral B cells must be increased in order to recognize CTCs in blood and other human fluids. Ligands and monoclonal antibodies are examples of active targeting moieties. Rituximab and other anti-CD20 antibodies target the CD20 antigen and are coupled with QDs or magnetic nanoparticles to remove lymphoma cells (Limongi et al., 2019).HumFt-PAMAM hybrid nanoparticle can carry miRNAs into cells expressing CD71 receptors (CD71-expressing cells). NB4 leukemia cells exhibit phenotypic changes comparable to early differentiation after internalizing pre-miRNA. With the ingestion of miRNA-containing nanoparticles, NB4 cells demonstrated increased expression of RAR and morphological alterations characteristic of granulocyte differentiation. This method might be used to create a new class of transfecting agents that target certain diseases (Palombarini et al., 2021) (Table 1) (Fig. 7).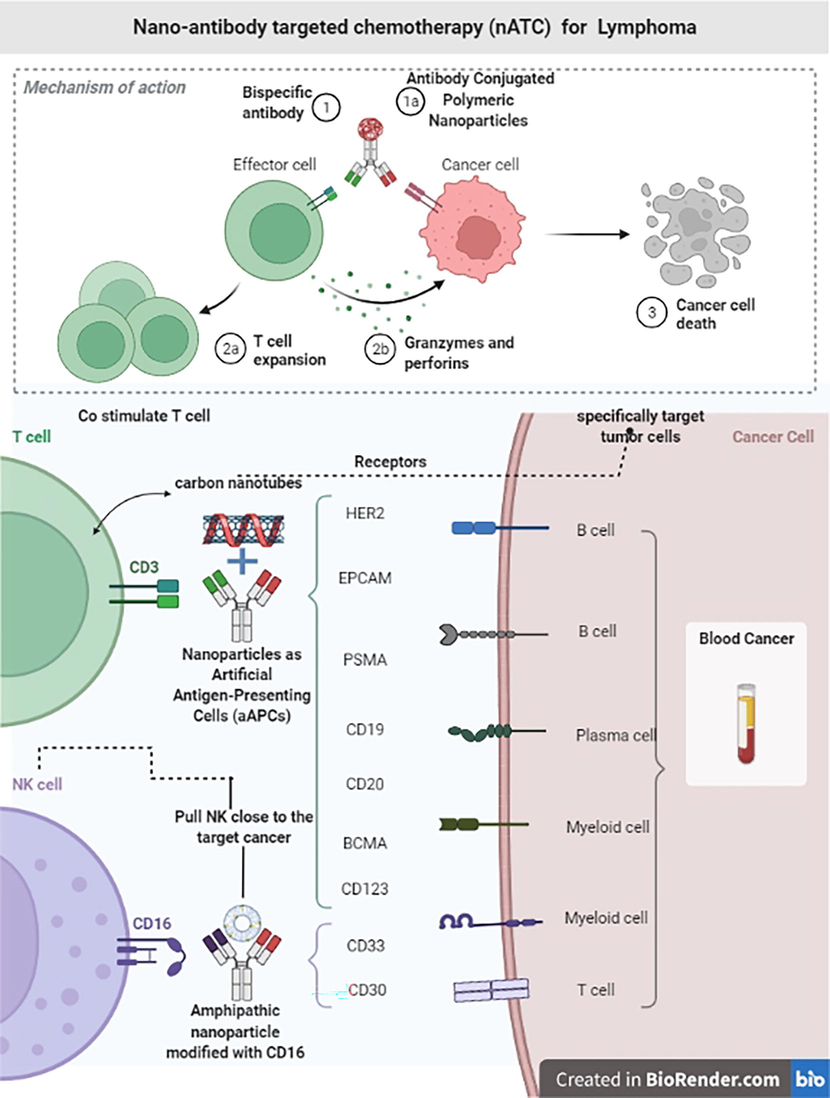
The Schematic diagram illustrates the designed Nanoantibody targeted chemotherapy that governs increased drug efflux, changes in cell metabolism, genetic alterations of the drug target, and eventually suppression of apoptosis.
2.16 Recent therapy for blood cancer
Blood cancer therapy is now one of the most significant medical fields of researching nanosized medication delivery and therapeutic technologies. The new technology has taken the research world by storm, changing the border between conceivable and impossible. Once CRISPR hit lab shelves and freezers, cancer researchers rushed to employ it. CRISPR is a convenient tool employed in numerous cancer biology investigations. CRISPR-edited CAR T cells have been shown to improve the defense against blood malignancy. The CRISPR/Cas9 method improved the modified T cells' capacity to remove blood malignancies by knocking out a protein known to inhibit T cell activity.
2.17 CAR T cells therapy
New developments in blood cancer therapy include CAR T-cell therapy, which requires a 15-month follow-up. The chimeric antigen receptor is targeted using CD19 lymphocytes. More than 80 % of patients with ZUMA-1 (large B-cell lymphoma) showed significant recovery after CAR T-cell treatment, while 54 % were totally cured (Vitale and Strati, 2020). One of the most common methods for separating T cells from white blood cells and re-engineering them with CAR T cells is called leukapheresis. AML patients respond well to a combination of targeted therapies. 50 % of the patients had a full response. Monoclonal antibodies and stem cell therapy were adapted to attack CD20 antigen, which embraces malignant cell growth in lymphoma. Whereas FLT3 is a gene that causes mutations in leukemia, there are drugs that suppress expression. Daratumumab, elotuzumab, and ixazomib boost the immune system, eliminating and inhibiting the growth of multiple myeloma cells. This therapy will enhance the patients' quality of life. After intensive chemotherapy, the patient's sibling's bone marrow is utilized, but not that of an identical twin. Bone marrow differences have a positive effect on survival. Bispecific antibodies bind to both T cells and tumor cells simultaneously. This is the most typical technique of therapy for every-one. A nanoparticle was employed to effectively transport cytochrome c, a pro-apoptotic basic protein, to AML cells (Macone et al., 2019).
2.18 CRISPR therapeutics
CRISPR genome editing has recently been performed using the PLGA nanomedicine platform. Cas9 nuclease, which is delivered into a cell through a synthetic guide RNA (gRNA), allows for genome slicing at a specific location, allowing for the silencing, deletion, or introduction of new genes. A breakpoint cluster region-abelson (BCR-ABL) fusion oncogene is translated into a BCR-ABL fusion protein in chronic myeloid leukemia (CML). pCas9/gBCR-ABL) encapsulated in a cationic lipid-assisted polymeric NP based on PEG-PLGA (Nadakuduti et al., 2021). In a CML mouse model, the gene of interest was effectively knocked down in vitro and in vivo. In comparison to naive and vehicle controls, the Nanocarrier reduced the number of myelogenous leukemia cells (K562) in blood and bone marrow and increased survival rates in mice, and also change the expression of pro-and anti-apoptotic genes and proteins (Liu et al., 2018). The role of NY-ESO-1 in redirected modified T cells in various cancers was explored (NCT03399448). Oncologists exploited the CRISPR/Cas9 system to delete endogenous TCR and B2M genes to make allogeneic chimeric antigen receptor (CAR) T cells in clinical trials for CD19 + leukemia and lymphoma (NCT03166878). As a result, graft-versus-host disease was minimized. But not all patients have CD19. It was designed to target CD19-negative cells and destroy those using CD20 or CD22 recognizing CAR-T cells (NCT03398967).
2.19 Antibody-targeted nanoparticles: An effective therapy
Antibody nanoparticle prodrugs have the ability to efficiently target and deliver therapeutic targets at the cancer site while limiting off-target negative effects of overdosing surrounding cells. AR160 nano-antibody targeted chemotherapy is more effective than chemotherapy alone or rituximab was given concurrently but sequentially to chemotherapy because higher concentrations of the chemotherapy can be targeted specifically to the tumor, increasing efficacy while limiting unwanted cytotoxic activity, which is the primary goal of antibody-targeted therapy (Jagadeesh and Smith, 2016). anti-CD20 antibodies (rituximab) are non-covalently bound to the albumin scaffold of nab-paclitaxel to form antibody-targeted paclitaxel nanoparticles for the treatment of CD20 + B-cell lymphoma (ABX) (Nevala et al., 2017). It was designed to drive ND particles to a particular cell surface antigen via an Apolipoprotein–antibody fusion. Cell surface protein CD20 is expressed in several B-cell lymphomas, including MCL (Witzig, 2005). Single-chain antibody fragments that maintain the original monoclonal antibodies antigen specificity and binding ability to CD20-expressing cells have been developed. This is because CD20 is a prominent surface antigen on MCL cells. Stable ND formation and CD20 recognition activity are two distinct functionalities of the recombinant CD20 scFv-based fusion protein. Nanodisk (ND) technology is a type of HDL that binds and distributes hydrophobic anti-cancer drugs to cells that express CD20. It is made up of apoA1 and a single-chain variable fragment antibody (Ryan, 2010).
2.20 Indigenous plants nanocomposite as a therapeutic agent
Annona muricata (Graviola) is a 5–6 m tall Annonaceae tropical fruit tree. Graviola was used to treat cancer. In vitro and in animals, the Graviola extract showed a potential selective tumorigenic suppressive effect. A significant positive agreement between p53, caspase 3, 9, Bax and NFkB gene expressions, and treatment modalities was seen in the presence of Graviola nanocomposite. Ensuring that Graviola nanocomposite has promise for sono-photodynamic leukemia therapy in vitro and in vivo. The use of nanoparticles also allows for targeted medication administration and enhances and amplifies the response to sono-phototherapy (Abu Rakhey et al., 2022). Nanoparticles (LdAuNPs) derived from lavender have been proven to be a promising treatment for K-562, a myelogenous leukemia cell line (Justus et al., 2019). Bimetallic silver–selenium (Ag-Se) nanoparticles produced with quercetin and gallic acid showed potential anticancer action against Dalton's lymphoma cells (Mittal et al., 2014).
Recently, photosynthesized nanoparticles were investigated for their ability to target cancer cells without damaging healthy cells. The use of plant viral nanoparticles to deliver cancer-fighting medications has been widely accepted. Non-lymphoma Hodgkin's was successfully treated using TMV-based nanoparticles. The antileukemic action of AgNPs produced by Spinacia oleracea leaf (spinach) was compared to doxorubicin in an animal model of AML (DOX). Spinacia oleracea extracts include over 13 flavonoids, which may function as anticancer and antioxidant agents, hence some interaction with AgNPs was predicted (Chandrakala et al., 2022). The synthesized nanoparticles showed strong antioxidant, anti-acute, and anticancer activity against myeloid leukemia (Green Zangeneh, 2020). The same techniques were used to synthesize AgNPs from Melissa officinalis, which showed antioxidant and anti-cancer effects against myeloid leukemia in animal models (mitoxantrone) (Mostafavi et al., 2022). Real-time PCR findings demonstrated a considerable rise in S1PR1 and S1PR5 mRNA expression following treatment with AgNPs and mitoxantrone, suggesting a possible synergy of both components. Moreover, AgNPs have comparable hematological qualities to mitoxantrone, boosting platelet, lymphocyte, and RBC parameters (Ahmeda et al., 2020). Viral antigen nanoparticles (vAgNPs) synthesized from Coptis chinensis enhance the expression of pro-apoptotic proteins Bax and Bak while decreasing the expression of anti-apoptotic Bcl-2 and Bcl-XL proteins (Pei et al., 2019).
2.21 Nanoparticles: Lead to future
Blood Cancer nanomedicine is quickly expanding our knowledge of its complexities as well as its potential benefits to cancer patients. In this review, we have discussed the necessity of bringing together nanotechnology and cancer biology for nanomedicine research and clinical translation. We believe that nanomedicines will revolutionize cancer therapy and that the ultimate aim of cancer nanomedicine: a huge increase in patient survival and will be realized in the near future.
The use of nanoparticles to enhance medication delivery is a novel strategy for treating cancer and a variety of other ailments. The primary objective of NPs is to expedite the delivery of medications without causing adverse effects. The FDA has authorized many liposomes and polymeric nanoparticles for therapeutic use in blood cancer, and several more are being explored in clinical trials for a variety of cancers, ranging from solid tumors to hematological malignancies such as acute myeloid leukemia. NPs enable us to deliver medicines with improved pharmacokinetics, safety profiles, and biodistribution, enabling us to reconsider chemotherapies that were previously discarded due to insufficient targeting ability, poor dissemination, or adverse effects.
3 Limitations of nanomedicine in cancer therapy
The lack of consistency in pre-clinical testing of nanoparticle-based delivery systems has impeded systematic comparison and has hampered the development of design principles for novel systems or specialized applications. Surprisingly few pre-clinical experiments offer quantitative data on factors that may be relevant in formulating design principles for nanomedicines. Poor experimental design and the variety of experimental settings further contribute to the field's delayed progress and lack of therapeutic effect. We emphasize newest technologies with pre-clinical trials may improve the effect of individual investigations.
4 Conclusion
Effective and accurate cancer screening and diagnostic procedures may help patients live longer and have an improved way of life. Surgery, chemotherapy, and radiation are the most common cancer treatments today. These treatments can only demonstrate limited effectiveness. Complex nanostructures with distinct physical characteristics and surface chemistry may now be manufactured thanks to recent breakthroughs in nanotechnology. In comparison to standard treatment agents, nanomedicines for cancer have shown remarkable promise.
Nanotechnology, biomaterials, and regenerative medicine revelations have resulted in advanced technologies in the area of nanomedicine. Cancer detection and therapy are in desperate need of modification, and nanomedicine may potentially enable significant advancements in both of these areas. Organic and inorganic nanoparticles encapsulating diagnostic or therapeutic materials have been developed and investigated for biomedical purposes, and they represent an exciting new nanomedicine method. Nanomaterials are comparable in size to biomolecules and cells.
Nanoparticle delivery systems may help cure blood cancer in two ways: 1) enhance potency and effectiveness of therapies by targeting the bone marrow niche where cancer cells formed. 2) NPs also lessen adverse effects in normal tissues by delivering medications directly to the target area. This will be possible by giving cancer cells bigger dosages and other tissues lesser levels. Hence, improving therapeutic potency or effectiveness while reducing side effects may improve illness outcomes while reducing drug side effects. In general, precision-designed nanomaterials, such as organelles and exosomes, may regulate cell state and function. In the last two decades, nanomaterials for cancer therapy have been extensively researched. Lipid nanoparticles feature cationic head groups that bind to anionic nucleic acids. They use hydrophobic drugs on the lipid membrane and hydrophilic drugs within the hollow center area. These nanoparticles may also interact with magnetic fields, light, and ionizing radiation to increase their biological value. Studies explored nanoparticles' potential for cancer treatment and diagnostics. Liposome nanoparticles with a promoter-enhancer and transcriptional activator have made significant contributions to the field of cancer treatment.
The future of stimuli-responsive nanoparticles can be achieved through a wide range of desirable properties such as pH variations, redox potential, enzymatic activation, thermal gradients, magnetic fields, light, and ultrasound, or they can even be responsive to dual or multicombinations of different stimuli that can eventually be developed for safe and efficient cancer therapy. We expect that further research on nanomedicine, and nanocomposite will result in the creation of sensitive, least invasive blood cancer treatments. Additionally, the merging of many scientific domains accelerates these breakthroughs, and we anticipate that these interdisciplinary efforts will have a significant impact on several fields of study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Folic acid conjugated graphene oxide graviola nanoparticle for sono-photodynamic leukemia treatment: up-to-date cancer treatment modality. J. Biosci. Appl. Res.. 2022;0(0):28-45.
- [Google Scholar]
- Preparation, formulation, and chemical characterization of silver nanoparticles using Melissa officinalis leaf aqueous extract for the treatment of acute myeloid leukemia in vitro and in vivo conditions. Appl. Organomet. Chem.. 2020;2:34.
- [CrossRef] [Google Scholar]
- Application of various types of liposomes in drug delivery systems. Adv Pharm Bull.. 2017;7:3-9.
- [CrossRef] [Google Scholar]
- The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
- [CrossRef] [Google Scholar]
- Liposomal carfilzomib nanoparticles effectively target multiple myeloma cells and demonstrate enhanced efficacy in vivo. J. Control. Release. 2014;196:113-121.
- [Google Scholar]
- Correction: BCMA peptide-engineered nanoparticles enhance induction and function of antigen-specific CD8+ cytotoxic T lymphocytes against multiple myeloma: Clinical applications. Leukemia. 2020;34(7)
- [Google Scholar]
- Identification and imaging of leukemia cells using dual-aptamer-functionalized graphene oxide complex. J. Biomater. Appl.. 2017;32(1):74-81.
- [Google Scholar]
- Daunorubicin/cytarabine liposome: A review in acute myeloid leukaemia. Drugs. 2018;78(18):1903-1910.
- [CrossRef] [Google Scholar]
- Sphingomyelin/cholesterol liposomal vincristine: a new formulation for an old drug. Expert Opin. Biol. Ther.. 2006;6(4):409-415. PMID: 16548767
- [CrossRef] [Google Scholar]
- Nanomedicine, nanotechnology in medicine. C.R. Phys.. 2011;12(7):620-636.
- [CrossRef] [Google Scholar]
- Colchicine-loaded lipid bilayer-coated 50 nm mesoporous nanoparticles efficiently induce microtubule depolymerization upon cell uptake. Nano Lett.. 2010;10(7):2484-2492.
- [Google Scholar]
- Chandrakala, V., Aruna, V. Angajala, G.2022. Review on metal nanoparticles as nanocarriers: current challenges and perspectives in drug delivery systems emergent mater. https://doi.org/10.1007/s42247-021-00335-x.
- Reformulating acute myeloid leukemia: liposomal cytarabine and daunorubicin (CPX-351) as an emerging therapy for secondary AML. OncoTargets Therapy. 2018;11:3425-3434.
- [CrossRef] [Google Scholar]
- Chapter 9 - Treating blood cancer with nanotechnology: A paradigm shift. In: Yadav Awesh K., Gupta Umesh, Sharma Rajeev, eds. Nano Drug Delivery Strategies for the Treatment of Cancers. Academic Press; 2021. p. :225-243. https://doi.org/10.1016/B978-0-12-819793-6.00010-2
- [Google Scholar]
- Binary targeting of siRNA to hematologic cancer cells in vivo using layer-by-layer nanoparticles. Adv. Funct. Mater.. 2019;29(20):1900018.
- [Google Scholar]
- Camptothecin loaded graphene oxide nanoparticle functionalized with polyethylene glycol and folic acid for anticancer drug delivery. J Drug Deliv Sci Technol.. 2018;43:333-342.
- [CrossRef] [Google Scholar]
- Nanomedicines for the treatment of hematological malignancies. J. Control. Release. 2018;287:194-215.
- [CrossRef] [Google Scholar]
- An update of current treatments for adult acute myeloid leukemia. Blood. 2016;127:53-61.
- [CrossRef] [Google Scholar]
- Major advances in the treatment of multiple myeloma in American Society of Hematology annual meeting 2020. Chronic Dis. Transl. Med.. 2021;2021(7):220-226.
- [CrossRef] [Google Scholar]
- Enhanced biostability and cellular uptake of zinc oxide nanocrystals shielded with a phospholipid bilayer. J Mater Chem B.. 2017;5(44):8799-8813.
- [Google Scholar]
- Antineoplastic activity of biogenic silver and gold nanoparticles to combat leukemia: Beginning a new era in cancer theragnostic. Biotechnol. Rep,. 2022;34:e00714.
- [Google Scholar]
- Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm.. 2017;528:675-691.
- [Google Scholar]
- Evidence-based clinical use of nanoscale extracellular vesicles in nanomedicine. ACS Nano. 2016;10:3886-3899.
- [Google Scholar]
- A microwave-assisted synthesis of zinc oxide nanocrystals finely tuned for biological applications. Nanomaterials (Basel). 2019;9(2):212.
- [Google Scholar]
- Synthesis and formulation a modern chemotherapeutic drug of Spinacia oleracea L. Leaf aqueous extract conjugated silver nanoparticles; chemical characterization and analysis of their cytotoxicity, antioxidant, and anti-acute myeloma. Appl. Organomet. Chem.. 2020;1):34
- [CrossRef] [Google Scholar]
- Affecting NF-κB cell signaling pathway in chronic lymphocytic leukemia by dendrimers-based nanoparticles. Toxicol. Appl. Pharmacol.. 2018;357:33-38.
- [CrossRef] [Google Scholar]
- Antibody drug conjugates (ADCs): changing the treatment landscape of lymphoma. Curr. Treat. Options Oncol.. 2016;17:55.
- [Google Scholar]
- Targeted drug delivery for the treatment of blood cancers. Molecules (Basel, Switzerland). 2022;27(4):1310.
- [CrossRef] [Google Scholar]
- Characterization and cytotoxic evaluation of silver and gold nanoparticles produced with aqueous extract of Lavandula dentata L. in relation to K-562 cell line. Braz. Arch. Biol. Technol.. 2019;62
- [CrossRef] [Google Scholar]
- Hollow core photonic crystal fiber for monitoring leukemia cells using surface enhanced Raman scattering (SERS) Biomed. Opt. Express. 2015;6:4599-4609.
- [Google Scholar]
- Amplified detection of leukemia cancer cells using an aptamer-conjugated gold-coated magnetic nanoparticle on a nitrogen-doped graphene modified electrode. Bioelectrochemistry. 2017;114:24-32.
- [Google Scholar]
- FDA approval summary: (daunorubicin and cytarabine) liposome for injection for the treatment of adults with high-risk acute myeloid leukemia. Clin. Cancer Res.. 2019;25:2685-2690.
- [CrossRef] [Google Scholar]
- CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J. Clin. Oncol.. 2018;36(26):2684-2692.
- [Google Scholar]
- Injectable coacervate hydrogel for delivery of anticancer drug-loaded nanoparticles in vivo. ACS Appl. Mater. Interfaces. 2018;10:13274-13282.
- [CrossRef] [Google Scholar]
- Nanoparticles for hematologic diseases detection and treatment. Hematol. Med. Oncol.. 2019;4:1000183.
- [Google Scholar]
- Anti-CD123 antibody-modified niosomes for targeted delivery of daunorubicin against acute myeloid leukemia. Drug Deliv.. 2017;24:882-890.
- [Google Scholar]
- Systemic delivery of CRISPR/Cas9 with PEG-PLGA nanoparticles for chronic myeloid leukemia targeted therapy. Biomater. Sci... 2018;6:1592-1603.
- [CrossRef] [Google Scholar]
- Ferritin nanovehicle for targeted delivery of cytochrome C to cancer cells. Sci. Rep.. 2019;9:11749.
- [CrossRef] [Google Scholar]
- Targeted drug delivery for chronic lymphocytic leukemia. Pharm. Res.. 2022;39(3):441-461.
- [Google Scholar]
- Nanoparticle-based strategy for personalized B-cell lymphoma therapy. Int. J. Nanomed.. 2016;11:6089-6101.
- [CrossRef] [Google Scholar]
- Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol.. 2019;21:9-17.
- [Google Scholar]
- Nanoparticles in medicine: A focus on vascular oxidative stress. Oxid. Med. Cell. Longevity. 2018;2018:1-20.
- [Google Scholar]
- Dual-action CXCR4-targeting liposomes in leukemia: Function blocking and drug delivery. Blood Adv.. 2019;3(14):2069-2081.
- [Google Scholar]
- Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov.. 2021;20(2):101-124.
- [Google Scholar]
- Quercetin and gallic acid mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential. J. Colloid Interface Sci.. 2014;431:194-199.
- [Google Scholar]
- Liposomes: an overview of manufacturing techniques. Cell. Mol. Biol. Lett.. 2005;10:711-719.
- [Google Scholar]
- Advances in genome editing with CRISPR systems and transformation technologies for plant DNA manipulation. Front. Plant Sci.. 2021;11
- [CrossRef] [Google Scholar]
- Integrin β7-mediated regulation of multiple myeloma cell adhesion, migration, and invasion. Blood. 2011;117(23):6202-6213.
- [Google Scholar]
- Antibody-targeted paclitaxel loaded nanoparticles for the treatment of CD20+ B-cell lymphoma. Sci. Rep.. 2017;7:45682.
- [CrossRef] [Google Scholar]
- High-grade B-cell lymphoma: How to diagnose and treat. Expert Rev. Hematol.. 2019;12:497-506.
- [CrossRef] [Google Scholar]
- Final overall survival results of a randomized trial comparing bortezomib plus pegylated liposomal doxorubicin with bortezomib alone in patients with relapsed or refractory multiple myeloma. Cancer. 2016;122(13):2050-2056.
- [Google Scholar]
- Self-assembling ferritin-dendrimer nanoparticles for targeted delivery of nucleic acids to myeloid leukemia cells. J. Nanobiotechnol.. 2021;19(1)
- [CrossRef] [Google Scholar]
- Biosynthesis, characterization, and anticancer effect of plant-mediated silver nanoparticles using Coptis chinensis. Int. J. Nanomed.. 2019;14:1969-1978.
- [Google Scholar]
- Molecular targets of curcumin and future therapeutic role in leukemia. J. Biosci. Med.. 2018;6(04):33-50.
- [Google Scholar]
- Nanobiotechnology applications of reconstituted high density lipoprotein. J. Nanobiotechnol.. 2010;8:28-38.
- [Google Scholar]
- Lymphoma cell isolation using multifunctional magnetic nanoparticles: antibody conjugation and characterization. RSC Adv.. 2017;7(36):22468-22478.
- [Google Scholar]
- Non-Hodgkin lymphoma. In: STaTPEaRls [INTERNET]. Treasure Island (FL): StatPearls Publishing; 2022.
- [Google Scholar]
- Hodgkin lymphoma: A review and update on recent progress. CA Cancer J. Clin.. 2018;68(2):116-132.
- [CrossRef] [Google Scholar]
- Immunofluorescent labeling of CD20 tumor marker with quantum dots for rapid and quantitative detection of diffuse large B-cell non-Hodgkin’s lymphoma. J. Cell. Biochem... 2019;120(3):4564-4572.
- [Google Scholar]
- Advances of Nanoparticles for Leukemia Treatment. ACS Biomater. Sci. Eng.. 2020;6(12):6478-6489.
- [Google Scholar]
- Cross-linked composite gel polymer electrolyte using mesoporous methacrylate-functionalized SiO2 nanoparticles for lithium-ion polymer batteries. Sci. Rep.. 2016;6(1)
- [CrossRef] [Google Scholar]
- Dual-receptor targeted strategy in nanoparticle design achieves tumor cell selectivity through cooperativity. Nanoscale.. 2019;11:4414-4427.
- [CrossRef] [Google Scholar]
- Engineered nanomedicine for myeloma and bone microenvironment targeting. PNAS. 2014;111(28):10287-10292.
- [Google Scholar]
- Passive and semi-active targeting of bone marrow and leukemia cells using anionic low cholesterol liposomes. J. Drug Target.. 2016;24:797-804.
- [CrossRef] [Google Scholar]
- Magnetic nanoparticles: current trends and future aspects in diagnostics and nanomedicine. Curr. Drug Metab... 2018;20(6):457-472.
- [CrossRef] [Google Scholar]
- Nanoparticles-emerging potential for managing leukemia and lymphoma. Front. Bioeng. Biotechnol.. 2017;5:79.
- [CrossRef] [Google Scholar]
- CAR T-Cell Therapy for B-Cell non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia: Clinical Trials and Real-World Experiences. Front. Oncol.. 2020;10:849.
- [CrossRef] [Google Scholar]
- Wang, X., Song, Y., Su, Y., Tian, Q., Li, B., Quan, J. 2015. Are PEGylated liposomes better than conventional liposomes? A special case for vincristine. 23:1092–100. https://doi.org/10.3109/1071754420151027015.
- Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J. Control. Release. 2015;200:138-157.
- [CrossRef] [Google Scholar]
- Current treatment approaches for mantle-cell lymphoma. J. Clin. Oncol.. 2005;23(26):6409-6414.
- [Google Scholar]
- Highly sensitive detection of leukemia cells based on aptamer and quantum dots. Oncol. Rep... 2016;36(2):886-892.
- [Google Scholar]
- Polysaccharide-based nanomedicines for cancer immunotherapy: A review. Bioact. Mater... 2021;6(10):3358-3382.
- [CrossRef] [Google Scholar]







