Translate this page into:
An efficient, sustainable approach to the chemo and regioselective synthesis of novel spiroindenoquinoxaline grafted piperidone hybrid heterocycles
⁎Corresponding author. anatarajan@ksu.edu.sa (Natarajan Arumugam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
An efficient, eco-friendly and sustainable approach for the synthesis of novel spiroindeno[1,2-b]quinoxaline-3-phenylspiro[4,3″]benzylidenepiperidone ring system has been developed by a one-pot four component [3 + 2] cycloaddition strategy. The 1,3-dipole generated in situ from quinoxalinone and l-phenylalanine reacts with the highly functionalized dipolarophiles, bisarylidene piperidones affording spirohybrid heterocycles in good yields. The unexplored novel class of dispirohybrid heterocycles obtained possess three C—N and two C—C bonds with four adjacent stereogenic carbons, out of which two is spirocarbons. The structure of compounds was elucidated using 1H, 13C and mass spectroscopic studies.
Keywords
Azomethine ylide
Spiroindenoquinoxalinone
Spiropyrrolidines
Benzylidene piperidone
Ionic liquids
1 Introduction
Exploring structurally intriguing hybrid heterocycles possessing important biological activities is a great challenge in organic and medicinal chemistry. Multicomponent 1,3-dipolar cycloaddition methodology is one of the efficient strategy for the construction of structurally complex architecture (Padwa, 2002; Karthikeyan et al., 2007) and holds prominent place in modern drug discovery research owing to their advantages such as avoiding isolation and purification of intermediates, improving atom economy, minimization of waste, selectivity and yield of product, formation of single product from three or more reactant in a single synthetic operation with multiple bond forming efficiency (Bortolini et al., 2007; Hong et al., 2007). These credentials make this strategy, economic and environmentally friendly. In this context, ionic liquids were widely accepted as green solvents due to their iconicity, stability, solvating properties, high thermal tolerance, acid and basic nature. In recent past years, many libraries of biologically significant spiroheterocyclic hybrids were synthesized via this green synthetic protocol using ionic liquids (Michael Rajesh et al., 2012; Arumugam et al., 2019)
Spiro compounds are attractive target in organic synthesis due to their exclusive three-dimensional structural nature that makes to interact more efficiently with binding pockets in designated enzyme of the biological system and have free solubility than planar aromatic ring system, a key property in the process of drug discovery. Among them, spiropyrrolidine is an important structural motif as its active moiety ubiquitous in several naturally occurring alkaloids (Trost and Brennan, 2009) and many other biologically potent synthetic compounds. For example, spirotryprostatin (Bhaskar et al., 2012), coerulescine (Reddy and Douglas, 2010), elecomine (Deppermann et al., 2010), pteropodine (Kang et al., 2002), horsfiline (Marti and Carreiram, 2003), formosanine (Singha et al., 2013), rychnophyilline, strychnofoline (Angenot, 1978), alstonisine (Garnick and Le Quesne, 1978), MI-219, M-219, MI-888. These compounds exhibited multifarious biological activities including potential antileukaemic (Abou-Gharbia and Doukas, 1979), anticonvulsant (Jiang et al., 2006), local anaesthetic (Kornett and Thio, 1976), antiviral (Lundahl et al., 1972), anticancer (Kathirvelan et al., 2015), antimycobacterial (Arun et al., 2014), anti-inflammatory, anti-microbial (Rajesh et al., 2011), cholinesterase inhibition activities (Kia et al., 2014), potent blocker of human NK-1 receptor (Kornett and Thio, 1976) and p53-mdm2 interaction (Skiles and Mc Neil, 1990).
Piperidone is also an important class of heterocyclic entity, being a useful candidate for the synthesis of naturally occurring alkaloids. Piperidone fused heterocyclic compounds displayed interesting biological activities including herbicidal, bactericidal, fungicidal activities (Dimmock et al., 1994, 1992) and act as potent in vitro blocker for human placental aromatase (Barondi et al., 1996). Piperidin-3-one derivatives are used as potential synthon for the synthesis of antimalarial agents viz., febrifugine and isofebrifugine (Takeuchi et al., 2000). In particular, 3,5-bis(arylidene)piperidin-4-ones showed potent cytotoxic, anticancer agents and are used as essential part for the synthesis of indolizidine alkaloids and tachykinin antagonists (Shintani et al., 2004; Lee et al., 2001). Furthermore, piperidin-4-one hydrochloride is an EPR active component, used as a spin trap in several EPR studies (Dzwigaj and Pezerart, 1995) and its hydrazine derivatives possess antioxidants properties. Besides, piperidin-2-one embedded heterocycles were also used as chiral intermediates in the synthesis of several synthetic and natural compounds with significant biological activities viz., anticancer (Fleet et al., 1989), anti-HIV (Winkler and Holan, 1989) and glycosidase inhibition activities (Fleet et al., 1988). On the other hand, quinoxaline is an active moiety in brimonidine and varenicline drugs which are potent drug candidate for the treatment of glaucoma (Danylkova et al., 2007) and smoking cessation therapy (Mohanasundaram et al., 2008). Besides, quinoxaline analogs act as in vitro Pim-3 kinase inhibitors (Gavara et al., 2010) and potent 5-HT3 receptor agonist (Campiani et al., 1999).
The aforementioned biological significance of spiropyrrolidine, piperidone and quinoxaline sub-structures in conjunction with our interest in the preparation of new class of hybrid heterocycles employing multicomponent 1,3-dipolar cycloaddition strategy, led us now to report the synthesis of hybrid heterocycles incorporating piperidone and spiro-pyrrolidinyl-indenoquinoxaline sub units. In the present work, the relatively less explored non-stabilized 1,3-dipole component derived from l-phenylalanine and indenoquinoxaline via decarboxylative condensation reaction has been employed. The synthetic strategy of the present and previous work has been described in Scheme 1.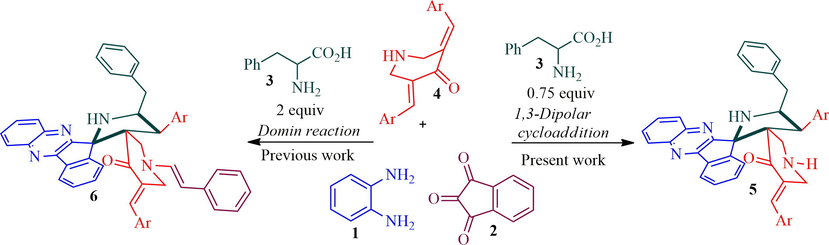
Present and previous work.
2 Materials and methods
2.1 Synthesis of spiropyrrolo-indenoquinoxaline tethered piperidone heterocyclic hybrids, 5a-f
A mixture of ninhydrin (1 mmol), 1,2-aryldiamine (1 mmol) and 1-butyl-3-methylimidazolium bromide [bmim]br (200 mg) were heated with stirring at 100 °C for 10 min. Then 2-amino-3-phenylpropanoic acid (0.75 mmol) and piperidone derivative (1 mmol) was added to the reaction mixture and stirred further for 50 min at same temperature. After completion of the reaction as evidenced by thin layer chromatography (TLC), EtOAc (2 × 5 mL) was added to the reaction mixture and stirred for 10 min. The organic phase was extracted and the excess EtOAc was removed under vaccum, the residue obtained was purified by column chromatography using hexane: ethyl acetate (3:7 v/v) as eluent to afford pure products 5 in good yield.
2.2 5-Benzyl-4-phenyl-spiro[2.3′]indenoquinoxalino-spiro[3.3′']-5′'benzylidenepiperidone-pyrrolidine, 5a
White color solid; 1H NMR (CDCl3, 400 MHz): δ/ppm 2.03 (d, J = 12.4 Hz, 1H), 2.85–2.90 (dd, J = 14.0, 7.2 Hz, 1H), 3.13–3.17 (m, 1H), 3.23–3.32 (m, 2H), 3.60 (d, J = 12.4 Hz, 1H), 4.58 (d, J = 11.2 Hz, 1H), 5.12–5.16 (m, 1H), 6.83–6.85 (m, 3H, ArH), 7.16–7.29 (m, 9H, ArH), 7.34–7.40 (m, 4H, ArH), 7.46–7.50 (m, 1H), 7.59–7.67 (m, 4H, ArH), 7.92 (d, J = 7.2 Hz, 1H), 8.00–8.02 (m, 1H), 8.14–8.16 (m, 1H, ArH); 13C NMR (CDCl3, 100 MHz): 39.3, 47.6, 50.0, 53.8, 61.5, 68.2, 73.1, 121.7, 121.8, 126.2, 126.9, 127.2, 128.1, 128.3, 128.4, 128.9, 129.0, 129.3, 129.7, 131.0, 134.7, 135.0, 136.6, 137.3, 138.2, 138.6, 140.6, 141.6, 148.4, 154.9, 166.2, 200.1; LC/MS(ESI): m/z = 611 (M+); Anal. Calcd for C42H34N4O: C, 82.60; H, 5.61; N, 9.17%; Found C, 82.71; H, 5.69; N, 9.24%.
3 Results and discussion
Recently, we have synthesized a small library of structurally unusual N-stryrylpiperidone tethered dispiropyrrolidine hybrid heterocycles 6 in good yields employing four component 1,3-dipolar cycloaddition cascade protocol (Almansour et al., 2019). Firstly, the cascade reaction sequence was carried out with an equimolar amount of 1,2-phenylenediamine 1, ninhydrin 2, l-phenylalanine 3 and arylidenepiperidone 4 under reflux in methanol, affording inseparable mixture of products on TLC analysis. Hence, the same reaction was attempted with different ratio of amino acid 3 (1 mmol to 1.75 mmol) and did not get fruitful results. The unusual N-styryl dispiropyrroldine hybrids 6 was obtained in moderate yield when two mole equivalents of amino acid 3 was employed. However, the reaction was optimized under the various solvent conditions including ionic liquids and product 6 was obtained in good yields in ionic liquids when compared to the other organic solvents. The present work describes the synthesis of dispiropyrrolidine tethered piperidone hybrid heterocycles 5. The previous reaction conditions and ratio of reactant as mentioned above kept in mind, the reaction was further investigated to get the desired product 5 instead of 6. In this context, the present work was performed with benzylidenepiperidone 4 (1 mmol) and non-stabilized 1,3-dipole component derived from quinoxalinone (1 mmol) and l-phenylalanine (0.5 mmol) under reflux in methanol, delightfully the expected dispiropyrrolidine hybrid heterocycles 5 afforded in moderate yield (Scheme 2). However, the starting substrate was not diminished even after prolonged reaction time. Further, the same four component reaction was attempted with 0.6 mmol equivalent of amino acid 3 and the product was obtained in a better yield. But the starting substrates were not completely disappeared even after several hours. Finally, the reaction was carried out with 0.75 mmol equivalent of l-phenylalanine and observed a substantial improvement in yield of product (55%). The four-component reaction was further investigated to improve the yield of product 5, in this direction the reaction was performed with 0.8–0.9 mmol equivalent of amino acid 3, the reaction leads to the formation of product 6 with inseparable mixture of the byproduct as evidenced by TLC analysis. As an alternative, the reaction was carried out with ionic liquid, [bmim]Br (Arumugam et al., 2019) and the product furnished in good yield. Thus, a mixture of o-phenylenediamine 1 (1 mmol), ninhydrin 2 (1 mmol), l-phenylalanine 3 (0.75 mmol) and dipolarophile 4 (1 mmol) were heated with constant stirring under [bmim]Br at 100 °C and the reaction progress has been monitored after every 10 min interval. After completion of the reaction, the mixture was diluted with ethyl acetate. The collected organic layer was removed under reduced pressure, the desired product 5 (65%) was obtained in good yields. The structurally complex dispiropyrrolidine hybrid heterocycles possess four adjacent stereocenters, out of which two are spirocarbons. In addition, the complex heterocycles possess two C—C bonds and three C—N bonds.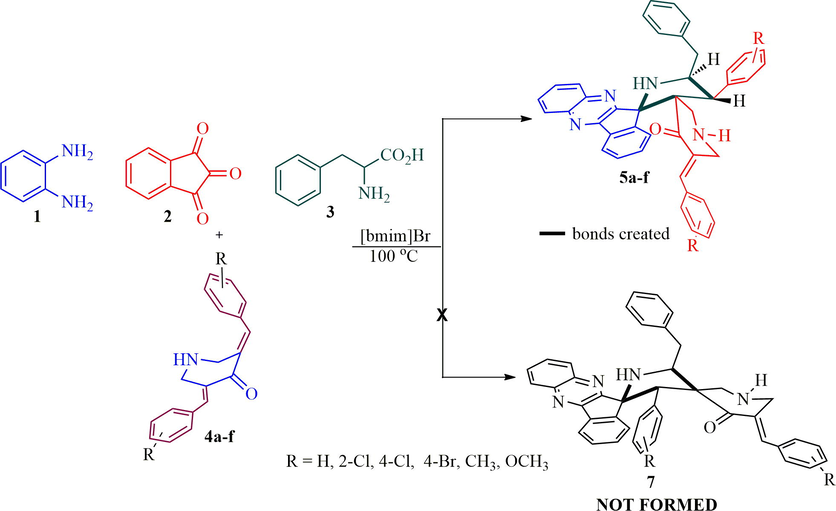
Synthesis of dispiroindenoquioxaline tethered piperidone hybrid heterocycles, 5.
The structure of all dispiropyrrolidine tethered piperidone hybrid heterocycles was characterized by 1H, 13C, mass spectroscopic and elemental analysis (Fig. 1). As a representative case, the structural assignment of 5a is described below. In its 1H NMR spectrum, the benzylic hydrogen (H-4) was assigned at δ 4.58 (J = 11.2 Hz) as doublet. The multiplet at δ 5.12–5.16 ppm was assigned to H-5 hydrogen of pyrrolidine ring. The doublet of doublets at δ 2.85–2.90 ppm and a multiplet at δ 3.13–3.17 were assignable to H-6 hydrogens. The two doublets at δ 3.60 and δ 2.03 ppm (J = 12.4 Hz) were belongs to piperidone H-2″ hydrogens and a multiplet at δ 3.23–3.32 ppm were assigned to H-6″ hydrogens of piperidone ring. All aromatic ring hydrogen appeared as multiplets from 6.83 to 8.16 ppm. The H-4 (J = 11.2 Hz) and H-5 hydrogens are trans to each other as evidenced by their coupling constant value. In its 13C NMR spectrum, the quinoxaline attached to pyrrolidine ring spirocarbon (C-2) resonated at δ 73.1 ppm and a signal at δ 68.2 ppm was assigned to piperidone ring attached spirocarbon (C-3″). The signals at 53.8 ppm and 61.5 ppm were assigned to C-4 and C-5 of pyrrolidine ring carbon, respectively. Similarly, the signals at δ 47.6 and δ 50.0 ppm were due to the C-2″ and C-6″ carbons of piperidone ring. The piperidone carbonyl carbon exhibited at δ 200.1 ppm. The structure of compound 5a was further confirmed by mass spectroscopic analysis.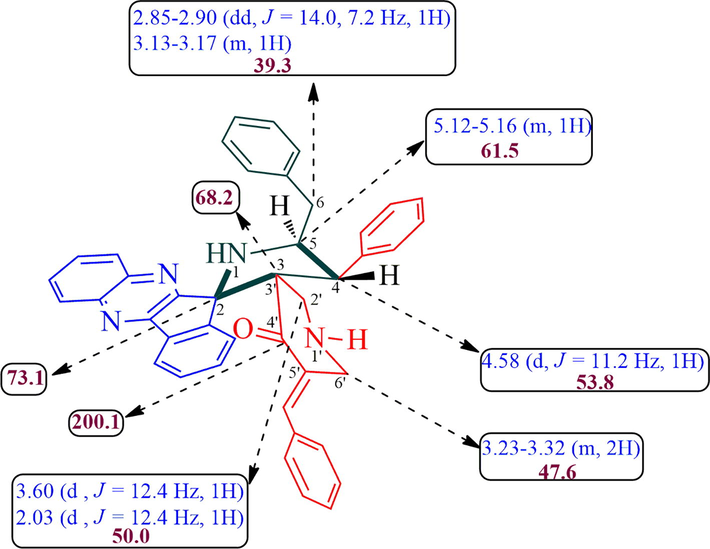
Selected chemical shift of 5a.
The other possible regioisomeric spiroheterocyclic hybrid 7 was not observed even in traces due to the possible secondary orbital interaction between 1,3-dipole component 11 and α, β-unsaturated exocyclic ketone of dipolarophile 4. In addition, the electron rich 1,3-dipole component 11 preferentially attacks electron deficient β-carbon of α, β-unsaturated dipolarophile to afford regioisomeric product 5. Further, we proved the formation of spiroheterocyclic hybrid 5 through computational investigation using energy minimization calculation (mm2) and found that compound 5 has lower energy 48.7470 kcal/mol when compared to other possible regioisomer 7 with higher energy 55.1475 kcal/mol, revealing that compound 5 is more stable than 7 as illustrated in Fig. 2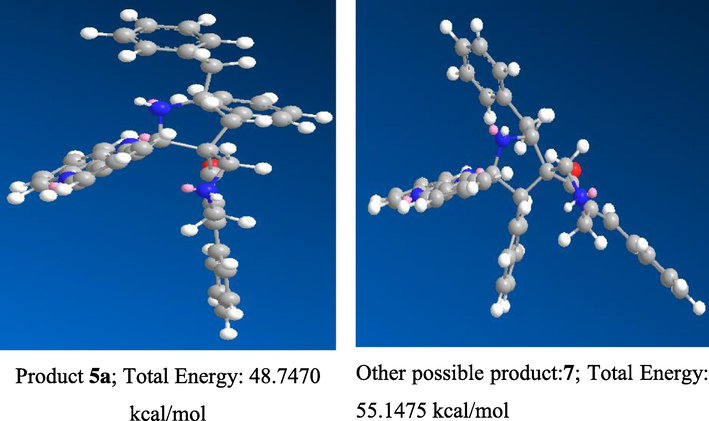
Energy mimization diagram of compound 5 and other possible regioisomer 7.
The four-component reaction presumably takes place via 1,3-dipolar cycloaddition sequence and reasonable mechanism for the construction of hybrid heterocycles 5a-f is demonstrated in Scheme 3. Initially, aryldiamine 1 and indene-1,2,3-trione 2 reacts with indeno[3,2-b]quinoxalin-11-one 7 with spontaneous elimination of two equivalents of H2O molecules. The indeno[3,2-b]quinoxalin-11-one intermediate 8 reacts with 2-amino-3-phenylpropanoic acid 4 to form spiro-oxazolidinone intermediate 10 via iminium component 9. The spiro-oxazolidinone 10 generates the highly reactive 1,3-dipole 11 via decarboxylative condensation. Simultaneously, the non-stabilized 1,3-dipole component of ylide 11 attacks one of the C⚌C bond of the electron deficient dipolarophile 4 by chemo and regioselectively furnishing the target heterocyclic hybrid 5. It is pertinent to note that ionic liquid, [bmim]Br play a vital role in the cycloaddition reaction sequence both as catalyst and solvent as described in Scheme 3. The electron deficient hydrogen of [bmim]br interact with the carbonyl unit of indene-1,2,3-trione 2, quinoxalinone 8, spiro-oxazolidinone 10 includes iminium intermediate 8 to form hydrogen bond that would increase the electrophilicity of the all components which further accelerated the nucleophilic attack of (i) o-phenylenediamine (ii) 2-amino-3-phenylpropanoic acid and (iii) formation of azomethine ylide via spiro-oxazolidinone intermediate (iv) formation of product via 1,3-dipole component and electron deficient dipolarophile (see Fig. 3).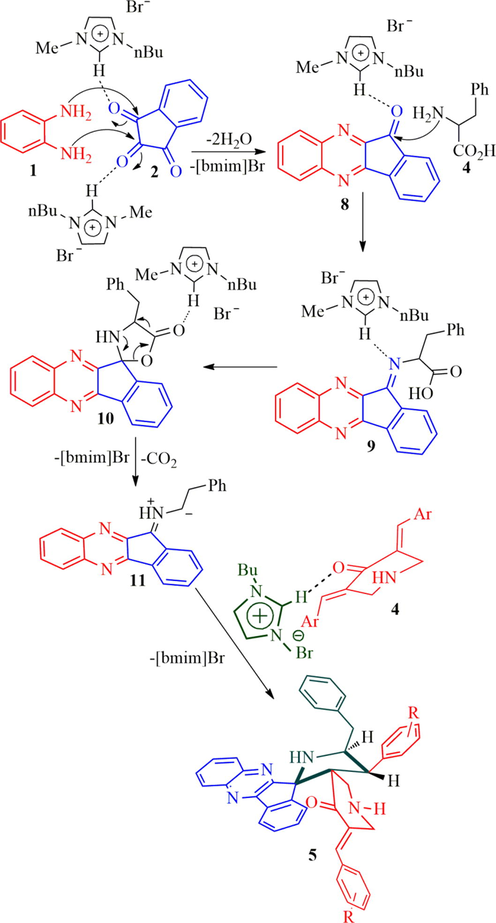
Plausible mechanism for the formation of dispiroindenoquinoxaline tethered piperidone heterocyclic hybrids, 5.
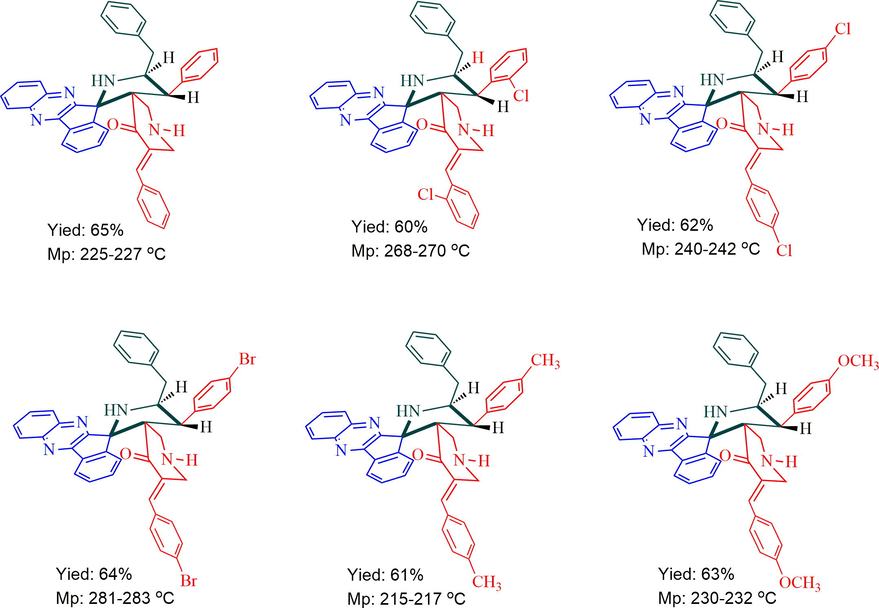
Synthesis of spiropyrrolidine-indenoquioxaline-piperidone hybrids heterocycles, 5a-f.
4 Conclusion
Hybrid heterocycles comprising spiroindenoquinoxaline, pyrrolidine and piperidone has been synthesized in good yields employing [bmim]Br assisted one pot four component [3 + 2] cycloaddition strategy. A relatively less explored non-stabilized 1,3-dipole component, the azomethine ylide derived from quinoxalinone and l-phenylalanine via decarboxylative condensation has been used. The formation of cycloadduct via two C—C and three C—N bonds in single synthetic transformation which possess four adjacent stereocenter, out of which two are spirocarbons. Further, the biological evaluation of these compounds will be published in due course.
Acknowledgement
The project was supported by Researchers Supporting Project number (RSP-2020/231), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis of tricyclic arylspiro compounds as potential Antileukemic and anticonvulsant agents. Heterocycles. 1979;12:637-640.
- [Google Scholar]
- Domino multicomponent approach for the synthesis of functionalized spiro-indeno[1,2-b]quinoxaline heterocyclic hybrids and their antimicrobial activity, synergistic effect and molecular docking simulation. Molecules. 2019;24:1962.
- [Google Scholar]
- Nouveaux alcaloïdes oxindoliques du Strychnos usambarensis GILG. Plant Med. Phytother.. 1978;12:123-129.
- [Google Scholar]
- Dispiropyrrolidinyl-piperidone embedded indeno[1,2-b] quinoxaline heterocyclic hybrids:Synthesis, cholinesterase inhibitory activity and their molecular docking simulation. Bioorg. Med. Chem.. 2019;27:2621-2628.
- [Google Scholar]
- Novel spirooxindole-pyrrolidine compounds: synthesis, anticancer and molecular docking studies. Eur. J. Med. Chem.. 2014;74:50-64.
- [Google Scholar]
- Imidazole derivatives of pyrrolidonic and piperidonic acids as potential inhibitors of human placental aromatase in vitro. J. Steroid Biochem. Mol. Biol.. 1996;57:73-77.
- [Google Scholar]
- Synthesis of novel spirooxindole derivatives by one pot multicomponent reaction and their antimicrobial activity. Eur. J. Med. Chem.. 2012;51:79-91.
- [Google Scholar]
- 1,3-Cycloaddition of nitrones in ionic liquids catalyzed by Er(III): an easy access to isozazolidines. Tetrahedron Lett.. 2007;48:7125-7128.
- [Google Scholar]
- Pyrroloquinoxaline derivatives as high-affinity and selective 5-HT3 receptor agonists: synthesis, further structure−activity relationships, and biological Studies. J. Med. Chem.. 1999;42:4362-4379.
- [Google Scholar]
- Neuroprotective effects of brimonidine treatment in a rodent model of ischemic optic neuropathy. Exp. Eye Res.. 2007;84:293-301.
- [Google Scholar]
- Pd-Catalyzed assembly of spirooxindole natural products: a short synthesis of horsfiline. J. Org. Chem.. 2010;75:5994-6000.
- [Google Scholar]
- 3, 5-Bis-arylidene-1-methyl-4-piperidone methohalides and related compounds with activity against L 1210 cells and DNA binding properties. Pharmazie. 1992;47:246-248.
- [Google Scholar]
- Cytotoxic evaluation of some 3, 5-diarylidene-4-piperidones and various related quaternary ammonium compounds and analogs. J. Pharm. Sci.. 1994;83:1124-1130.
- [Google Scholar]
- Singlet oxygen-trapping reaction as a method of 1O2 detection: role of some reducing agents. Free Rad. Res.. 1995;23:103-115.
- [Google Scholar]
- δ-Lactams: synthesis from D-glucose, and preliminiary evaluation as a fucosidase inhibitor, of L-fuconic-δ-lactam. 1988. J. Chem. Soc., Chem. Commun.. 1988;7:483-485.
- [Google Scholar]
- Practical synthesis of deoxymannojirimycin and mannonolactam from L-gulonolactone.: Synthesis of L-deoxymannojirimycin and L-mannonolactam from D-gulonolactone. Tetrahedron. 1989;45:319-326.
- [Google Scholar]
- Biomimetic transformations among monomeric macroline-related indole alkaloids. J. Am. Chem. Soc.. 1978;100:4213-4219.
- [Google Scholar]
- Synthesis and biological activities of pyrazolo [3, 4-g] quinoxaline derivatives. Eur. J. Med. Chem.. 2010;45:5520-5526.
- [Google Scholar]
- A dipolar cycloaddition approach toward the kopsifoline alkaloid framework. Tetrahedron. 2007;63:5962-5976.
- [Google Scholar]
- Stereoselective preparation of 1,2,4-oxadiazole derivatives substituted by pentafluorophenyl by 1,3-dipolar cycloaddition reaction. Tetrahedron. 2006;62:11008-11011.
- [Google Scholar]
- Pteropodine and isopteropodine positively modulate the function of rat muscarinic M1 and 5-HT2 receptors expressed in Xenopus oocyte. Eur. J. Pharmacol.. 2002;2002(444):39-45.
- [Google Scholar]
- Diastereoselective syntheses of pyrazolyl isoxazolidines via 1, 3-dipolar cycloaddition. Tetrahedron. 2007;63:10581-10586.
- [Google Scholar]
- Facile and diastereoselective synthesis of 3,2′-spiropyrrolidine-oxindoles derivatives, their molecular docking and antiproliferative activities. Bioorg. Med. Chem. Lett.. 2015;25:389-399.
- [Google Scholar]
- Synthesis and discovery of highly functionalized mono- and bis-spiro-pyrrolidines as potent cholinesterase enzyme inhibitors. Bioorg. Med. Chem. Lett.. 2014;24:1815-1819.
- [Google Scholar]
- Oxindole-3-spiropyrrolidines and -piperidines. Synthesis and local anesthetic activity. J. Med. Chem.. 1976;19:892-898.
- [Google Scholar]
- Facile transformation of 3, 4-disubstituted 2-azetidinones to chiral 5, 6-dihydro-2-pyridones. Tetrahedron Lett.. 2001;42:3483-3486.
- [Google Scholar]
- Synthesis and antiviral activities of adamantane spiro compounds. 1. Adamantane and analogous spiro-3'-pyrrolidines. J. Med. Chem.. 1972;15:129-132.
- [Google Scholar]
- Construction of spiro[pyrrolidine-3,3′-oxindoles] − recent applications to the synthesis of oxindole alkaloids. Eur. J. Org. Chem. 2003:2209-2219.
- [Google Scholar]
- Multi-component, 1,3-dipolar cycloaddition reactions for the chemo-, regio and stereoselective synthesis of novel hybrid spiroheterocycles in ionic liquid. Tetrahedron Lett.. 2012;53:5367-5371.
- [Google Scholar]
- Smoking cessation therapy with varenicline. Int. J. Chron. Obstruct. Pulmon. Dis.. 2008;3:239-251.
- [Google Scholar]
- Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry toward Heterocycles and Natural Products. New York: John Wiley & Sons; 2002.
- Antimycobacterial activity of spirooxindolo-pyrrolidine, pyrrolizine and pyrrolothiazole hybrids obtained by a three-component regio- and stereoselective 1,3-dipolar cycloaddition. Med. Chem. Commun.. 2011;2:626-630.
- [Google Scholar]
- Highly enantioselective intramolecular cynoamidation: (+)-horsfiline, (-)coerulescine, (-)-esermethole. Org. Lett.. 2010;12:952-955.
- [Google Scholar]
- A new entry of nucleophiles in rhodium-catalyzed asymmetric 1, 4-addition reactions: addition of organozinc reagents for the synthesis of 2-aryl-4-piperidones. J. Am. Chem. Soc.. 2004;126:6240—6241.
- [Google Scholar]
- Cu-mediated 1,3-dipolar cycloaddition of azomethine ylides with dipolarophiles: a faster access to spirooxindoles of potential pharmacological interest. Tetrahedron Lett.. 2013;54:5448-5452.
- [Google Scholar]
- Spiro indolinone beta-lactams, inhibitors of poliovirus and rhinovlrus 3C-proteinases. Tetrahedron Lett.. 1990;31:7277-7280.
- [Google Scholar]
- Asymmetric synthesis of febrifugine and isofebrifugine using yeast reduction. Chem. Commun. 2000:1643-1644.
- [Google Scholar]
- Assymetric syntheses of oxindole and indole spirocyclic alkaloids natural products. Synthesis 2009:3003-3025.
- [Google Scholar]
- Design of potential anti-HIV agents. 1. Mannosidase inhibitors. J. Med. Chem.. 1989;32:2084-2089.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.08.013.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







