Translate this page into:
Alpha-linolenic acid rich Allium porrum methanolic extract potentially inhibits HT-115 human colon cancer cells proliferation via mitochondria mediated apoptotic mechanism
⁎Corresponding author. aghedeir@KSU.EDU.SA (Ghedeir M. Alshammari)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Colon cancer is the third most devastating cancer type in morbidity and mortality associated with severe comorbidities in developing and developed countries. Dietary supplementation of alpha-linolenic acid has a beneficial effect on intestinal dysfunction. In the present study, we processed Allium porrum (Leek) for extraction and identified the bioactive metabolites with the potential of antiproliferative effect in HT-115 (human colon carcinoma) cells. We preliminarily analyzed the cytotoxic effects of methanol, chloroform, and hexane extracts of leek on HT-115 cells. In vitro cytotoxicity assay revealed that leek methanol extract (LME) significantly prohibited cell proliferation in a dose-as well as time-dependent manner, compared with hexane and chloroform extracts of leek. We found that LME at 0.4 and 0.2 μg/mL inhibited HT-115 cell proliferation by 50% at both 24 and 48 h. Cell morphological analysis and nuclear staining indicated that 22% of cells became apoptotic and ∼4% were necrotic after treatment with 0.4 μg/mL of LME. RT-PCR and western blot analysis showed that mRNA and protein expression levels of apoptotic markers such as p53, Bax, caspase-9, and caspase-3 were up-regulated, whereas Bcl-2 was down-regulated in cells exposed with 0.4 μg/mL LME, compared with the levels in untreated control cells. Lipid peroxidation marker (LPO) was significantly elevated in LME-treated cell lysates. The activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) were significantly decreased in LME-treated cell lysates when compared with control HT-115 cells. In conclusion, the observed results demonstrate that LME significantly inhibits colon cancer cell growth via the mitochondria mediated caspase-dependent apoptosis mechanism.
Keywords
Colon cancer
HT-115
Cell apoptosis
A. porrum
Methanol extract
Anticancer agent
- ANOVA
-
Analysis of variance
- AO
-
Acridine orange
- Et/Br
-
Ethidium bromide
- FBS
-
Fetal bovine serum
- LME
-
Leek methanol extract
- PI
-
Propidium iodide
- ROS
-
Reactive oxygen species
- SCR
-
Saudi Cancer Registry
- TBARS
-
Thiobarbituric acid-reactive substances
Abbreviations
1 Introduction
Mortality due to cancer remains high in both developed and developing countries, even though scientific advances have increased the human lifespan. Lifestyles are also changing globally which contributes to the progression of diseases. Recently, the incidence of colon cancer has increased because of changes in the diet system. This increased incidence has made colon cancer the third leading reason of mortality due to cancer (Siegel et al., 2012). World Health Organization (WHO) reported that 0.774 million mortality occurred due to colon cancer, among 8.8 million people who died due to different types of cancer (Arnold et al., 2017; Siegel et al., 2014). It was also reported that the number of cases of colon cancer globally expected to rise about 60% by the year 2030. Moreover, it was calculated that there would be around 2.2 million new patients affected annually and 1.1 million demises by 2030.
According to the Saudi Cancer Registry (SCR), colon cancer is the generally widespread cancer in the Saudi population. Further SCR reports state that its incidence does not differ significantly between the sexes, although its rate is ∼10% upper in males (∼62%) than in females (51%), which is lower than the levels globally (males 65%, females 77%). Since its incidence is high, colon cancer is also the most important reason for cancer-associated mortality in Saudi men and women (Al-Shahrani et al., 2017; Ibrahim et al., 2008). Recently, the range of tools for diagnosing colon cancer has increased, which has raised awareness about this condition among the population of Saudi Arabia (Khayyat and Ibrahim, 2014). However, the mortality rate is not altered, which indicates the need for chemopreventative rather than chemotherapeutic approaches to tackle this form of cancer. One of the best ways to move toward chemoprevention is to use phytochemicals because cancer develops through a multi-step process. Precisely, phytochemicals are thought to have multiple functions in cancer cells, including inhibition of cancer development and retarding and/or proliferation of cancer.
Furthermore, phytochemicals have fewer unfavorable side effects than man-made medicines. Indeed, among currently available cancer chemotherapy drugs, over 60% were initially derived from natural sources (Newman and Cragg, 2012). At present, various natural phytochemicals act through cancer cell mechanisms to inhibit or kill different proliferating cancer cells. These active natural compounds may be further modified to increase the intensity of the anticancer effect. Based on this approach, a wide range of anticancer compounds derived from natural products has accumulated (Cooper, 1993). Leek (Allium porrum) is a common vegetable used worldwide, which has a high number of bioactive constituents. It was previously reported that leek and its bioactive components exhibited several biological properties, including antiplatelet, antioxidant, antifungal, anti-hepatitis, and antiproliferative effects (Mikaili et al., 2013). Allium porrum contains high levels of sulphur compounds, which inhibit microbial growth by inhibiting the formation of para-amino benzoic acid, a key component in the synthesis of folic acid, which is required for the continuous development and multiplication of microbial cells. Antibacterial characteristics of Allicin has antibacterial properties make it efficient against a wide variety of bacteria. Allicin was found to be effective against multidrug-resistant E. coli strains (Ankri and Mirelman, 1999). Dietary uptake of leeks lowers serum triglycerides in hypercholesterolemia, may be having the beneficial effect on reducing the risk of prostate cancer, colorectal cancer, stomach cancer, and avoids neural tube defects and other disorders (Bianchini and Vainio, 2001; Hsing et al., 2002). However, in a literature review, until now no experimental proof has been available on the impact of leek extract on colon cancer cells. Since leek is a commonly consumed as a vegetable in traditional and continental food, we aimed to evaluate its effect on inhibition of colon cancer cells proliferation. Hence, in the current investigation, we assessed the antiproliferative effect of leek extract(s) on HT-115 cells via mitochondria mediated, caspase dependent apoptosis mechanisms.
2 Materials and methods
2.1 Chemicals and reagents
Cell culture plates, ethidium bromide (EB), acridine orange (AO), propidium iodide (PI), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), dimethylsulfoxide (DMSO), 4,6-diamidino-2-phenylindole (DAPI), fetal bovine serum (FBS) and Roswell Park Memorial Institute 1640 medium (RPMI-1640) were obtained from Sigma-Aldrich, St. Louis, MO, USA. The Fast Lane Cell cDNA kit and QuantiFast SYBR Green PCR kits were procured from Qiagen, Hilden, Germany. From Promega, Madison, WI, USA, TUNEL examine kit was obtained. All other chemicals and reagents utilized in the current investigation were of molecular biology research ranking.
2.2 Allium porrum L: collection and preparation of organic solvent extract
Fresh Allium porrum L. whole plants were collected in September 2020, Riyadh, Saudi Arabia. Leek (Allium porrum) aerial parts were sieved, milled, and grounded using a commercial blender. 500 gm of fresh leek was soaked in 1500 mL of n-hexane for 72 h with frequent shaking using a shaker. Leek hexane extract was filtered, condensed using a rotary evaporator, weighed, and stored at −20 °C until further use. The residue was again soaked with 1500 mL of chloroform and methanol for 72 h, respectively, as sequential extraction. The n-hexane, ethyl acetate, and methanolic extract of leek were filtered and stored at −20 °C until further use.
2.3 Gas-chromatography and mass spectroscopy analysis
The identification of metabolites in n-hexane, chloroform, and methanolic extract of leek was carried out by GC/MS method. Briefly, GC–MS analysis was performed on a PerkinElmer Clarus 600 GC System, fitted with a Rtx-5MS capillary column (30 m × 0.25 mm inner diameter, × 0.25 μm film thickness; maximum temperature, 350 °C), coupled to a Perkin Elmer Clarus 600C MS. Ultra-high purity helium (99.99%) was used as carrier gas at a constant flow rate of 1.0 mL/min. The injection, transfer line and ion source temperatures were fixed as 290 °C. The ionizing energy was 70 eV. Electron multiplier voltage was obtained from autotune. The oven temperature was programmed and held at 60 °C for 2 min, then continued until 280 °C at a rate of 3 °C/min. The crude samples were diluted with an appropriate solvent (1/100, v/v) and filtered. Using 0.22 µm syringe filter, the leek extract(s) filtered and diluted with methanol were taken in a syringe and injected into the injector with a split ratio 30:1. All data were obtained by collecting the full-scan mass spectra within the scan range 40–550 amu. The percentage composition of the leek extract constituents were expressed as a percentage by peak area (PerkinElmer Clarus, 600 GC System).
2.4 Cell culture
The HT-115 (human colon carcinoma) cell line was procured from the ATCC, Manassas, VA 20110 USA. The obtained cells were grown in a 5% CO2 (Thermo Scientific, USA) atmosphere at 37 °C with the supplementation of RPMI-1640 medium containing 10% FBS for regular subcultures and procedures to all additional experiments.
2.5 Assessment of in vitro cytotoxicity through MTT assay
In 96-well cell culture plates, HT-115 cells were plated at a concentration of 1 × 104 cells/well. Cell were maintained for 24 h in a normal RPMI-1640 medium. Then, the cells were treated with various doses of leek hexane, chloroform, and methanol extracts (0, 0.1, 0.2, 0.4, 0.8, 1.2, and 2.4 µg/mL) for 24 and 48 h. After the incubation, 20 µL of MTT solution at a prescribed amount of 5 mg/mL (in PBS) was added to all wells. This incubation was continued for 4 h at 37 °C in the dark (Mosmann, 1983). The purple formazan created was suspended by adding 100 μL of DMSO. A plate reader from Bio-Rad (CA, USA) was used to measure the absorbance at 630 and 570 nm. The viability of HT-115 cells exposed to hexane, chloroform, and methanol leek extracts is presented as the proportion of live cells when compared with untreated cells (control cells), which was obtained using the following formula: [(Mean OD of control cells - Mean OD of different treated cells)/(Mean OD of control cells)] × 100. From the initial observation, we obtained the IC50 of different leek extracts for 24 and 48 h. The IC50 of leek methanol extract (LME) was found to be lower than those for hexane and chloroform extracts. From these observations, LME was selected to understand the probable mechanism of action on HT-115 cells. The cell viability experiments were repetitive of triplicates.
2.6 Morphological studies by fluorescence microscopy
Control and LME-treated cells were fixed with ice-cold ethanol. Necrotic cells and apoptotic cells were discriminated through PI and AO/EtBr staining. To calculate the level of necrotic cells, cells were treated and fixed as mentioned above and stained with PI (1 mg/mL). After the incubation of cells for 15 min in the dark, unbound PI was removed through three washes. From ten different fields, ∼300 PI-stained cells were scrutinized under an inverted fluorescence microscope (200× magnification). The PI-negative and positive cells were calculated by an individual blinded to the experimental set-up. The experiment was repeated four times in triplicate.
AO/EtBr staining was used to examine typical apoptotic morphological alterations and unusual chromatin associations (Leite et al., 1999). Viable and nonviable cells could be differentiated through AO/EtBr staining. In detail, experimental cells were exposed to different doses of LME (0, 0.1, 0.2, and 0.4 µg/mL) for 48 h in a 24-well plate. After the treatment period, cells were washed once with PBS and exposed to staining solution for 2 min (500 µL of AO/EtBr). After the cells were rinsed with PBS and immediately observed under an inverted fluorescent microscope fixed with excitation (530 nm), and emission (620 nm) filters and images of the samples were captured (200x magnification). In the same way, nuclear damage (condensed) was observed through staining the different experimental cells with an appropriate nuclear stain.
2.7 Analysis of oxidative stress, apoptosis, and tumor suppressor responsive genes through quantitative PCR
After the treatment period, RNA was isolated from control and LME-treated cells. After confirming the purity of RNA, the cDNA was prepared from treated cells by using the commercially available kit mentioned in the materials section. The manufacturer’s instructions were precisely followed to prepare cDNA. Gene expression levels of two major tumor suppressor genes, namely, Bcl-2 (forward-GTGGATGACTGAGTACCT; reverse - CCAGGAGAAATCAAACAGAG) and p53 (forward – CCTCAGCATCTTATCCGAGTGG; reverse - TGGATGGTGGTACAGTCAGAGC); and three major apoptotic genes, namely, Bax (forward- TCAGGATGCGTCCACCAAGAAG; reverse- TGTGTCCACGGCGGCAATCATC), caspase-3 (forward- CCAGGAGAAATCAAACAGAG; reverse- AAGGACTCAAATTCTGTTGCCACC), and caspase-9 (forward- GCTCTTCCTTTGTTCATC; reverse- CTCTTCCTCCACTGTTCA) were analyzed. To compare the expression of the different genes against their respective control expression, the expression of a reference gene, GAPDH, was also measured. The manufacturer's protocol was followed step by step to generate a qPCR reaction in a volume of 25 μL. The Ct data were utilized to assay the expression levels of LME-treated and control HT-115 cells. The differences in gene expression patterns were assayed as described previously (Yuan et al., 2006). The relative expression levels of the different genes were assayed using the following equation: 2ΔΔCt (relative threshold) = ΔCt (experimental sample) × ΔCt (untreated control). Therefore, the different gene expression levels are presented as fold change compared with that of GAPDH. The calculated 2−ΔΔCt values were used to plot the differentially expressed genes.
2.8 Quantification of different apoptotic protein expression by western blot analysis
After exposing the HT-115 cells to LME or leaving them untreated for 48 h, they were washed once with PBS. By adopting the previously published protocol, Western blot assay was executed (Naveen Kumar et al., 2015). Immediately thereafter, cells were lysed with RIPA buffer, including protease cocktail inhibitor (15 min on ice). At 4 °C, the lysates were centrifuged at 8000 rpm for 20 min and clear supernatant was collected. By using the Bradford method (Bradford, 1976), the protein concentration was quantified. A total of 25 µg of protein under each experimental condition was separated by 12% SDS-PAGE. A semi-dry transfer unit (Hofer TE77XP) was used to transfer the separated proteins to a PVDF membrane. After rinsing the membrane with PBS, the nonspecific sites were blocked with 2% BSA in 1 × TBST for 2 h at room temperature. After blocking, the membranes were washed twice with PBS to get off surplus 2% BSA. The blocked PVDF membrane was incubated with primary antibodies: p53 and active caspase-9 (from Santa Cruz Biotechnologies) and Bax, Bcl-2, and active caspase-3 (from Cell Signaling Technology). The primary antibodies were diluted to a 1:1000 ratio and hatched all night at 4 °C. After this incubation period, they were washed three times with TBST. Subsequently, for 2 h, they were incubated with appropriate secondary antibodies at room temperature. These secondary antibodies were either IgG-horseradish peroxidase rabbit antimouse or IgG-horseradish peroxidase goat anti-rabbit (1:20,000 dilution). The protein expression was observed through a chemiluminescence method. For equal loading of different samples, the same membrane was stripped and incubated with β-actin (Santa Cruz Biotechnology). Finally, Image J software was used to quantify the protein intensity.
2.9 Assay of lipid peroxidation and antioxidant levels
After treatment with LME, the cells were homogenized in Tris-HCl buffer (pH 7.0), and the cell lysate total protein was calculated by the Bradford protocol (1976). Lipid peroxidation was calculated using the thiobarbituric acid-reactive substances (TBARS), where malondialdehyde served as a standard (Ohkawa et al., 1979). The activity of antioxidant enzymes was measured by previously described methods SOD (Marklund and Marklund, 1974), GPx (Carlberg and Mannervik, 1975), and catalase (Sinha, 1972).
2.10 Intracellular assay of reactive oxygen species (ROS)
Intracellular ROS generation was assayed as reported previously (Alshammari and Balakrishnan, 2019). In detail, cells were loaded with 2′,7′-dichlorohydrofluorescein diacetate (DCFH-DA). This nonfluorescent cell-permeable composite undergoes hydrolysis via cellular esterases to deliver dihydro-2′,7′-dichlorofluorescein. The fluorescent 2′,7′-dichlorofluorescein (DCF) forms through the auto-oxidation of dihydro-2′,7′-dichlorofluorescein by H2O2 present inside the cells. The fluorescence level of DCF in the cell extract was quantified at an excitation wavelength of 485 nm and an emission wavelength of 530 nm. To obtain the absolute ROS produced intracellularly, the normal cells without DCFH-DA were maintained, the data from which were considered as the background fluorescence intensity, with the same being subtracted in all cases.
2.11 Statistical analysis
The SPSS/28.5 software package was used to statistically evaluate the grouped data. The data were analyzed through a one-way analysis of variance (ANOVA) followed by Tukey’s test. All data are presented as the mean ± SD. p ≤ 0.01 and p ≤ 0.001were considered statistically significant for all comparisons.
3 Results
3.1 GC–MS based phytochemical profiling of leek methanol extract (LME)
In the present study, GC–MS analysis of LME has confirmed that the presence of major phytochemical components such as 74.42% peak area of 9, 12, 15 – Octadecatrienoic acid, 4.38% peak area of 9, 12 – Octadecadienoic acid, and 6% peak area of Cyclotrisiloxane group of compounds along with other 13 trace components. The results of GC–MS peak and list of components matched references with 90 % above in NIST01 & W8N08 library have been presented in the supplementary file (supplementary Fig. 1 and supplementary table 1).
3.2 In vitro cytotoxic effect of leek extracts on HT-115 cells
The effects of different leek extracts on the multiplication of human HT-115 cells are shown in Fig. 1. Concentration- and duration-dependent cytotoxic potentials of leek extract were observed in HT-115 cells vs. vehicle control (0 μg/mL). In the current investigation, significant inhibitory effects were noticed in the different leek extract cells. However, more inhibition was observed in LME-treated cells than for the hexane and chloroform extracts. Chloroform extract showed higher IC50 values (1.1 μg/mL at 24 h and 0.76 μg/mL at 48 h) than hexane (0.78 μg/mL at 24 h and 0.73 μg/mL at 48 h) and methanol extracts (0.4 μg/mL at 24 h and 0.2 μg/mL at 48 h). The IC50 value for LME was found to be minimal [0.4 μg/mL at 24 h (Fig. 1A) and 0.2 μg/mL at 48 h (Fig. 1B)] when compared with those of the other extracts. These results show that LME has a greater antiproliferative effect than the other two extracts. We thus selected LME for use in the subsequent studies.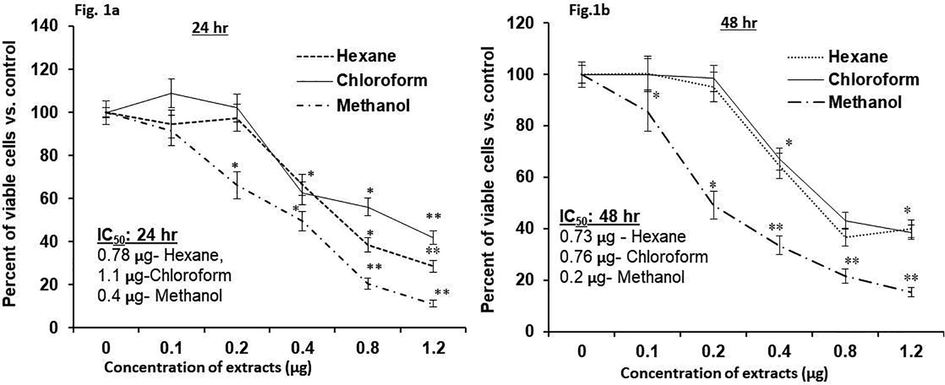
In vitro cytotoxic potential of hexane, chloroform, and methanol extracts of leek on HT-115 cells (Fig. 1a, 24 h; Fig. 1b, 48 h). The experiment was performed four times in triplicate. Outcome are articulated as mean ± S.D. (n = 6). *p ≤ 0.001 vs. control (0 μg is untreated control).
3.3 Induction of apoptosis by LME in HT-115 cells
LME-treated cells were scrutinized under a light microscope (Fig. 2-i). The phase contrast images indicated the morphological changes in LME-treated cells when compared with untreated cells (control). The PI- and AO/EtBr-stained cells were observed under a fluorescent microscope. In this case, the LME-exposed cells showed the typical features of apoptosis mediated cell death. They featured an abnormal horseshoe-shaped nucleus, along with a fragmented nucleus and condensed chromatin. These features represent the apoptotic mode of cell death because they indicate golgi complex linearization and endoplasmic reticulum stress (Fig. 2-i, 2-ii, and 2-iii).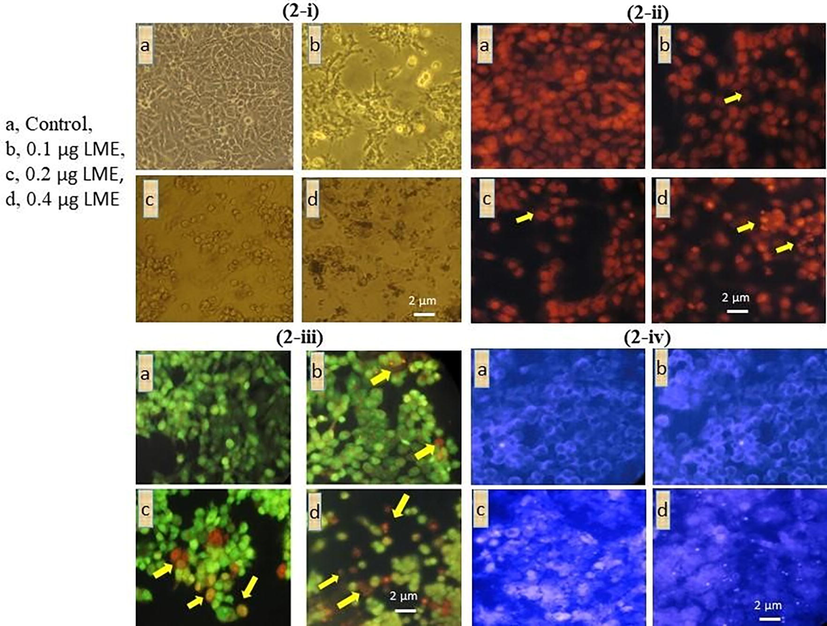
Cellular and nuclear damage analysis of LME in HT-115 cells using (2-i) light microscopy, (2-ii) propidium iodide staining, (2-iii) AO/EtBr staining and (2-iv) DAPI staining. a, control; b, 0.1 μg/mL LME; c, 0.2 μg/mL LME; d, 0.4 μg/mL LME. PI staining (200×) indicated abnormal nuclei, particularly featuring the disintegration of chromatin/nuclei and a horseshoe-shaped nucleus, which reflects cellular stress and cell apoptosis. Staining with AO/EtBr (200×) indicated the following findings were indicated by arrow heads: bright green, proapoptotic; light green, early apoptotic; orange, late apoptotic; and red, necrotic cells. In Fig. 2-iii, D indicates around 22% light green and orange staining pattern upon LME treatment representing early and late apoptotic cells. DAPI staining (200×) showed an abnormal and fragmented nucleus with bright florescence, which indicated nuclear damage and stress.
AO/EtBr staining of the HT-115 cells exposed to 0.4 μg/mL LME showed that ∼22% of the cells were apoptotic and ∼5% were necrotic. When the LME concentration was increased, the number of apoptotic cells also increased (p ≤ 0.001). Similarly, when the duration of LME increased, the quantity of cells undergoing apoptosis is elevated [lower dose (p ≤ 0.001) of 0.2 μg/mL vs. higher dose of 0.4 μg/mL at 48 h]. To investigate the relationship between different doses and durations of LME exposure, we observed the following changes: dark green, pre-apoptosis; light green, early apoptosis; orange, late apoptosis; and red, necrosis (Fig. 2-iii).
Furthermore, to distinguish the LME-induced apoptosis in HT-115 cells, they were exposed to 0.1, 0.2, and 0.4 μg/mL LME (IC50) for 48 h. Later than the treatment duration, cells were stained with the DNA binding dye DAPI (Fig. 2-iv) and observed by an individual blinded to the groups in this study. In the current investigation, no noteworthy cell death was identified at an LME concentration of 0.2 μg/mL (below). However, the IC50 concentration (0.4 μg/mL) of LME produced apoptotic changes similar to nuclear pyknosis. Chromatin condensation and asymmetrical boundaries were observed around the nucleus at 48 h of treatment (Fig. 3). On the other hand, untreated cells emerged with round, clearly circumscribed, and homogeneously stained nuclei.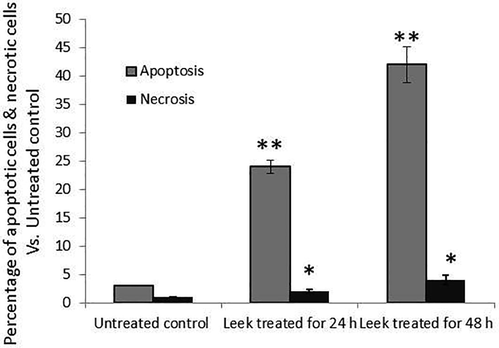
The percentage of apoptotic and necrotic cells in untreated control and LME-treated HT-115 cells, as revealed by the manual counting of propidium iodide staining. The experimentation was executed three times. Values are presented as mean ± S.D. (n = 4). * p ≤ 0.05 vs. control and** p ≤ 0.001 vs. control.
3.4 Effects of LME on mRNA expression levels of Bax, p53, Bcl-2, and caspases
We witnessed different expression patterns of apoptosis-associated genes in the untreated control, 0.2 and 0.4 μg/mL of LME (IC50) treated HT-115 cells after 48 h. Significant changes in the mRNA levels of Bax, Bcl-2, caspase-3, and caspase-9 were observed in the LME-treated and control HT-115 cells (Fig. 4). Twofold increase were found in the mRNA contents of Bax and caspase-3, while there was also a significant reduction (p ≤ 0.001) in the level of Bcl-2 expression in HT-115 cells exposed to 0.4 μg/mL LME compared with the level in healthy control HT-115 cells. The level of a key tumor suppressor gene, p53 was upregulated in the cells after suppressing mdm2. Approximately twofold elevation in the p53 expression level (p ≤ 0.001) was observed in 0.4 μg/mL LME-treated cells compared with the level of untreated cells.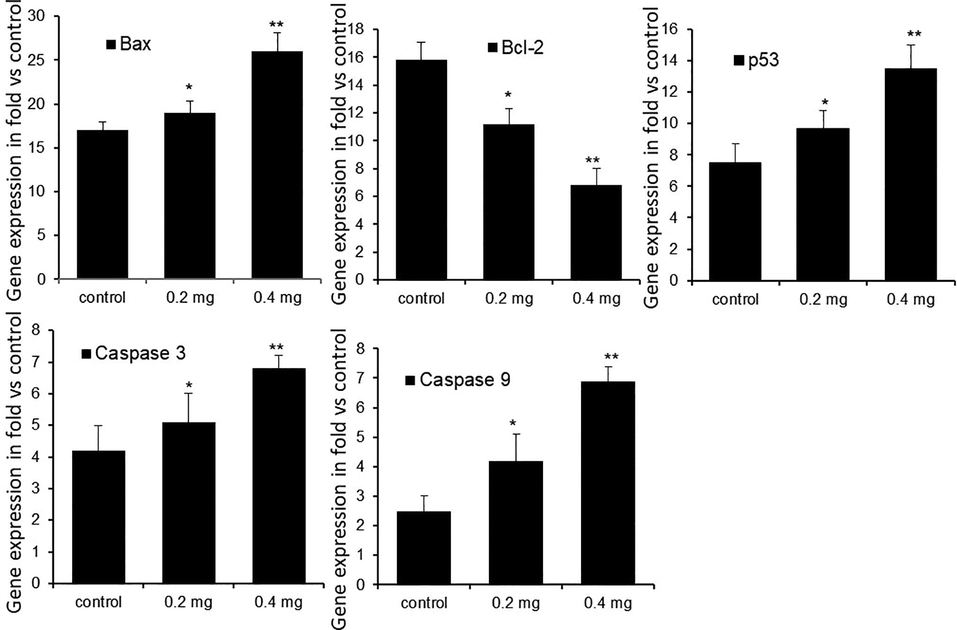
The mRNA expression levels of tumor suppressor and apoptosis related genes in control and LME-treated HT-115 cells after 48 h. Values are presented as mean ± S.D. (n = 4). *p ≤ 0.05 vs. control and **p ≤ 0.001 vs. control.
3.5 Protein expression of Bax, p53, Bcl-2, and caspases in LME-treated HT-115 cells
The protein contents of the apoptotic indicators p53, Bax, caspase-3, and caspase-9 and the anti-apoptotic marker Bcl-2 were assayed. As shown in Fig. 5, we observed a twofold increase of Bax and fourfold increases of p53, caspase-3, and caspase-9 proteins (p ≤ 0.05). In calculation, there was a significant (p ≤ 0.05) decrease in the Bcl-2 protein intensity with 0.4 μg/mL LME compared with that of control cells. The increases in the levels of apoptotic proteins appeared in a concentration-dependent mode for Bax, p53, caspase-3, and caspase-9, with the findings being significantly (p ≤ 0.001) lower even at 0.2 μg/mL LME when compared with 0.4 μg/mL LME at 48 h.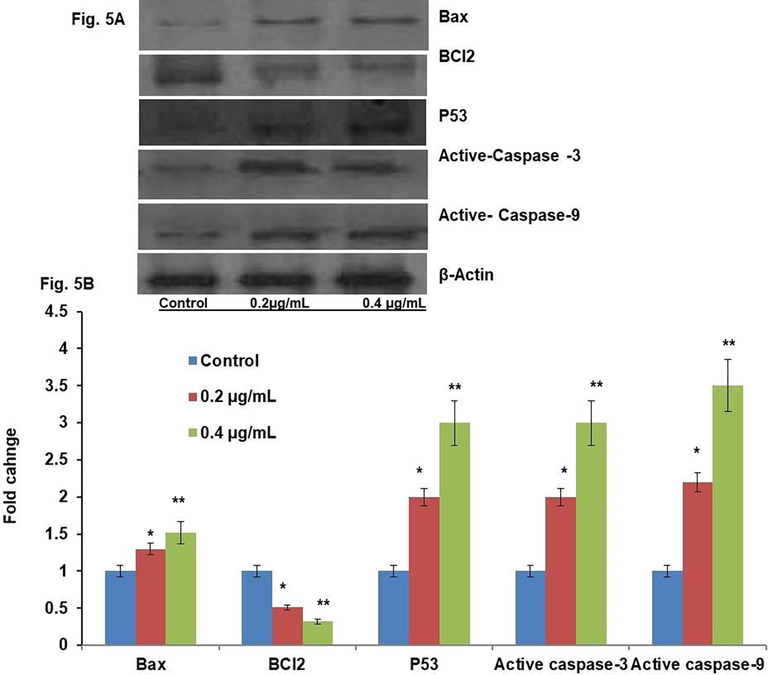
Western blot result for tumor suppressor and apoptotic pathways related protein levels of control and LME-treated HT-115 cells for 48 h. Values are presented as mean ± S.D. (n = 4). * p ≤ 0.05 vs. control and ** p ≤ 0.001 vs. control.
3.6 Lipid peroxidation and antioxidant status in LME-treated HT-115 cells
The levels of lipid peroxidation (LPO) and antioxidant activities of CAT, SOD, and GPx and the non-enzymatic antioxidant GSH were measured in LME-treated and untreated HT-115 cell lysates (Fig. 6). LPO was significantly elevated (p ≤ 0.001) in the cells exposed to 0.4 μg/mL LME, when compared with that of untreated cells (control). The levels of antioxidants such as SOD and GPx and non-enzymatic antioxidants such as GSH were significantly decreased (p ≤ 0.001) in cells exposed to 0.2 μg/mL LME, compared with the levels of untreated control HT-115 cells. More significant reductions were observed in cells exposed to 0.4 μg/mL LME than in control cells. Surprisingly, CAT activity did not show any statistically significant difference between LME and control HT-115 cells. The increase in lipid peroxidation and decreased antioxidant activities appeared in dose-dependent manners, except for the case of CAT. The lower dose (0.2 μg/mL) used in this study showed a significant difference in antioxidant changes than the higher concentration (0.4 μg/mL) at 48 h.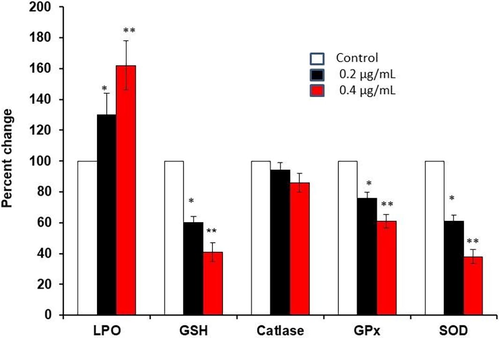
Lipid peroxidation, GSH level and antioxidant (catalase, GPx, SOD) enzymes activities in control and LME-treated human HT-115 colon cancer cells for 48 h. Values are presented as the mean ± S.D. (n = 5). *p ≤ 0.05 vs. control and **p ≤ 0.001 vs. control.
3.7 ROS in control and LME-treated HT-115 cells
As we observed significant differences in antioxidant status in untreated (control) and LME-treated cells (Fig. 7), we tested whether these differences in antioxidant status are due to the elevation of intracellular ROS in LME-treated cells. The change in ROS level was statistically significant even in the cells exposed to a low dose of LME. The levels were increased further in the cells treated with a higher dose of LME. These findings clearly show that LME could prohibit the multiplication of cancer cells through the elevation of intracellular ROS.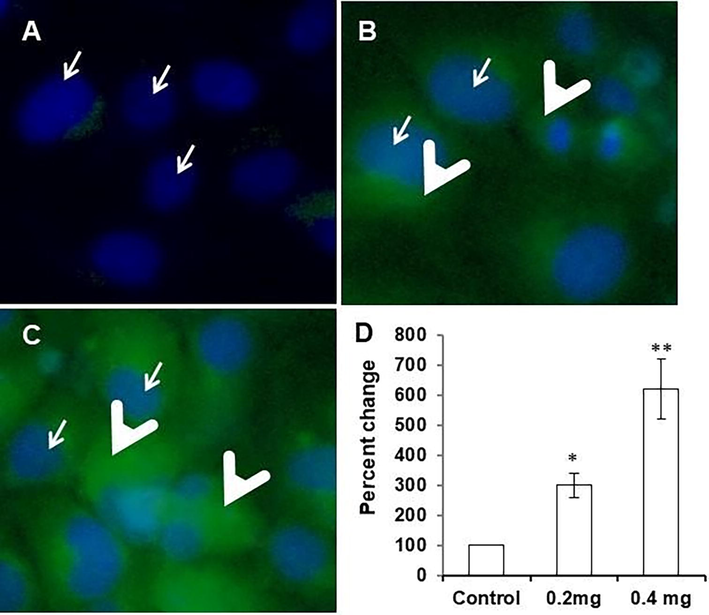
Level of intracellular reactive oxygen species in control (A) and LME-treated (B, 0.2 μg/mL & C, 0.4 μg/mL) human HT-115 colon cancer cells for 48 h. Outcomes are presented as mean ± S.D (n = 4). *p ≤ 0.05 vs. control and ** p ≤ 0.001 vs. control.
4 Discussion
Phytochemicals are known to have multiple effects in biological systems to serve as the starting materials for various synthetic drugs. However, cancers such as colon cancer develop through multi-step processes, so targeting these diseases with a single molecule may not be particularly beneficial. Monotherapies typically have just a single mode of action. In contrast, plant extracts containing various bioactive compounds may be the optimal choice for treating cancers (Xu et al., 2009). Some anticancer drugs have been withdrawn from the usage because of its toxicity to normal cells. Against this background, crude plant extracts considered to have both cytotoxic and noncytotoxic components should be effective via synergistic and antagonistic actions in cancerous and noncancerous cells (Ryu and Chung, 2015). Thus, in the current investigation, we examined the effects of leek extracts against colon cancer.
Cell proliferation and differentiation are of paramount importance for the growth, development, and regeneration of eukaryotic organisms. In the case of normal growth of an organism, this is tightly regulated. In contrast, excessively proliferating and undifferentiated cells are considered to be cancerous (Diaz-Moralli et al., 2013). Cytotoxic assays are commonly used to detect the ability of a given compound or extract to induce cell death. Even though this is the broad spectrum specificity, but a primary assay to know whether a given extract has anticancer property or not. In this study, the cytotoxicity of leek extracts against HT-115 cells was studied through an MTT cell proliferation experiment. Cells were exposed to media containing various leek extracts at concentrations of 0, 0.1, 0.2, 0.4, 0.8, and 1.2 μg/mL. The associated antiproliferative effects of the leek extracts were analyzed at 24 and 48 h. All the three extracts were found to inhibit the proliferation of HT-115 cells in a dose and time dependent manner. However, LME was found to be more effective, with a low IC50 value. The results of the present investigation are in agreement with in vitro studies by different investigators. If a plant extract inhibits cell growth, this suggests that that extract contains pharmacologically active substances (Chadalapaka et al., 2008; Imai et al., 2009; Palanivel et al., 2013). Exposure for a short duration such as 24 h requires a high concentration of LME to induce 50% cell death, while a longer period (48 h) is required with lower concentrations of LME.
Many approaches are available to detect apoptotic cell death in situ and in vitro. Double staining methods for detecting apoptosis (AO/EtBr and PI) provide consistent and reproducible observations. As reported by different investigators, apoptotic cells can be clearly visualized with fluorescent compounds such as AO/EtBr. This method is accepted as a suitable experimental procedure for assessing differences in nuclear morphology (Gasiorowski et al., 2001; Savitskiy et al., 2003). In the current investigation, AO/EtBr staining of LME-treated cells showed different cytological modifications such as binucleation, chromatin fragmentation, vacuolation, and plasma membrane blebbing. These observations indicate that LME inhibits cancer cell proliferation through apoptosis-mediated cell death. We assayed lactate dehydrogenase activity in the medium of LME-treated cells. Such cells did not demonstrate any statistically significant divergence in such activity when compared with untreated cells. This finding confirms that LME-treated cells undergo apoptosis-mediated cell death.
The Bcl-2 genes encode a family of proteins known to modulate apoptosis. In normal cells, Bcl-2 serves as a cyto-protector by acting as an intracellular down regulator of apoptosis (Lin et al., 2000), which functions by heterodimerizing with its pro-apoptotic relative Bax (Oltval et al., 1993). The ratio of Bcl-2 to Bax is connected with the vulnerability or resistance of a cell to apoptotic motivation (Oltvai and Korsmeyer, 1994). In the current study, we noticed that the exposure of cells to LME lowered the Bcl-2 level while elevating Bax. Therefore, we concluded that LME-induced apoptosis might be initiated by suppressing the Bcl-2 and Bax ratio. We also observed that the p53 protein content was elevated, whereas the Bcl-2 protein concentration was lowered in LME-treated cells compared with the levels in normal cells. It was also observed that a decreased Bcl-2 level led to a p53-associated increase in Bax content. In summary, a pro-apoptotic or anti-apoptotic mechanism in LME-treated cells may result from the collaboration between Bcl-2- and p53-associated pathways.
Apoptosis is triggered by either an intracellular or an extracellular pathway. In both of these pathways, the family of proteases called the caspases is involved. These proteases are synthesized in latent form and stored intracellularly as pro-enzymes. Conversion of these proenzymes to active enzymes is tightly regulated. Based on the caspase proteases involved, the apoptotic pathway is divided into caspase-3-dependent and independent pathways (Green and Amarante-Mendes, 1998). The current outcome shows that LME enhanced apoptosis by promoting the commencement of caspase-3 and caspase-9. In addition, alteration in the ratio of Bax to Bcl-2 initiated the caspase signaling mechanism. Overall, in this study, LME-treated cells exhibited dysregulation of the ratio of Bax to Bcl-2, ensuing in the commencement of caspase-3 and caspase-9.
ROS are important intracellular signaling molecules regulating a wide range of metabolic processes. When ROS levels become too high, this is detrimental to cells. Therefore, the level of ROS inside and outside cells is tightly regulated by a group of molecules named antioxidants.
Decreased antioxidant defense systems in the cell lead to oxidative stress (Roessner et al., 2008). One way or another, oxidative stress is responsible for the development of cancer and cancer treatment. We noticed that LME-treated cells showed an increased LPO level compared with control cells in the current investigation. Furthermore, the concentration of LME and the duration of LME treatment were directly proportional to the LPO level. On the other hand, enzymatic antioxidants such as SOD, CAT, and GPx were decreased in LME-treated cells. Decreased activities of antioxidant enzyme pilot to a further increase in intracellular ROS, leading to cell death. Such decreased activities can lead to various harmful properties payable to the gathering of H2O2 and superoxide radicals in the mitochondria. Increased mitochondrial ROS prompt cells to undergo mitochondria-mediated apoptosis in LME-treated cells. LME could have anticancer effects by decreasing antioxidant defense. These effects could be connected to the prevention of cell propagation, initiation of cancer cell death, and modulation of the contents of oxidative stress indicators. It was also observed that LME treatment could enhance intracellular ROS. The current observations align with the findings of various other research groups (Green and Kroemer, 2004; Juan et al., 2008; Kerr et al., 1994).
5 Conclusion
The present observations indicate that the availability of major portion such as 74.42% of 9, 12, 15 – Octadecatrienoic acid in LME extract, potentially stimulate the antiproliferation mechanism against HT-115 cells. Also the mechanistic effect of this antiproliferative effect have found to be mitochondrial mediated caspase dependent apoptotic mechanisms. Although further in vivo studies are warranted, since leek is an edible vegetable, higher consumption could be recommended in different populations to reduce the incidence of colon cancer.
Funding
This work was supported by the Researchers’ Support Project [grant number RSP2021/84], King Saud University, Riyadh, Kingdom of Saudi Arabia.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Al-Shahrani, Z.S., Al-Rawaji, A.I., Al-Madouj, A.N., Hayder, M.S., 2017. Cancer Incidence Report, Saudi Arabia 2014, Saudi Cancer Registry.
- Pumpkin (Cucurbita ficifolia Bouche) extract attenuate the adipogenesis in human mesenchymal stem cells by controlling adipogenic gene expression. Saudi J. Biol. Sci.. 2019;26:744-751.
- [CrossRef] [Google Scholar]
- Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691.
- [CrossRef] [Google Scholar]
- Allium vegetables and organosulfur compounds: do they help prevent cancer? Environ. Health Perspect.. 2001;109:893-902.
- [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [CrossRef] [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem.. 1975;250:5475-5480.
- [Google Scholar]
- Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res.. 2008;68:5345-5354.
- [CrossRef] [Google Scholar]
- The Cancer Book (First edition). USA: Jones & Bartlett Learning; 1993.
- Targeting cell cycle regulation in cancer therapy. Pharmacol. Therapeut.. 2013;138:255-271.
- [CrossRef] [Google Scholar]
- A comparison of the methods applied to detect apoptosis in genotoxically-damaged lymphocytes cultured in the presence of four antimutagens. Cell. Mol. Biol. Lett.. 2001;6:141-159.
- [Google Scholar]
- The point of no return: mitochondria, caspases, and the commitment to cell death. Results Probl. Cell Differ.. 1998;24:45-61.
- [Google Scholar]
- The pathophysiology of mitochondrial cell death. Science. 2004;305:626-629.
- [CrossRef] [Google Scholar]
- Allium vegetables and risk of prostate cancer: a population-based study. J. Natl Cancer Inst.. 2002;94:1648-1651.
- [Google Scholar]
- Past, present and future of colorectal cancer in the Kingdom of Saudi Arabia. Saudi J Gastroenterol.. 2008;14:178-182.
- [CrossRef] [Google Scholar]
- Cytotoxic effects of flavonoids against a human colon cancer derived cell line, COLO 201: a potential natural anticancer substance. Cancer Lett.. 2009;276:74-80.
- [CrossRef] [Google Scholar]
- Resveratrol induces apoptosis through ROS-dependent mitochondria pathway in HT-29 human colorectal carcinoma cells. J. Agr. Food. Chem.. 2008;56:4813-4818.
- [CrossRef] [Google Scholar]
- Apoptosis - its significance in cancer and cancer-therapy. Cancer. 1994;73:2013-2026.
- [CrossRef] [Google Scholar]
- Public awareness of colon cancer screening among the general population: a study from the Western Region of Saudi Arabia. Qatar. Med. J.. 2014;2014:17-24.
- [CrossRef] [Google Scholar]
- Critical evaluation of techniques to detect and measure cell death–study in a model of UV radiation of the leukaemic cell line HL60. Anal. Cell. Pathol.. 1999;19:139-151.
- [CrossRef] [Google Scholar]
- Antioxidant and hepatoprotective effects of Anoectochilus formosanus and Gynostemma pentaphyllum. Am. J. Chinese. Med.. 2000;28:87-96.
- [CrossRef] [Google Scholar]
- Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem.. 1974;47:469-474.
- [CrossRef] [Google Scholar]
- Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iran. J. Basic. Med. Sci.. 2013;16:1031-1048.
- [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55-63.
- [CrossRef] [Google Scholar]
- Unconjugated bilirubin exerts pro-apoptotic effect on platelets via p38-MAPK activation. Sci. Rep.. 2015;5
- [CrossRef] [Google Scholar]
- Natural products as sources of new drugs over the 30 Years from 1981 to 2010. J. Nat. Prod.. 2012;75:311-335.
- [CrossRef] [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [CrossRef] [Google Scholar]
- Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell. 1993;74:609-619.
- [CrossRef] [Google Scholar]
- Verrucarin A, a protein synthesis inhibitor, induces growth inhibition and apoptosis in breast cancer cell lines MDA-MB-231 and T47D. Biotechnol. Lett.. 2013;35:1395-1403.
- [CrossRef] [Google Scholar]
- Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol. – Res. Practice. 2008;204:511-524.
- [CrossRef] [Google Scholar]
- [10]-Gingerol induces mitochondrial apoptosis through activation of MAPK pathway in HCT116 human colon cancer cells. In Vitro Cell Dev-An.. 2015;51:92-101.
- [CrossRef] [Google Scholar]
- Comparative measurement of spontaneous apoptosis in pediatric acute leukemia by different techniques. Cytom Part B-Clin Cy. 2003;56B:16-22.
- [CrossRef] [Google Scholar]
- Siegel, R., DeSantis, C., Jemal, A., 2014. Colorectal cancer statistics, 2014. CA: A Cancer Journal for Clinicians. 64, 104-117. 10.3322/caac.21220.
- Cancer statistics for Hispanics/Latinos, 2012. CaCancer. J. Clin.. 2012;62:283-298.
- [CrossRef] [Google Scholar]
- Corosolic acid induces apoptosis through mitochondrial pathway and caspases activation in human cervix adenocarcinoma HeLa cells. Cancer Lett.. 2009;284:229-237.
- [CrossRef] [Google Scholar]
- Statistical analysis of real-time PCR data. Bmc Bioinformatics. 2006;7:85.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101736.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







