Translate this page into:
Adaptive response to ionizing radiation and the role of vitamin B12 in amelioration radiation protection standards
⁎Corresponding author. Address: Zoology Department, Faculty of Science, King Saud University, P.O. Box 22452, Riyadh 11495, Saudi Arabia. Tel./fax: +966 14767296. o_elhabit@yahoo.com (Ola H.M. El-Habit)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 11 July 2010
Peer-review under responsibility of King Saud University.
Abstract
This work was carried out to study the protective role of vitamin B12 on the induced cytotoxicity and genotoxicity of ionizing radiation on male mice, as assessed using micronucleus technique and by analyzing the morphological abnormalities of spermatozoa.
Male mice were injected intramuscularly with vitamin B12, at a dose of 0.8 mg/kg body weight, 1 h prior to a whole body exposure to 2 Gy gamma irradiation, followed by 2 Gy as a challenged dose. Animals were sacrificed 6, 24, and 72 h post-irradiation. The frequency of micronucleated polychromatic erythrocytes (PCEs) and normochromatic erythrocytes (NCEs); the ratio of PCEs/NCEs; and the mitotic index (MI) of bone marrow cells and sperm, head, and tail abnormalities were evaluated. Administration of vitamin B12 pre-irradiation resulted in a significant inhibition in the frequency of radiation-induced MNPCE and MNNCE as well as in the ratio of PCEs/NCEs and MI of bone marrow cells and a significant amelioration in sperm head and tail abnormalities.
The results suggested that vitamin B12 in a dose of 0.8 mg/kg body weight plays a protective role when administered prior to irradiation.
Keywords
Genotoxicity
Maximum tolerated dose
Micronucleus assay
Ionizing radiation
Vitamin B12
1 Introduction
Dietary vitamin supplementation is an emerging trend, with the consumption of vitamins dramatically increasing. This increase can be attributed partially to an increased awareness of the function of micronutrients in the body and the role they appear to have in maintaining general health and treating disease. Supplements are widely available, and marketing of vitamins has developed into a considerable consumer industry largely driven by the media (Rowe and Toner, 2008; Woodside et al., 2005). A large number of micronutrients (vitamins, essential minerals and other compounds required in small amounts for normal metabolism) are required in the human diet (Saltman et al., 1993).
Folic acid and vitamin B12 play an important role in DNA metabolism (Eto and Krumdieck, 1986). Folic acid and vitamin B12 are also required for the synthesis of methionine and S-adenosyl methionine, the common methyl donor required for the maintenance of methylation patterns in DNA that determine gene expression and DNA conformation (Eto and Krumdieck, 1986). Deficiencies in folic acid and vitamin B12 therefore lead to elevated DNA damage rate and altered methylation of DNA, both of which are important risk factors for cancer, and an increased level in homocysteine (HC) status is an important risk factor for cardiovascular diseases (Zingg and Jones, 1997).These same defects may also play an important role in development and lead to neurological abnormalities. The blood levels of folate and vitamin B12 required to prevent anemia and hyperhomocysteinemia are properly defined, however, it is still uncertain whether such accepted levels of sufficiency are in fact adequate to minimize chromosome damage rate and optimize DNA methylation status (Wilson and Jones, 1983). A series of studies to investigate the interrelationship between DNA damage in somatic cells and blood status for folate, vitamin B12, and HC were carried out (Fenech, 2003).
The importance of identifying dietary factors that minimize DNA damage rate is underscored by recent evidence from two epidemiological prospective studies indicating that a reduced level of chromosome damage in lymphocytes is a relevant biomarker of reduced future cancer risk, (Fenech et al., 1998; Hagmar et al., 1994). Most studies have considered the combined effect of both folic acid and vitamin B12, while the sole effect of vitamin B12 has been addressed in a few epidemiological studies on men. Preliminary studies in young men (Fenech et al., 1998) indicated that there was a significant negative correlation between the micronucleus frequency in lymphocytes and plasma vitamin B12 status. Results from studies in men aged 50–70 years (Bonassi et al., 1995) have shown that the micronucleus index correlates negatively with vitamin B12 in subjects who are not vitamin B12 deficient and that the micronucleus index is significantly and positively correlated with plasma HC status in men who are not folate or vitamin B12 deficient. These studies suggested that the plasma levels of HC and vitamin B12 that correspond to the minimization of chromosome damage require better definition. The first study reporting the interrelationship of chromosome damage rate, DNA methylation, HC, folate and vitamin B12 status in young Australians was reported (Fenech et al., 1998). No experimentally controlled study on the protective role of vitamin B12 has been reported. The mammalian in vivo micronucleus assay is widely used as part of the genotoxicity testing battery required during the development of new drugs (Fenech, 2000; Wyrobek et al., 1984).
Sperms are important target cells in reproductive toxicology for the assessment of spermatogenic damage, fertility and heritable genetic mutations (Krzanowska, 1976; Adler, 1977). Although not widely used in mutagenicity testing, the sperm morphology test proves to be a sensitive one. Sperm tests have also been used to study chemically induced sperm – mutagenic dysfunction in other mammalian species, including humans (Adler, 1977).
Two lines of evidence suggest that the induced changes in sperm morphology reflect genetic damage in male germ cell. First, considerable evidence indicates that sperm shaping is polygenetically controlled by numerous autosomal and sex-linked abnormalities. Second, all mouse germ cell mutagens (including ionizing radiations) so far tested in the mouse sperm morphology test showed positive responses. This observation is based on comparison of the ability of chemical agents to induce abnormal-shaped sperm besides inducing dominant lethal, heritable translocations, and specific locus mutations (Bruce and Heddle, 1979).
This study was carried out to evaluate the role of vitamin B12 on radiation-induced cytotoxicity and genotoxicity.
2 Materials and methods
2.1 Experimental animals
Swiss albino mice, strain SWR/J, were used in these studies. Adult Swiss albino male mice were obtained from the Central Animal House of King Saud University (Womens Branch – Malaz, Riyadh, KSA). The animals were maintained in hygienic conditions with good ventilation and under normal temperatures and humidity. Food and water were provided ad libitum. Male mice were used in order to avoid hormonal interference in females during the different stages of the estrous cycle. All the animals were from 10 to 12 weeks of age with an approximate body weight of 25 g, and were randomly selected for experimental and control series.
Animals were divided into six groups, each of 20 mice. Group 1: animals served as control, received no treatment. Group 2: animals received a whole body exposure of gamma rays at a dose of 2.0 Gy. Group 3: animals were injected i.m. with a single dose of vitamin B12 (Vit. B12) (0.8 mg/kg body weight). Group 4: animals were injected i.m. with a single dose of Vit. B12, one hour before exposure to a dose of 2.0 Gy of gamma radiation. Group 5: animals were exposed to an initial dose of 2 Gy gamma radiation followed after 6.0 h with a second challenge dose of 2.0 Gy. Group 6: animals were treated in the same way as group 5 except for being injected i.m. with Vit. B12 (0.8 mg/kg body weight) 1 h. before the second challenging dose of gamma radiation.
Mice (n = 5) were sacrificed (0 h) 1 h after the first dose of radiation and 6, 24 and 72 h post-challenge dose of radiation or post-treatment with vitamin B12.
Irradiation was carried out using Cobalt-60 source (Gamma cell 220-Nordion International Inc., Kanata, Canada) at Research Center of King Saud University, Faculty of Science, Riyadh, KSA.
Each animal received a whole body exposure of 2 Gy and an additional 2 Gy (challenged dose) at a dose rate of 0.667 Gy/min.
Both femurs were removed and the bone marrow was extracted for cytogenetic analysis and sperm samples for sperm head and tail abnormalities.
2.2 Vitamin B12 toxicity
Vitamin B12 was purchased from sigma and dissolved in saline and given to the animals by intramuscular injection.
Five groups of male mice, each consisting of 10 animals, were used to assess the toxicity of Vit. B12. One group served as control and the other groups each received one of the following doses of Vit. B12: 0.8, 1.2, 1.6 or 1.8 mg/kg body weight injected i.m. daily for three consecutive weeks. The animals were observed for any signs of health deterioration or mortality and the results were recorded.
2.3 Cytogenetic analysis (micronucleus MN assay) (Schmid, 1975, 1982; Heddle et al., 1983)
Bone marrow smears were prepared and allowed to dry overnight. Differential staining to distinguish polychromatic erythrocytes (PCE) and normochromatic erythrocytes (NCE) with May-Gruenwald and Giemsa stains was preformed.
The polychromatic erythrocytes (PCEs) stained bluish purple due to their high content of RNA in the cytoplasm. The normochromatic erythrocytes (NCEs) stained orange-yellow and were slightly smaller than PCEs. The micronuclei (MNs) were recognized as deep purple-stained bodies in the cytoplasm.
Five hundred PCEs and the number of MNPCEs were counted for each individual mouse using 100× (oil immersion). The number of NCEs was also counted in the fields of recorded PCEs and scored for MNs and the ratio of PCE/NCE was determined. The data from individual animals were reported as presented in Figs. 3–5.
2.4 Morphological abnormalities of spermatozoa (Wyrobek et al., 1984; Krzanowska, 1976; Wyrobek and Bruce, 1975)
This experiment was carried out to evaluate the effect of vitamin B12 and gamma radiation on the morphological changes of sperm head and tail abnormalities.
2.4.1 Slide preparation and staining
Slides were made by placing one drop of the solutions on a slide and spreading by three passes of another slide and then they were air dried. Then, the slides were stained with 1% Eosin (Sigma) for 10–15 min and differentiated in distilled water.
2.4.2 Microscopic analysis and data recording
One thousand spermatozoa from each animal were counted from different fields. The percentages of abnormal sperm were calculated. Sperms with abnormal morphology were categorized into sperm with head and tail abnormality. The data from each individual animal were reported and tabulated.
2.5 Statistical analysis of data
Student’s t-test was applied for the statistical analysis of the cytogenetic results (Byrkit, 1980).
3 Results
3.1 Lethality and toxicity studies
These studies determined the optimum dose of vitamin B12 with no apparent toxic effect. Four different doses were investigated: 0.8, 1.2, 1.6, and 1.8 mg/25 kg body weight, animals used weights (20–25 g) injected intramuscular for three consecutive weeks.
The results demonstrate that treatment of mice with vitamin B12 at the previous indicated doses caused no death during 30 days after administration of the last dose. No significant changes were observed in body weights, also no morphological changes during the whole experiments.
The results in (Fig. 1) demonstrate the percentage of mortality rate in different animal groups. The dose equivalent to the human is 0.8 mg/kg g body weight injected intramuscularly 1 h before irradiation with 2 Gy, in addition to another group pre-treated with vitamin B12 and irradiated with an additional dose of 2 Gy.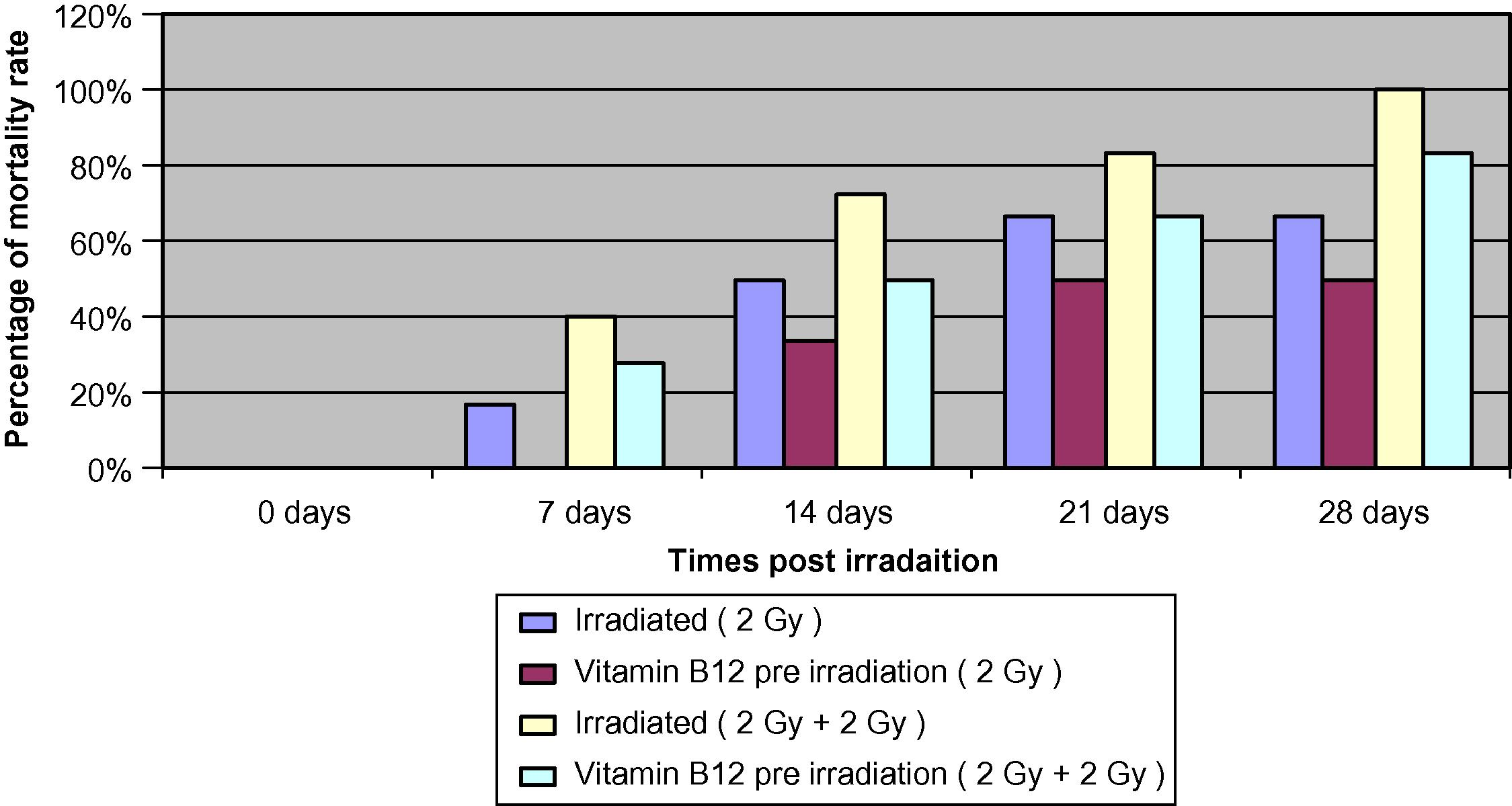
The percentage of mortality rate in different animal groups.
The results demonstrate that 2 Gy irradiation showed a mortality rate of 16.6%, 50%, 66.6%, and 66.6% at 7, 14, 21, and 28 days post-irradiation, respectively. While the vitamin B12 pre-treated group showed a mortality rate of 0%, 33.3%, 50%, and 50%, respectively, at 0 h.
Results of 2 Gy additional dose demonstrate that irradiation of mice increased the percentage of mortality to 40%, 72.2%, 83.3%, and 100% at 7, 14, 21, and 28 days, respectively, post-irradiation, while the group pre-treated with Vit. B12 (0.8 mg/kg body weight) pre-irradiation resulted in a mortality rate of 27.7%, 50%, 66.6%, and 83.3% at the same experimental times, which indicates a lower mortality rate of about 12.3%, 22.2%, 16.7%, and 16.7% at 7, 14, 21, and 28 days, respectively.
The results of observation of the surviving animals in the irradiated groups revealed that all animals showed irritated nose, ears, loss of hair, swollen feet and lesioned tails. While the groups pre-treated with Vit. B12 (0.02 mg/25 g body weight) before irradiation were normal and did not show any symptoms of sickness.
The results obtained indicated that the optimum radio-protective action of Vit. B12 against mortality and morphological changes induced by whole body gamma irradiation was at day 14.
3.2 Effect of vitamin B12 and gamma radiation on cytogentic parameters (mitotic index MI)
The control for 2 Gy at 0 h showed a mitotic index of 49.82% assessed at 0, 6, 24 and 72 h, while Vit. B12 pre-treated group revealed a mitotic index ranging between 47.22% and 50%. No significant changes were noted between Vit. B12 pre-treated group and the control at different time intervals (P1 > 0.05).
The frequency of the changes in the mitotic index for the irradiated group was 32.9% at 0 h, which indicates a highly significant delay (P1 < 0.001) in the MI compared to the control group. While the irradiated group pre-treated with Vit. B12 showed a highly significant delay (P2 < 0.001) in the mitotic index, compared to Vit. B12 pre-treated groups, but less than the effect induced by irradiation alone. Pre-treatment of the irradiated group with vitamin B12, resulted in a relatively remarkable recovery of about 15.7% (P3 < 0.001) when compared to the irradiated group at 0, 6, 24 and 72 h. The additional challenged dose of 2 Gy resulted in a highly significant delay in the mitotic indices compared to the data obtained from control group at 6, 24 and 72 h. Also, pre-treated Vit. B12 irradiated groups showed a relative recovery of about 22.7%, 19.8%, and 22.6% at 6, 24, and 72 h, respectively, when compared to the irradiated group reaching its maximum at 6 and 72 h (Fig. 2).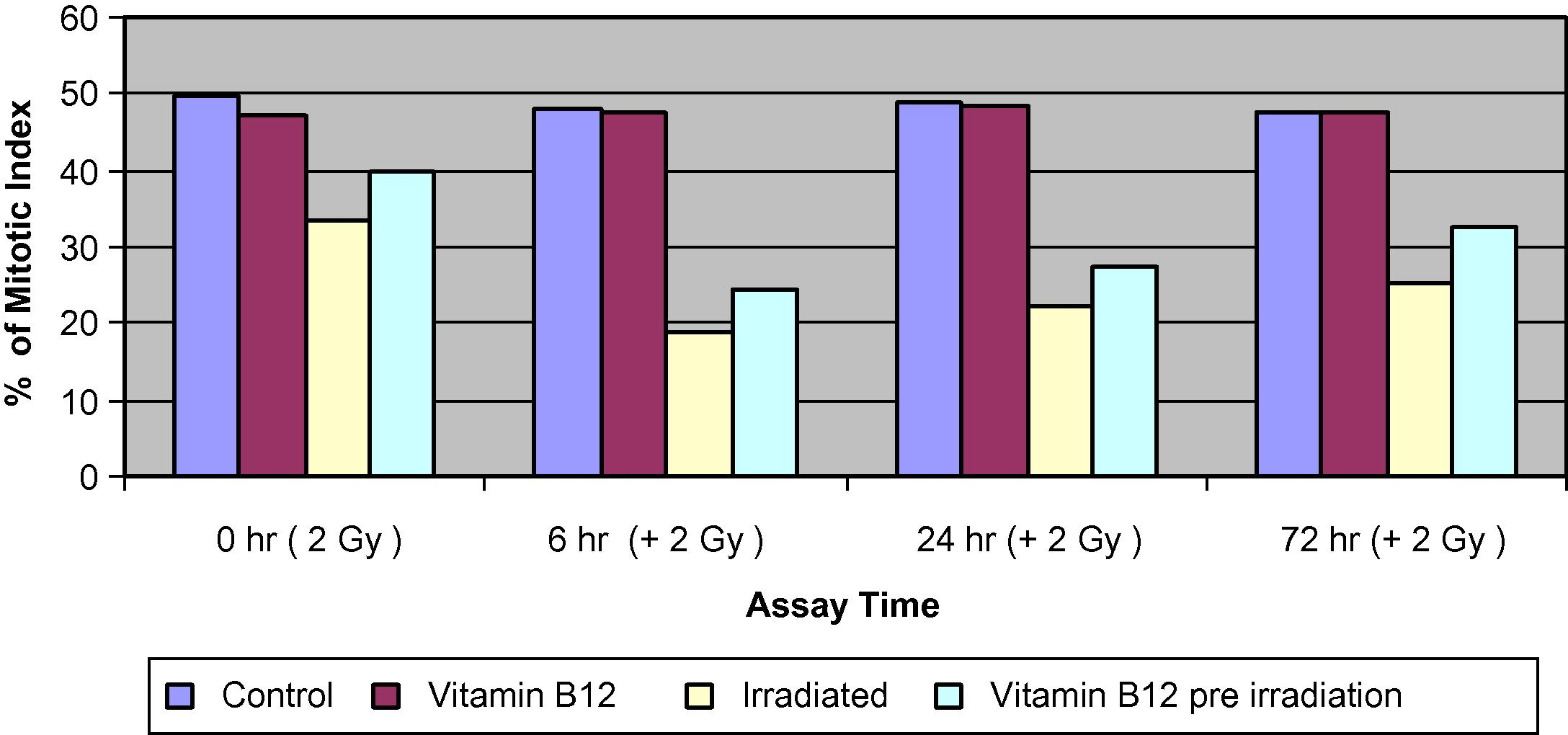
Percentage of mitotic index (% MI) of bone marrow cells in different groups of mice assessed at 0, 6, 24 and 72 h.
4 Induction of micronuclei
4.1 The frequency of micronuclei in polychromatic erythrocytes (MNPCEs)
The MNPCEs, MNNCEs and the ratio of PCEs/NCEs data obtained from bone marrow smears are presented in (Figs. 3–5).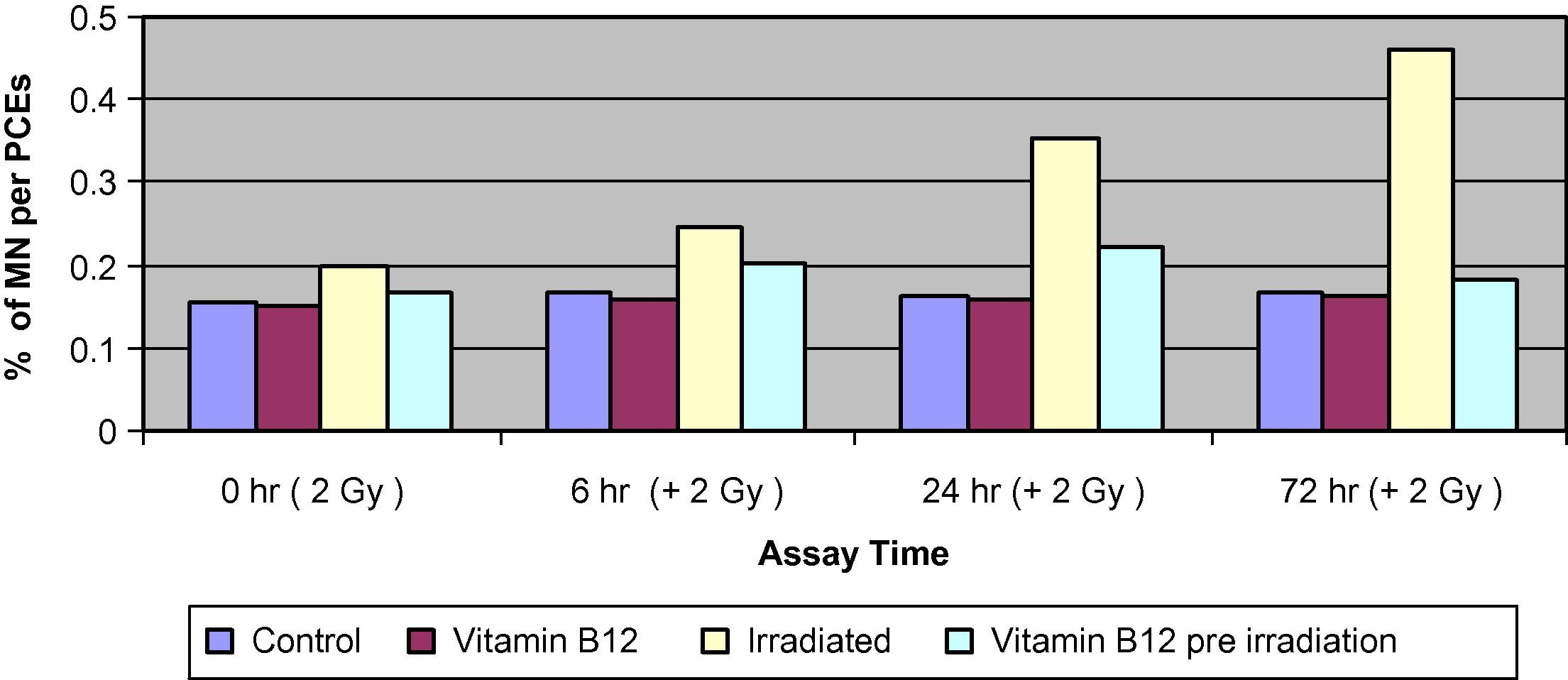
Changes of the percentage of MNPCEs of bone marrow cells in different groups of mice assessed at 0, 6, 24 and 72 h.
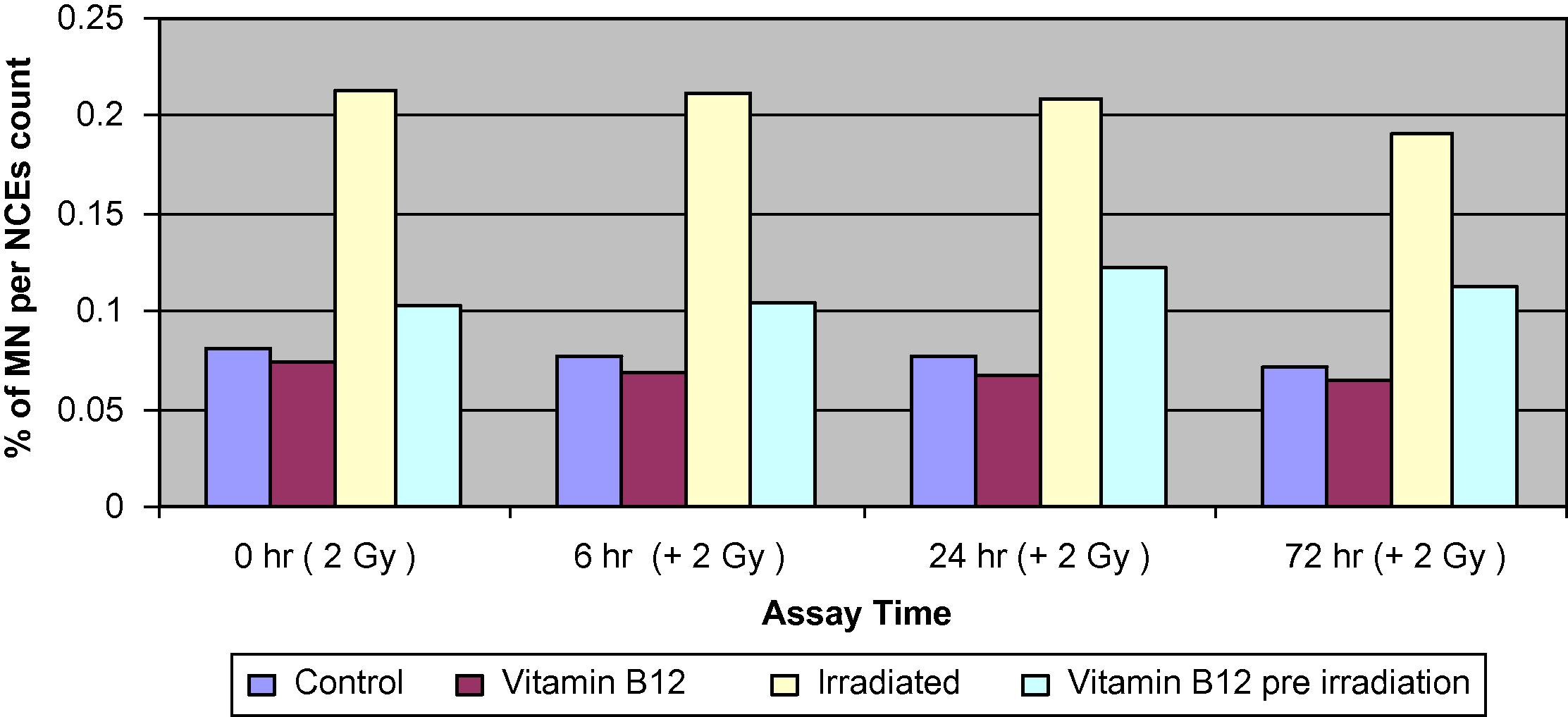
Changes of the percentage of MNNCEs of bone marrow cells in different groups of mice assessed at 0, 6, 24 and 72 h.
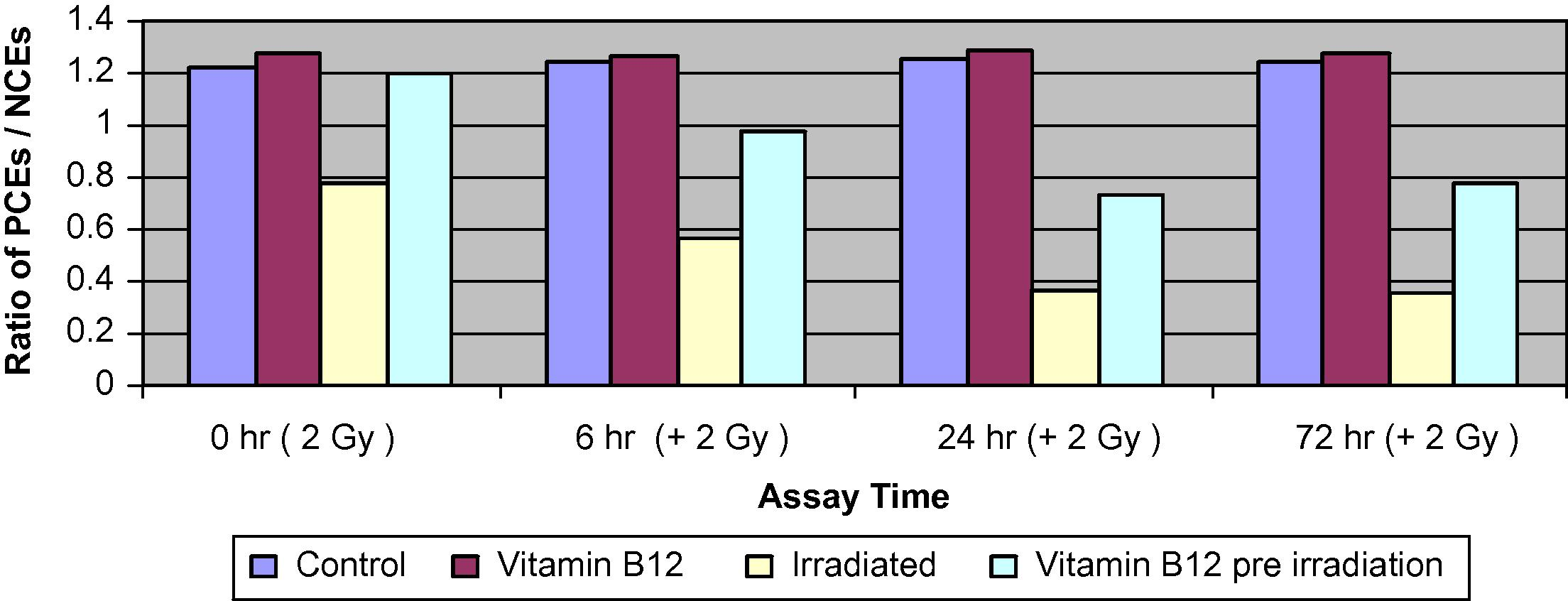
Changes in the ratio of PCEs to NCEs of bone marrow cells in different groups of mice assessed at 0, 6, 24 and 72 h.
4.2 The frequency of micronuclei in normochromatic erythrocytes (MNNCEs)
4.2.1 The changes in the ratios of polychromatic erythrocytes to normochromatic erythrocytes (PCEs/NCEs)
Comparison of the control and the Vit. B12-treated groups showed no significant differences in the percentage of total MNPCE at different assay times. The irradiated group showed a significant increase in PCEs compared with both control and Vit. B12-treated groups. The differences increased steadily to reach 3-folds by 72 h. The frequency of PCEs with more than one MN was even higher than 3-folds suggesting that cells with one MN are more liable to more damage than non-affected cells.
Pre-treatment of irradiated mice with Vit. B12 has resulted in a significant reduction in the percentage of MNPCEs to about 35–45% of those values induced by irradiation alone at 24 and 72 h, respectively. The frequency of MNNCEs was greatly increased by exposure to 2 Gy and the increase was time dependent, it reached almost 3-folds by 72 h. Pre-treatment of irradiated groups with Vit. B12 caused significantly less damage and resulted in a much lower frequency of MNPCEs which reached about 50% of those inflected by radiation alone. The PCE/NCE ratio was similar between the control and the Vit. B12-treated groups. Irradiation resulted in a significant decrease in PCE relative to NCE. Pre-treatment with Vit. B12 resulted in a moderate decrease of PCEs relative to NCE.
4.3 Effect of vitamin B12 and gamma radiation and their combination on sperm head and tail abnormalities
The frequencies of sperm head and tail abnormalities of mice from control; vitamin B12; irradiated group to a priming dose of 2 Gy γ-irradiation and vitamin B12 pre-treated irradiated group, assessed at 0 h, are illustrated in (Fig. 6A).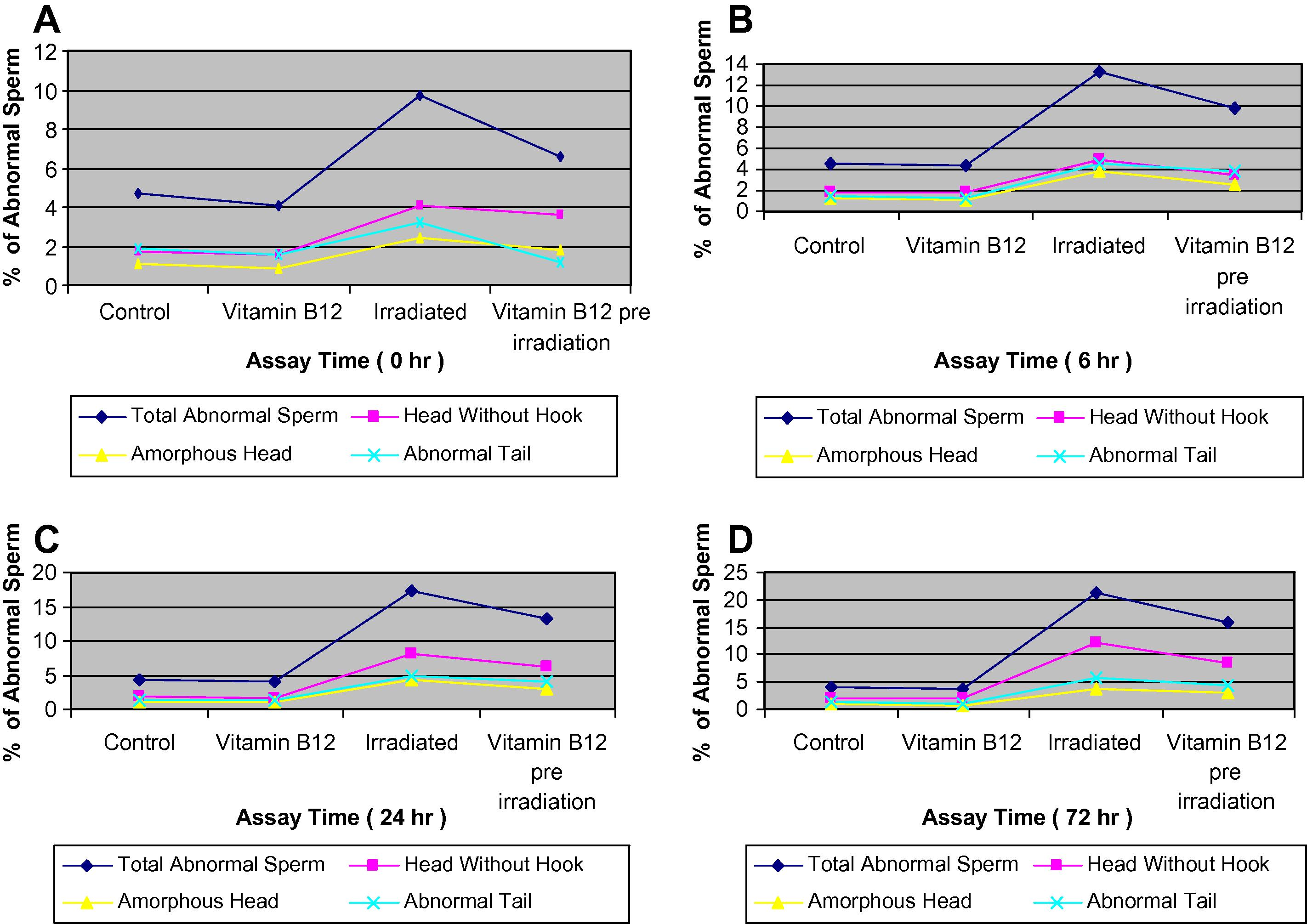
(A–D) Effect of gamma rays in the percentages of sperm head and tail abnormalities in different groups of mice assessed at 0, 6, 24 and 72 h.
The data from the irradiated group to an additional (challenge dose) dose of 2 Gy and a group of mice pre-treated with vitamin B12 and irradiated to the challenge dose of 2 Gy were compared with the non-treated (control) and vitamin B12 group, and assessed at 6, 24 and 72 h, and are illustrated in (Fig. 6B–D). The results suggest that there is an increase in the frequencies of total abnormal sperms, reaches its maximum at 72 h assay time post-irradiation (21.3%) in the group of mice exposed to the additional challenge dose of 2 Gy and 13.3% and 17.3% when assessed at 6 and 24 h, respectively, which indicates an increment with the time of assessment post-irradiation. The total frequency of abnormal sperms was 9.7% for the group of mice exposed to a priming dose of 2 Gy only and assessed at 0 h. It means that time plays an important factor in increasing the incidence of total abnormal sperms with increasing dose.
Pre-treatment with vitamin B12 resulted in a decrease in the frequency of the total abnormal sperms and the differences were very highly significant (P3 < 0.001) when compared to the irradiated groups at all assay times, and highly significant changes (P3 < 0.01) in the total number of sperms with amorphous head and abnormal tail were noted only at 24 and 72 h of the assay time (Fig. 6). Pre-treatment with vitamin B12 prior to irradiation resulted in a decrease in the total abnormal sperms by 1.3-folds, and 1.5-, 1.3- and 1.4-folds for the total abnormal head. A decrease by 1.1-, 1.2- and 1.3-folds were noted in the total sperms with abnormal tail when compared with the irradiated groups and assessed at 6, 24 and 72 h, respectively. The types of abnormalities scored were mainly head without hook, amorphous head and abnormal tail (Plate 1a–f).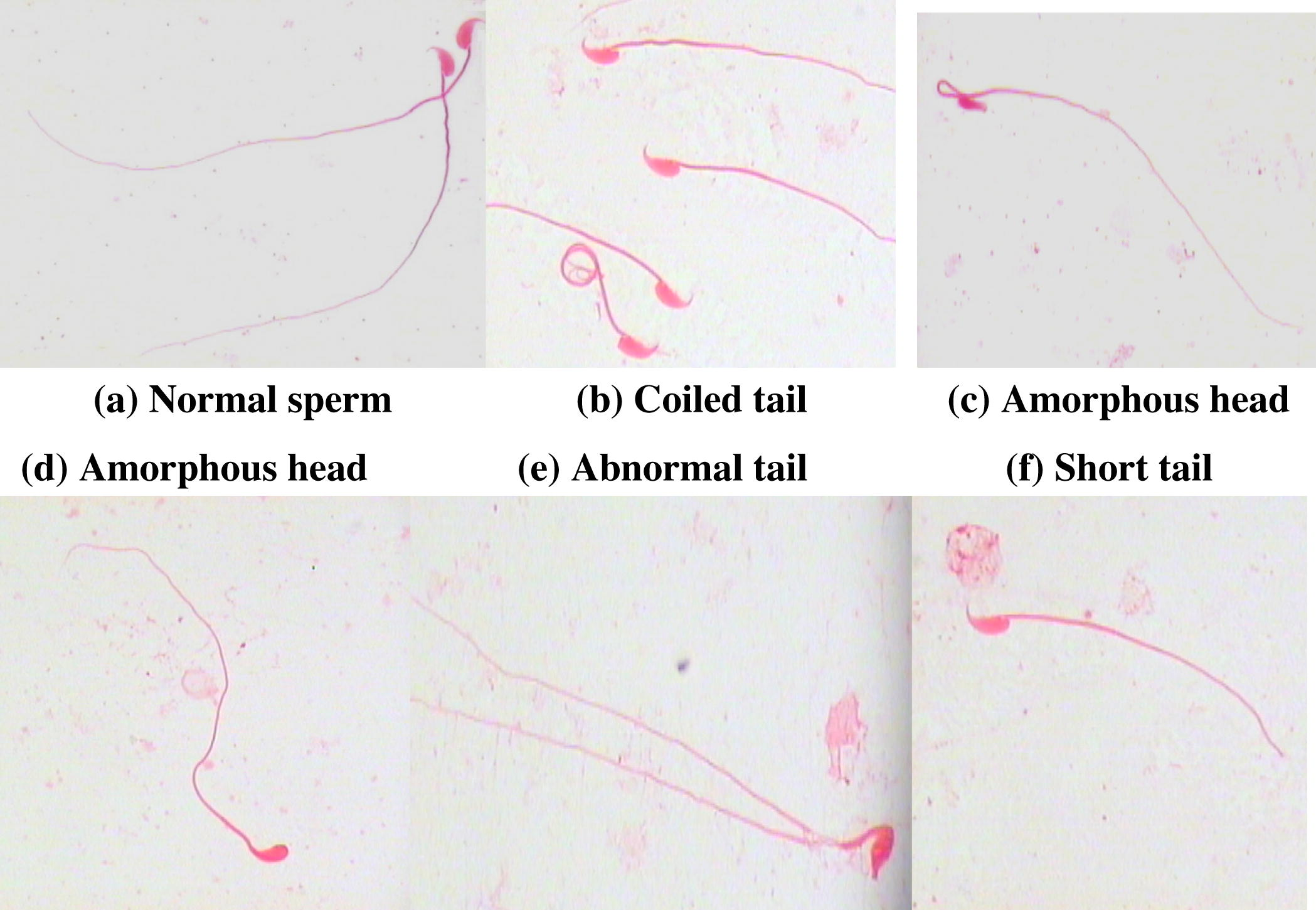
Effect of gamma rays on the frequencies of sperm head and tail abnormalities in different groups of mice, assessed at 0, 6, 24, and 72 h.
5 Discussion
It is well documented that ionizing radiation increased the incidence of MN in vivo (Salvadori et al., 1996). In the present study, whole body gamma irradiation of mice raised the incidence of MNPCEs very significantly. The MNPCE that appeared in the case of mice represents erythroblasts in G2 phase of the cell cycle during irradiation, while the ones that appeared may be at the G1/S phase of the cell cycle. During irradiation both hydrogen peroxide and hydroxyl free radicals are found within the cell which can directly or indirectly cause DNA damage (Fenech, 2000, 2002). The radio-protective effect of vitamin B12 was assessed in this study by estimating the frequency of induced MN in PCEs and NCEs of bone marrow in vivo. The protective action of vitamin B12 against Gamma irradiation depends on the dose and rate of exposure. Vitamin B12 plays an important role in DNA metabolism (Hultdin et al., 2005) and it is also required for the synthesis of methionine and S-adenosyl methionine, the common methyl donor required for the maintenance of methylation patterns in DNA that determine gene expression and DNA conformation (Zingg and Jones, 1997). It has also been reported that vitamin B12 plays an important protective role in cervical carcinogenesis (Hernandez et al., 2003) and an effective role in patients with rheumatoid arthritis (Hornung et al., 2004).
In this study, the administration of Vit. B12 did not alter the incidence of MN of PCEs and NCEs. However, the pre-treatment of irradiated group with vitamin B12 at different time intervals showed a very significant decrease in the frequency of MNNCEs. Similar results (Fenech et al., 1998) were reported, where Vit. B12 caused a significant decrease in the MN of NCEs induced by Gamma rays (Fenech and Rinaldi, 1994; Fenech et al., 1997; El-Habit et al., 2000; Mortazavi et al., 2003). The ratio of PCEs to MNCEs decreased significantly at 6 and 72 h post-irradiation. This was due to a decline in the number of PCEs relative to NCEs in the bone marrow after irradiation. Pre-treatment with Vit. B12 prior to irradiation reestablished the relative proportion of PCEs/NCEs. Protection of bone marrow tissue by Vit. B12 against radiation toxicity is expressed as an increase in the ratio of PCEs/NCEs (Salvadori et al., 1996).
Sperm DNA damage has been associated with high levels of reactive oxygen species which have been detected in the semen of 25% of infertile men (Irvine et al., 2000; Oliva, 2006). Although low levels of reactive oxygen species are necessary for normal sperm functions, high levels are generated by defective spermatozoa and by semen leukocytes, which result in sperm dysfunction.
The association between sperm DNA damage and sperm-derived reactive oxygen species suggests that DNA damage may be caused by a defect in spermatogenesis (Gomez et al., 1996), whereas the association between sperm DNA damage and leukocyte-derived reactive oxygen species suggests that DNA damage may be caused by a post-testicular defect (Ochsendorf, 1999; Zhang et al., 2008).
In this study, sperm abnormalities are used to measure the mutagenic effect in the germ cells of mice treated with Vit. B12, irradiated and pre-treated with Vit. B12, irradiated with a dose of 2 Gy of γ-rays and to an additional challenged dose of 2 Gy and assessed at 0, 6, 24 and 72 h.
The significant increase in sperm shape abnormalities may reflect chromosome abnormalities in primary spermatocytes and spermatids and has always been associated with infertility (Wyrobek and Bruce, 1978; Wyrobek et al., 1984).
Two lines of evidence suggest that the induced changes in sperm morphology reflect genetic damage in the male germ cell. First, considerable evidence indicate that sperm shaping is polygenetically controlled by numerous autosomal and sex-linked abnormalities. Second, effects of all the mouse germ cell mutagens (including ionizing radiations) so far tested on the mouse sperm morphology showed positive responses. This observation is based on comparison of the ability of chemical agents to induce abnormal sperms morphology besides inducing dominant lethal, heritable translocations, and specific locus mutations (Adler, 1977; Wyrobek et al., 1984). The protective role of vitamin B12 against the induced morphological changes in sperm morphology was represented in the data of the Vit. B12 pre-treated irradiated groups of mice.
The data obtained demonstrated a definite and pronounced clastogenic effect of ionizing radiation on the mouse bone marrow cells represented by induction of micronuclei and sperm head and tail abnormalities. However, a modulatory effect was noted in Vit. B12 pre-treated irradiated groups, which suggest that Vit. B12 alone is likely to be one of the most important micronutrients which exert a vital protective role against gamma irradiation.
The priming dose may be as large as 2 Gy and would have the same effect as the small doses. Therefore, it may have the capacity of inducing a radio-adaptive response as the effect of the larger doses. The intake of antioxidants as supplements in the diet will confer greater protection against potential damage incurred by exposure to radiations or other environmental hazards.
Splitting of radiation dose could have the same effect as the initial priming dose and the subsequent challenge dose in conferring adaptive responses by the initial dose as recently reported (Zhang et al., 2008). The results suggest that the priming dose can be as large as 2.0 Gy using an animal model.
Acknowledgements
The authors would like to thank King Saud University Kingdom of Saudi Arabia, Riyadh, for their financial support and to the Research Center, College of Science, for providing the radiation and all their laboratory facilities.
References
- Stage-sensitivity and dose-response study after γ-irradiation of mouse spermatocytes. Int. J. Rad. Biol.. 1977;31:79-85.
- [Google Scholar]
- Are chromosome aberrations in circulating lymphocytes predictive of future cancer onset in humans? Cancer Genet. Cytogen.. 1995;79:133-135.
- [Google Scholar]
- The mutagenic activity of 61 agents as determined by the micronucleus, Salmonella, and sperm abnormality assay. Can. J. Genet. Cytol.. 1979;21:319-334.
- [Google Scholar]
- Elements of Statistics (third ed.). New York: Van Nostrand; 1980.
- The modifying effect of β-carotene on gamma radiation-induced elevation of oxidative reactions and genotoxicity in male rats. Mutat. Res.. 2000;466:179-186.
- [Google Scholar]
- Role of vitamin B12 and folate deficiencies in carcinogenesis. In: Poirier L.A., Newberne P.M., Patiza M.W., eds. Essential Nutrients in Carcinogenesis. Plenum Press; 1986. p. :313-331.
- [Google Scholar]
- Chromosomal biomarkers of genomic instability relevant to cancer. Res Focus.. 2002;7:1228-1237.
- [Google Scholar]
- Nutritional treatment of genome instability: a paradigm shift in disease prevention and in the setting of recommended dietary allowances. Nutr. Res. Rev.. 2003;16:109-122.
- [Google Scholar]
- The relationship between micronuclei in human lymphocytes and plasma levels of vitamin-C, vitamin-E, vitamin B-12 and folic acid. Carcinogenesis. 1994;15:1405-1411.
- [Google Scholar]
- Folate, vitamin B12, homocysteine status and chromosome damage rate in lymphocytes of older men. Carcinogenesis. 1997;18:1329-1336.
- [Google Scholar]
- Folate, vitamin B12, homocysteine status and DNA damage in young Australian adults. Carcinogen. 1998;19(7):1163-1171.
- [Google Scholar]
- Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: correlation with biochemical markers of the cytoplasmic space, oxidative stress and sperm function. J. Androl.. 1996;17:87-276.
- [Google Scholar]
- Cancer risk in humans predicted by increased levels of chromosomal aberrations in lymphocytes: Nordic study group on health risk of chromosomal damage. Cancer Res.. 1994;54:2919-2922.
- [Google Scholar]
- The induction of micronuclei as a measure of genotoxicity. A report of the US environmental protection agency Gene-Tox program. Mutat. Res.. 1983;123:61-188.
- [Google Scholar]
- Diet and premalignant lesions of the cervix: evidence of a protective role for folate, riboflavin, thiamin and vitamin B1. Cancer Causes Cont.. 2003;14:859-870.
- [Google Scholar]
- Folate, homocysteine, and cobalamin status in patients with rheumatoid arthritis treated with methotrexate, and the low effect of low dose folic acid supplement. J. Rheumatol.. 2004;31:2374-2381.
- [Google Scholar]
- Plasma folate, vitamin B12, and homocycstein and prostate cancer risk: a prospective study. Int. J. Cancer. 2005;20:819-824.
- [Google Scholar]
- DNA integrity in human spermatozoa: relationships with semen quality. J. Androl.. 2000;21:33-44.
- [Google Scholar]
- Inheritance of sperm head abnormality types in mice and the role of the Y chromosome. Genet. Res.. 1976;28:189-198.
- [Google Scholar]
- Variability of chromosomal radioadaptive response in human lymphocytes. Iran J. Res.. 2003;176:55-61.
- [Google Scholar]
- Infections in the male genital tract and reactive oxygen species. Hum. Reported Update. 1999;5:399-420.
- [Google Scholar]
- Quality of care and standard setting in general practice. Aust. Fam. Physician. 1993;22:1109-1709.
- [Google Scholar]
- Radioprotection of β-carotene evaluated on mouse somatic and germ cells. Mutat. Res.. 1996;356:163-170.
- [Google Scholar]
- The micronucleus test: an in vivo bone marrow method. In: Hsu T.C., ed. Cytogenetic Assays of Environmental Mutagens. Osmum, NJ, Totowa: Allanheld; 1982.
- [Google Scholar]
- DNA methylation decreases in aging but not in immortal cells. Science. 1983;220:1055-1057.
- [Google Scholar]
- Micronutrients: dietary intake v. supplement use. Proc. Nurt. Soc.. 2005;64(4):543-553.
- [Google Scholar]
- Chemical induction of sperm abnormalities in mice. Proc. Natl. Acad. Sci.. 1975;72:4425-4429.
- [Google Scholar]
- Induction of sperm shape abnormalities in mice and humans. In: Hollaender A., De Serres F.J., eds. Chemical Mutagens Principles and Methods for Their Detection. Vol vol. 5. Plenum Press; 1978. p. :257-285.
- [Google Scholar]
- Sperm morphology testing in mice. In: Kilbey B.J., Nichol M., Ramel C., eds. Handbook of Mutagenicity Test Procedures (second ed.). Amsterdam: Elsevier Science; 1984. p. :739-750.
- [Google Scholar]
- Induction of cytogenetic adaptive response in spermatogonia by pre-exposure of mouse testis to low-dose 12C6+ ions. Mutat. Res.. 2008;653:109-112.
- [Google Scholar]
- Genetic and epigenetic aspects of DNA methylation on genome expression, evolution, mutation and carcinogenesis. Carcinogen. 1997;18:869-882.
- [Google Scholar]
- Genetic and epigenetic aspects of DNA methylation on genome expression, evolution, mutation and carcinogenesis. Carcinogenesis. 1997;18:869-882.
- [Google Scholar]







