Translate this page into:
Acidotolerant Streptomyces sp. MBRL 10 from limestone quarry site showing antagonism against fungal pathogens and growth promotion in rice plants
⁎Corresponding authors. Fax: +91 385 2435 145/831. tammasi2009@gmail.com (K. Tamreihao), debananda.ningthoujam@gmail.com (Debananda S. Ningthoujam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Acidotolerant Streptomyces sp. MBRL 10 isolated from limestone deposit site on Gauze’s medium No. 1 (pH 5.3) showed significant antagonism against the tested fungal pathogens. It exhibited the highest mycelial growth inhibition by diffusible and volatile compound(s) production against Rhizoctonia solani. Culture filtrates also exhibited significant inhibition zone but the inhibition activities vanished when sterilized. The strain produced chitinase, β-1,3-glucanase, lipase, protease and ammonia but not β-1,4-glucanase. It could produce 25 μg/ml of indole acetic acid, solubilize up to 140 μg/ml of phosphate with a concomitant decrease in pH of the medium. The bioactive actinomycete strain produced hydroxamate type of siderophore. Casamino acid was found to be the best medium for siderophore production (87% siderophore units).
MBRL 10 showed the highest rice seedlings vigor index corresponding to an inoculum size of 0.3 × 108 cfu/ml. Strain treated rice seeds at an inoculum size of 0.3 × 108 cfu/ml showed higher germination percentage and significantly enhanced (P ⩽ 0.05) the growth of seedlings. Strain treated rice seedlings challenged with pathogens also exhibited higher germination percentages and significantly enhanced (P ⩽ 0.05) growth over seedlings challenged with pathogen alone in the absence of the bioinoculant. Rice plants treated with the strain significantly promote (P ⩽ 0.05) the growth under nethouse conditions.
Keywords
Streptomyces sp. MBRL 10
Acidotolerant
Antagonism
Plant growth promoting traits
Vigor index
Rice plants
1 Introduction
Rice (Oryza sativa) is one of the most important staple foods for more than three billion people i.e. over half the world’s population (IRRI, 2006) and this cereal crop influences the livelihoods and economies of several billion people across the world. It provides 27% of dietary energy and 20% of dietary protein in the developing world (Redoña, 2004). The majority of the global rice production (88%) is done in Asian countries, with China and India being the major producers (IRRI, 2008). However, since 2000, the world’s average growth rate in rice production has not kept up with population increases and demand for rice has outstripped its production (FAO, 2000). Intensive research on plant growth promoting bacteria (PGPB) is underway worldwide for developing biofertilizers and biocontrol agents as better alternative to agrochemicals, as the latter harm the environment and human health besides demanding high costs (Ningthoujam et al., 2009).
Actinomycetes are prolific producers of several agriculturally important secondary metabolites and several members have been considered as plant growth promoting agents (Goodfellow and Williams, 1983; Nimaichand et al., 2013). Until the investigations of Corke and Chase (1964), and Khan and Williams (1975) had been published, all soil actinomycetes were believed to be neutrophilic. Acidophilic isolates grow in the pH range 3.5–6.5, with optimum growth between pH 4.5 and 5.5 (Khan and Williams, 1975). The most frequently encountered acidophilic/acidotolerant actinomycetes belong to the genus Streptomyces (Hagedorn, 1976; Poomthongdee et al., 2015). Soil pH can drop below 5.0 after prolonged use of ammonia-based fertilizers or acid rain and this can cause considerable reduction in bacteria and actinomycetes population and increase the relative abundance of fungi in soil (Ventura, 2000; Haney et al., 2000). Acidophilic actinomycetes may be a potential source of new effective agents for controlling fungal plant diseases and plant growth promotion for sustainable agricultural product where soil has been contaminated with the excessive use of agrochemicals and environmental factors.
Streptomyces sp. has played an important role in chitin decomposition in acidic soil and litter, where fungi are important colonizers (Williams and Robinson, 1981). Release of ammonia by the deacetylation and deamination of N-acetylglucosamine residues may raise the pH of the soil (Williams and Robinson, 1981) making the way for the other neutrophilic PGPB to colonize and compete with the pathogens. In an acidic soil environment, they probably involved in competition with fungi, and therefore, it is logical that acidophilic actinomycetes possess an antifungal activity (Zakalyukina and Zenova, 2007). Acidophilic/acidotolerant actinomycetes that can inhibit the growth of different Fusarium sp. have been reported (Zakalyukina and Zenova, 2007). But there is limited report for their potential as biocontrol and plant growth promoting bacteria especially for rice. Acidophilic/acidotolerant Streptomyces sp. has been reported to show antagonistic activity against rice fungal pathogens such as Fusarium moniliforme, Helminthosporium oryzae and Rhizoctonia solani (Poomthongdee et al., 2015).
Hundung limestone deposit site is a unique, non-rhizospheric habitat in Ukhrul, Manipur, India falling under the Indo-Burma Biodiversity hotspot. The present investigation aimed to study the native acidotolerant actinomycetes, Streptomyces sp. MBRL 10 showing in vitro antagonistic activity against important rice fungal pathogens as well as plant growth promoting activity. It also aimed to study rice plant growth promotion under nethouse conditions.
2 Materials and methods
2.1 Isolation
Soil samples were collected from Hundung limestone deposit, Ukhrul District, Manipur. Isolation was performed on Gauze’s Medium No. 1 (GM 1, pH 5.3) by serial dilution technique (10−3 to 10−6) as described earlier (Nimaichand et al., 2012). After incubation at 30 °C for 1 week, colonies obtained with distinct morphologies were selected and subcultured in the same medium to get pure cultures. The purified cultures were preserved as agar slants (4 °C) and glycerol stocks (20% v/v, −20 °C) for further use.
2.2 Preliminary antagonistic bioassays
The isolates obtained were subjected to preliminary antagonistic assays by dual culture method (described in the following section) against fungal pathogens. Of the isolates showing antagonistic activity, the best isolate MBRL 10 was characterized and further screened for other antagonistic and plant growth promoting traits.
2.3 Genomic DNA isolation and strain characterization
Genomic DNA extraction and PCR amplification of the 16S rRNA gene was performed as described by Li et al. (2007). The almost complete 16S rRNA gene sequence of the strain was identified using the EzTaxon-e server database (Kim et al., 2012) and aligned with the 16S rRNA gene sequences of related species using CLUSTAL X version 2.1 (Larkin et al., 2007). Phylogenetic analyses were performed using the software package MEGA version 5 (Tamura et al., 2011). Phylogenetic distances were calculated with the Kimura two-parameter model (Kimura, 1983) and tree topologies were inferred using the neighbor-joining method (Saitou and Nei, 1987). To determine the support of each clade, bootstrap analysis was performed with 1000 resamplings (Felsenstein, 1985).
2.4 Antagonistic assay
2.4.1 Fungal pathogens
The rice fungal pathogens, viz, Rhizoctonia solani (MTCC 4633), Rhizoctonia oryzae-sativae (MTCC 2162), Bipolaris oryzae (MTCC 3717), Pyricularia oryzae (MTCC 1477), Fusarium oxysporum (MTCC 287) and Curvularia oryzae (MTCC 2605) were procured from Microbial Type Culture Collection (MTCC), Institute of Microbial Technology (IMTECH), Chandigarh, India. The strains were grown and maintained on Potato Dextrose Agar (PDA).
2.4.2 Antagonism assay by diffusible and volatile compounds
The antifungal bioassays due to diffusible compound(s) were done by dual culture method (Khamna et al., 2009). Agar plugs (6 mm) of 5 d old strain MBRL 10 grown on GM1 were placed at the corners of the PDA plates leaving 1 cm from the margins. The plates were incubated at 30 °C for 24 h. Fungal plugs (6 mm) were then placed at the centers of the plates. Plates containing fungal plugs without the isolates were kept as controls.
The antagonism due to production of volatile compound(s) (VOCs) was screened according to Trivedi et al. (2008) with some modifications. Strain MBRL 10 was allowed to grow on GM1 until full growth was observed. The lids of the plates were then replaced with PDA plates containing fungal plugs (6 mm) at the centers. In the control plates, lids of the GM1 plates without the isolate were replaced with PDA plates containing fungal plugs (6 mm) at the centers. Plates were wrapped with parafilm and kept incubated at 30 °C.
The inhibition zone was measured after the fungal mycelia in the control plates reached the edges of the plates. Colony growth inhibition was calculated using the formula: (C − T)/C × 100, where C represent the radial growth of the test pathogen in the control plate (measured in mm), and T is the radial growth of test pathogen in the test plates (mm).
2.4.3 Antagonism assay by culture filtrates
For bioassays of antifungal compounds in the culture filtrates, the strain was inoculated in GM1 broth (GMB) and incubated with shaking conditions for 5 d (150 rpm, 30 °C). Culture broth was then centrifuged (10,000 rpm, 10 min) (Centrifuge 5810, Eppendorf) and the supernatants collected were filtered through a membrane filter (0.2 μm pore size). The filtrates were then divided into two portions; one portion was autoclaved at 121 °C for 20 min (Sterile) and other was maintained at room temperature (Non-sterile). Hundred μl of each of the treated culture filtrate were then incorporated in a well on each side of PDA plates. Fungal plugs were then placed at the centers of the plates. The inhibition zone was measured in mm.
2.4.4 Assay for fungal cell wall degrading enzyme production
Colloidal chitin from chitin (shrimp shells) was prepared according to Reid and Ogrydziak (1981). A loop full of 5 d old culture was transferred into sterile vials containing 2 ml of semi-solid GM1 (0.1% agar), and gently shaken for uniformity. Then it was streaked on Colloidal Chitin agar plate and chitinase production was screened according to Hsu and Lockwood (1975). β-1,3-Glucanase and β-1,4-glucanase production were assayed according to Srividya et al. (2012) and Ariffin et al. (2006) respectively. Lipase and protease production was screened on Tributyrin agar and Skim Milk agar according to Cappucino and Sherman (1992).
2.4.5 Ammonia production
Ammonia production was studied in peptone water according to Cappucino and Sherman (1992).
2.5 Plant growth promoting (PGP) assay
2.5.1 Indole acetic acid (IAA) production
IAA production was determined according to the method of Bano and Musarrat (2003). Strain MBRL 10 was inoculated on Starch Casein Nitrate broth (SCNB) containing 2 mg/ml of l-tryptophan (trp) and incubated in a shaker (150 rpm, 30 °C) for 5 d. One ml of the culture supernatant was mixed with 2 ml of Salkowski reagent. Appearance of pink color indicated IAA production.
For quantitative assay of IAA production, strain was allowed to grow on SCNB containing 2 mg/ml of trp under shaking conditions (150 rpm, 30 °C). Five ml aliquot was withdrawn periodically from each culture flask at 24 h intervals and centrifuged (10,000 rpm, 10 min). One ml supernatant was mixed with 2 ml Salkowski reagent and kept incubated for 20 min at room temperature. Absorbance was measured at 530 nm (BioSpectrometer, Eppendorf) and the amount of IAA produced was calculated by comparing with the standard IAA curve. The amount of IAA produced was compared with the dry cell mass.
2.5.2 Siderophore production
Siderophore production was determined according to You et al. (2005) with few modifications. An agar plug of strain MBRL 10 fully grown on GM 1 were inoculated on Starch Casein Nitrate agar (SCNA) (without iron) amended with CAS-substrates and kept incubated at 30 °C for 7 d. Formation of orange zone surrounding the colony indicates siderophore production. Catecholate or hydroxamate type of siderophore was determined according to the method of Arnow’s (1937) and Meyer et al. (1995).
Quantitative estimation of siderophore production was done by CAS-shuttle assay (Payne, 1994). Strain was inoculated on five different iron deficient liquid media according to Sayyed et al. (2005) viz, SCN, Casamino acid medium (CAA), Nutrient broth (NB), Succinic acid medium (SM) and Bharbhiaya and Rao medium (BR), and kept incubated under shaking condition (150 rpm, 30 °C). A 5 ml aliquot was withdrawn periodically at 24 h interval, centrifuged (10,000 rpm, 10 min) and 1 ml supernatant was mixed with an equal volume of CAS reagent. Absorbance was measured at 630 nm against a reference consisting 1 ml uninoculated broth and 1 ml CAS reagent. The amount of siderophore produced (% siderophore units) was calculated using the formula: (Ar − As)/Ar × 100, where, Ar represent absorbance of reference at 630 nm and As absorbance of sample.
2.5.3 Phosphate (P) solubilization
P solubilization assay was done as described by Mehta and Nautiyal (2001) using NBRIP-BPB medium. Quantitative estimation of P solubilization was done according to Kapri and Tewari (2010).
2.5.4 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase production
ACC deaminase activity was screened according to El-Tarabily (2008) using the nitrogen-free Dworkin and Foster’s salts minimal agar medium (DF, Dworkin and Foster, 1958).
2.6 In vitro seed germination test (vigor index)
MBRL 10 was grown on GMB medium for 5 d and harvested by centrifugation (10,000 rpm, 10 min) and the pellet was washed thrice with sterile distilled water (SDW). The pellet was dissolved in SDW and different inoculum sizes were prepared (0.3 × 108, 0.6 × 108, 1.2 × 108, 1.8 × 108, 2.4 × 108, 3 × 108 cfu/ml). Different inoculum sizes were prepared by setting the inoculum at different optical density (OD600) and measuring the cfu/ml using standard plate count method. Rice seeds (Variety: Jatra) were surface sterilized with 70% ethanol for 5 min followed by 0.2% sodium hypochlorite for 5 min and rinsed four times with SDW. Sterilized seeds were soaked in the cell suspensions prepared earlier and kept under shaking conditions (150 rpm, 2 h). Sterilized seeds soaked in SDW were taken as control. The seeds were then transferred to sterile plates containing wetted filter papers at the rate of 10 seeds per plate. Plates were incubated at 28–30 °C and after 3 d, the number of germinated seeds, root lengths and shoot lengths were noted and compared with controls. Four replications were done per treatment and the experiments were repeated twice. Vigor index was calculated using the formula (Baki and Anderson, 1973): Percent germination × Seedling length (i.e. shoot length + root length).
Vigor indices were also calculated under pathogen challenged conditions. Rice seeds were sterilized with the same method as stated above. Sterilized seeds were soaked in cell suspensions corresponding to the highest vigor index (0.3 × 108 cfu/ml) in the above experiment and kept incubated under shaking conditions (150 rpm, 2 h). Ten seeds were then placed in the petri plates containing a modified PDA (HiMedia, 0.48% w/v) and agar (0.8% w/v). Fungal plugs were placed in the center of the plates. Sterilized seeds soaked in SDW were also placed in plates containing only the fungal plugs and in control plates. Plates were incubated at 28–30 °C and after 8 d, the number of germinated seeds, root lengths and shoot lengths were noted and compared with fungal challenged and control seedlings. Four replications were done per treatment and the experiments were repeated twice. Vigor index was calculated using the same formula.
2.7 Evaluation of PGP activity under nethouse conditions
Rice seeds were allowed to grow for 24 d in a pot containing sandy-loamy soils. Rice plants were uprooted and washed thoroughly under running water to remove the adhering soils of the roots. Roots were then dipped into a culture suspension corresponding to the highest vigor index (prepared as stated above) and kept for 2 h. For control the roots were dipped into SDW. Four plants were planted in each pot containing dried sandy-loamy soils (kept dried for 1 month). Six pots were kept for each treatment in randomized block design. Pots were watered daily with tap (nonsterile) water. The plants were harvested after 30 d and selected parameters (root length, shoot length, leaf length, leaf width, number of leaflets, fresh root weight, dry root weight, fresh shoot weight and dry shoot weight) were measured and compared with the controls.
2.8 Statistical analysis
All data were tested for significance by a one-way ANOVA followed by independent t-test at P ⩽ 0.05 using the SPSS 17 software package (SPSS Inc., USA).
3 Results
3.1 Strain selection and characterization
A total of 22 acidotolerant actinobacteria were isolated from a limestone deposit sample collected from Hundung, Ukhrul, Manipur and preserved as described earlier. These strains were screened for biocontrol activities against six rice fungal pathogens, of which 4 (four) isolates (18%) exhibited growth inhibition against almost all the indicator pathogens. Strain MBRL 10 was further selected as it showed maximum antagonistic activity against all the tested pathogens.
On the basis of 16S rRNA gene sequence analysis, strain MBRL 10 shared 100% sequence similarities with Streptomyces manipurensis MBRL 201T while sharing 99.8% similarities with Streptomyces cirratus NRRL B-3250T, Streptomyces noijiriensis LMG 20094T, Streptomyces racemochromogenes NRRL B-5430T and Streptomyces polychromogenes NBRC 13072T, and 99.7% similarities with Streptomyces amritsarensis 2AT and Streptomyces cinnamonensis NBRC 15873T. However, neighbor-joining dendrogram with the 16S rRNA gene sequences of closely related Streptomyces strains retrieved from EzTaxon-e server database (Fig. 1) indicated strain MBRL 10 form a separate clade within the closely related species of the genus Streptomyces. Due to the complex nature for species affiliation of Streptomyces strains and absence of polyphasic taxonomic data, the strain is hereby assigned as Streptomyces sp. MBRL 10.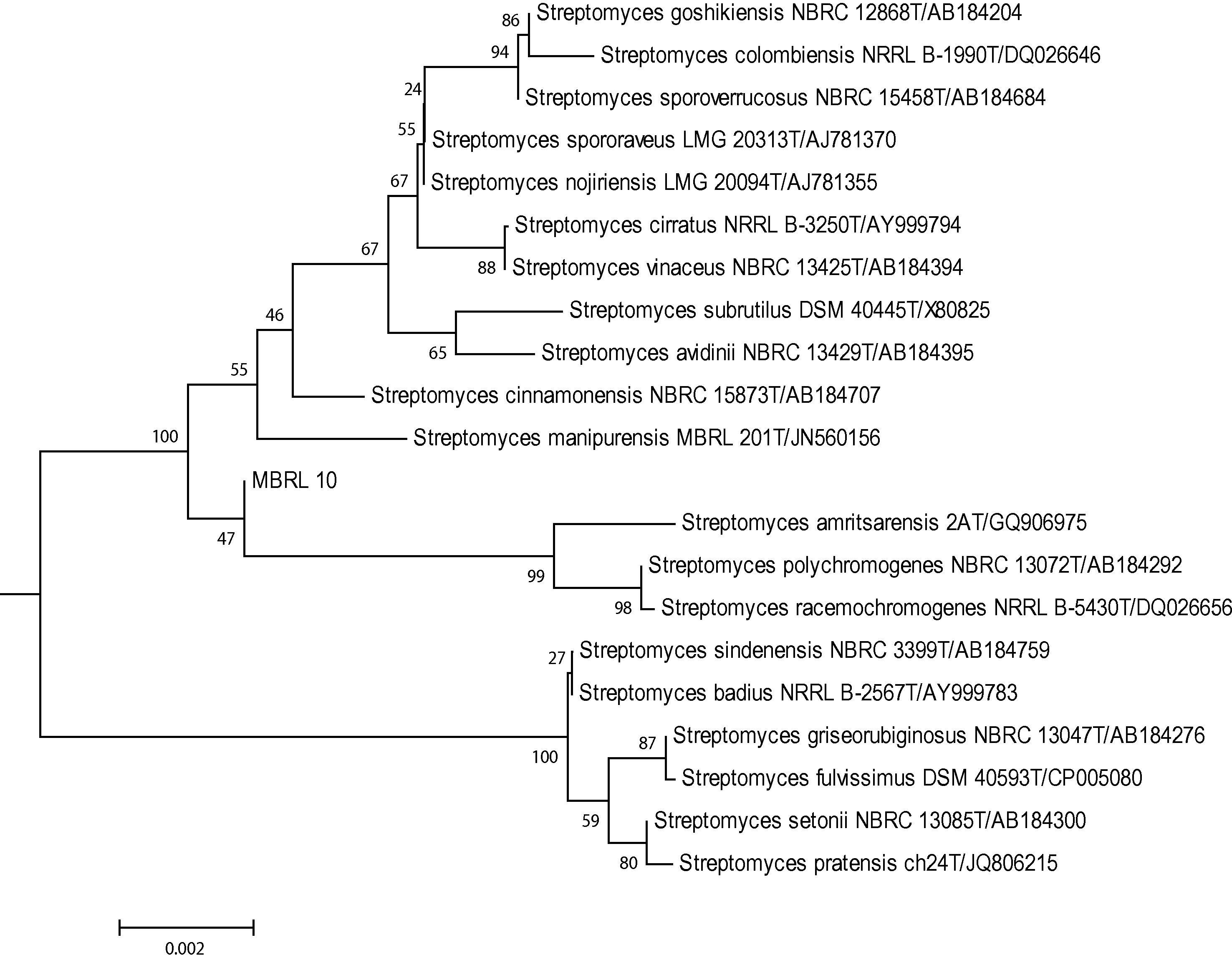
Neighbor-joining tree showing phylogenetic relationship of strain MBRL 10 with its closely related Streptomyces strains.
3.2 Antagonistic activities
Streptomyces sp. MBRL 10 showed significant antagonism against all the indicator fungal pathogens. It exhibited the highest mycelial growth inhibition by diffusible compound(s) against R. solani (69%) and the lowest antagonism was observed against R. oryzae-sativae (50%) and C. oryzae (50%). Inhibition by VOCs was the highest against R. solani (63%) and the lowest against F. oxysporum (32%) (Fig. 2). Culture filtrates also exhibited significant inhibition zone (15 to 23 mm), but it fails to exhibit an inhibition on R. oryzae-sativae. The inhibition activities of culture filtrates vanished when sterilized (Table 1). The strain produced chitinase, β-1,3-glucanase, lipase and protease but not β-1,4-glucanase. MBRL 10 showed positive results for ammonia production (Table 2). CP, Chitinase production; 1,3-GP, β-1,3-glucanase production; 1,4-GP, β-1,4-glucanase production; LP, Lipase production; PP, Protease production; AP, Ammonia production; PS, Phosphate solubilization; IP, Indole acetic acid production; SP, Siderophore production; ACCP, ACC deaminase production.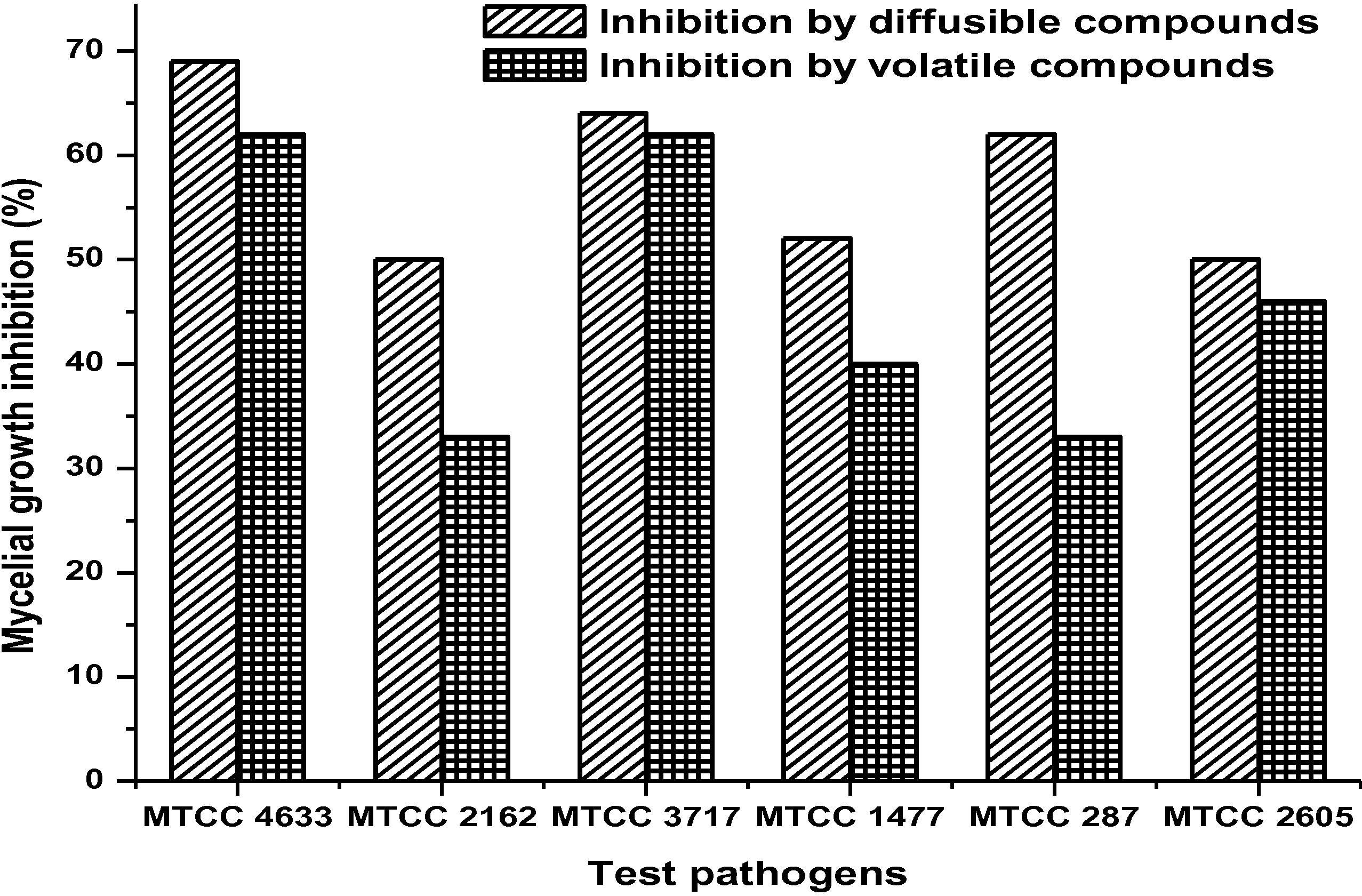
Mycelial growth inhibition of fungal test pathogens by strain MBRL 10 due to diffusible and volatile compound(s). MTCC 4633, Rhizoctonia solani; MTCC 2162, Rhizoctonia oryzae-sativae; MTCC 3717, Bipolaris oryzae; MTCC 1477, Pyricularia oryzae; MTCC 284, Fusarium oxysporum; MTCC 2605, Curvularia oryzae.
Fungal pathogens
MBRL-10
Non-sterile (in mm)
Sterile
MTCC 4633
20
–
MTCC 2162
–
–
MTCC 3717
23
–
MTCC 1477
22
–
MTCC 287
20
–
MTCC 2605
17
–
Characteristics
CP
1,3-GP
1,4-GP
LP
PP
AP
IP
PS
SP
ACCP
MBRL 10
+
+
−
+
+
+
+
+
+
−
3.3 Plant growth promoting activities
Streptomyces sp. MBRL10 showed positive results for almost all the PGP traits tested (Table 2). It produced maximum amounts of IAA (25 μg/ml) after 12 d of incubation (Fig. 3), solubilized P (up to 140 μg/ml) after 8 d of incubation with concomitant decrease in pH (from pH 7–4.22) of the medium (Fig. 4). The bioactive actinomycete strain produced hydroxamate type of siderophore and CAA was found to be the best medium for siderophore production (87% siderophore units, after 5 d of incubation). Little or no siderophore production was observed in GM, GP and NB media (Fig. 5). MBRL 10 gave negative results for ACC deaminase (Table 2).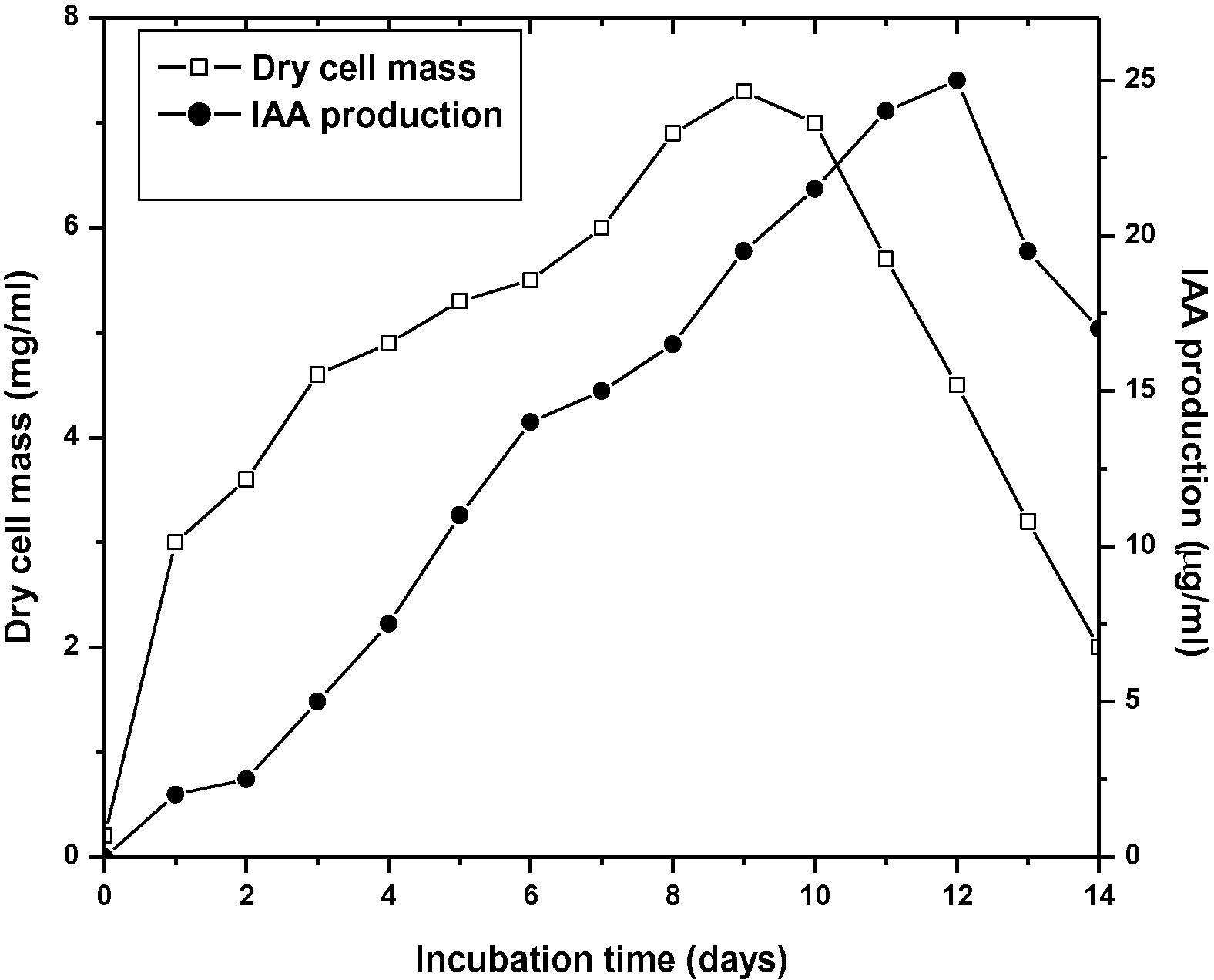
IAA production by MBRL 10 in the presence of 2 mg/ml of tryptophan.
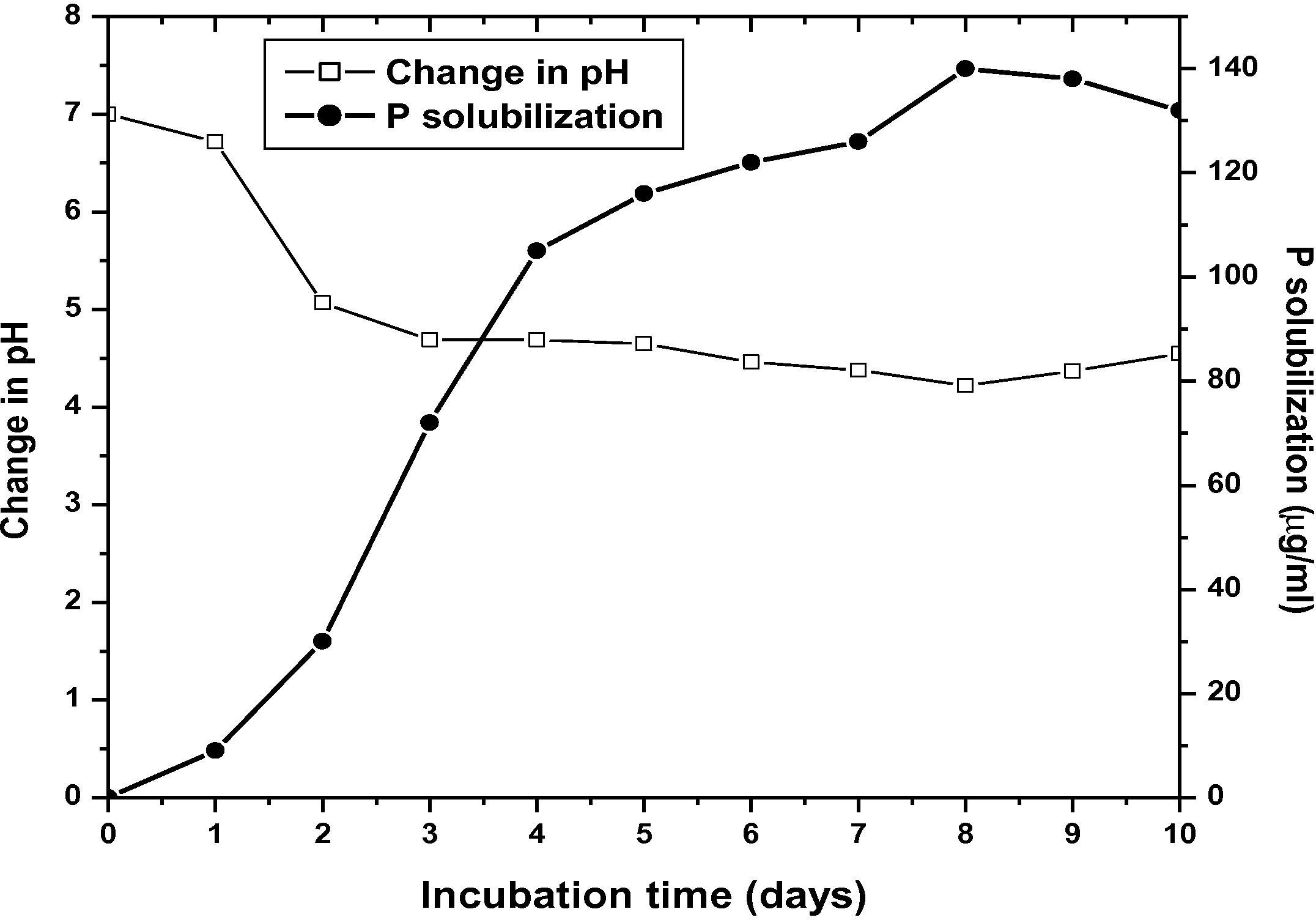
P solubilization by MBRL 10 with corresponding change in medium pH.
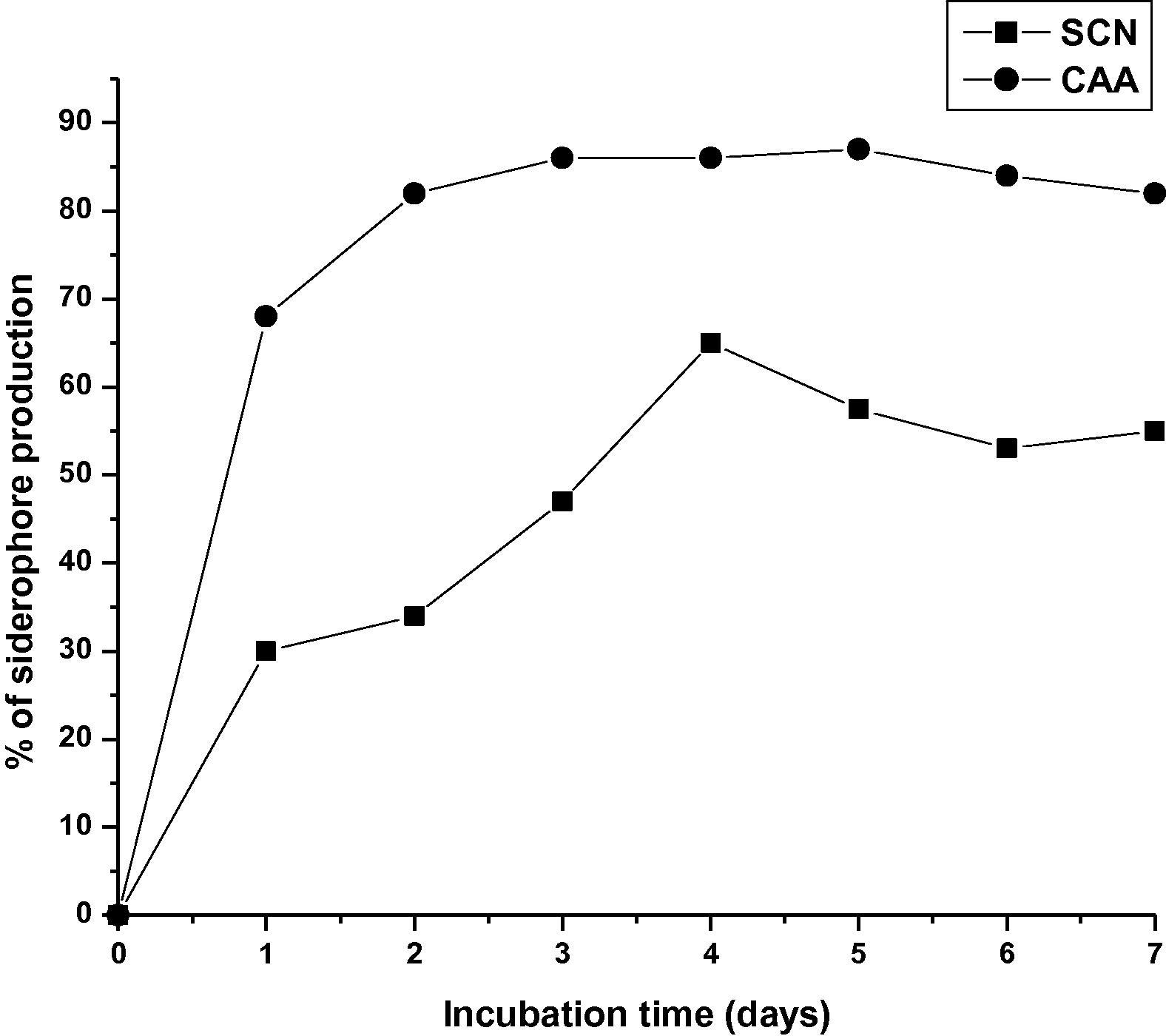
Siderophore production by MBRL 10 in different media.
3.4 In vitro seed germination test (vigor index) and rice plant growth promotion under nethouse conditions
Among the different inoculum densities, MBRL 10 showed the highest vigor index (632) corresponding to inoculum size of 0.3 × 108 cfu/ml. There was a decrease in vigor index with corresponding increase in the concentration of culture suspension (Table 3). Strain treated rice seeds at inoculum size of 0.3 × 108 cfu/ml showed higher germination percentage and significantly enhance (P ⩽ 0.05) the growth of root and shoot length over control. Strain treated rice seedlings challenged with pathogens also exhibited higher germination percentages and significant increase (P ⩽ 0.05) in root and shoot lengths over seedlings challenged with pathogen alone in the absence of the bioinoculant (Table 4) (Supplementary Fig. S1).
Treatment
Inoculum size (×108 cfu/ml)
Germination percent
Root length⁎ (cm)
Shoot length⁎ (cm)
Vigor index
Control
00
95
4.07 ± 0.34a
1.2 ± 0.59a
500.6
MBRL 10
0.3
100
4.86 ± 0.23c
1.45 ± 0.05c
632
0.6
100
4.66 ± 0.19b
4.66 ± 0.19b
599
1.2
100
4.42 ± 0.12b
1.25 ± 0.05b
567
1.8
100
4.79 ± 0.30c
1.32 ± 0.08b
560
2.4
100
4.68 ± 0.46b
1.21 ± 0.08a
550
3
100
4.30 ± 0.14b
1.19 ± 0.05a
549
Treatment
Germination percent
Root length⁎ (cm)
Shoot length⁎ (cm)
Vigor index
Control
93.3
1.96 ± 0.87c
3.99 ± 0.97b
555.2
MBRL 10
100
1.69 ± 0.20c
4.44 ± 0.50c
613
MTCC 4633
75
0.86 ± 0.73c
1.90 ± 1.78a
207
MTCC 4633 + MBRL 10
100
1.43 ± 1.05c
4.81 ± 0.61c
624
MTCC 2162
75
0.98 ± 0.77c
2.68 ± 2.05a
274.5
MTCC 2162 + MBRL 10
96.6
0.50 ± 0.33a
3.79 ± 1.38b
414.4
MTCC 3717
83.3
0.46 ± 0.40a
2.52 ± 1.32a
248.2
MTCC 3717 + MBRL 10
96.6
0.65 ± 0.40a
3.94 ± 1.53b
443.3
MTCC 1477
80
1.04 ± 0.93a
2.38 ± 1.57a
273.6
MTCC 1477 + MBRL 10
100
1.20 ± 1.08c
4.73 ± 0.34c
593
MTCC 287
86.6
1.79 ± 1.12c
3.44 ± 1.95b
452.9
MTCC 287 + MBRL 10
100
0.81 ± 0.54b
4.45 ± 0.92c
526
When assayed for growth promotion under nethouse conditions, bioinoculant rice plants showed significant increase (P ⩽ 0.05) in root, shoot and leaf length, leaf width, fresh and dry shoot weight and fresh root over control plants (Table 5) (S2).
Treatment
Root length (cm)⁎
Shoot length (cm)⁎
Leaf length (cm)⁎
Leaf width (cm)⁎
Leaflet⁎
Fresh shoot weight (g)⁎
Dry shoot weight (g)⁎
Fresh root weight (g)⁎
Dry root weight (g)⁎
Control
8.5 ± 2.1a
52 ± 5.7a
33.5 ± 4.9a
0.55 ± 0.1a
4a
0.8 ± 0.4a
0.35 ± 0.2a
0.16 ± 0.06a
0.12 ± 0.07a
MBRL 10
12.5 ± 4.9b
59 ± 0b
34.9 ± 6.9b
1.05 ± 0.1b
5a
2.71 ± 0.9b
0.59 ± 0.2b
0.37 ± 0.04b
0.14 ± 0.04a
4 Discussion
Intensive screening for new secondary metabolites is focusing on minor groups of actinomycetes, including species that are difficult to isolate and culture, and those that grow under extreme conditions (i.e. alkaline and acidic conditions) (Goodfellow and O’Donnell, 1989; Lazzarini et al., 2000; Zakalyukina and Zenova, 2007). Since many actinomycetes can produce spores, that help dissemination and confer resistance to many adverse conditions (Chater, 1993), they can be promising agents for development as novel biofertilizers and bio-control products under extreme environmental conditions.
In the present study, Streptomyces sp. MBRL 10 exhibited biocontrol activities against several important rice fungal pathogens. The strain showed mycelial growth inhibition by diffusible and volatile antifungal metabolites. Another possible factor contributing to the antifungal activity of the strain could be the production of cell wall degrading enzymes. Streptomyces sp. producing chitinase, β-1,3-glucanse, lipase and protease exhibited antagonism against R. solani, Colletotrichum gloeosporioides, Alternaria brassicae and Phytophthora capsici (Srividya et al., 2012). The antifungal compound(s) present in the culture filtrates must be heat labile as antagonistic activity was lost when sterilized. Antagonistic activity of the culture filtrates of MBRL 10 may be largely due to the presence of thermolabile enzymes such as chitinase and β-1,3-glucanase as reported by Prapagdee et al. (2008). MBRL 10 could produce ammonia. Ammonia produced by Enterobacter cloacae, has been reported to suppress the disease caused by Pythium sp. (Howell et al., 1988).
The bioactive actinomycete strain also showed promising PGP traits. MBRL 10 could produce significant amounts of IAA, a phytohormone essential for the growth and development of plants. IAA producing Streptomyces spp. promoted seed germination and plant growth in maize and cowpea (Khamna et al., 2010). The strain could solubilize inorganic P and increase in the level of solubilization corresponded with the decrease in the pH of the medium. This may be due to production of low molecular weight organic acids as reported by Rodriguez et al. (2004). MBRL 10 showed positive results for siderophore production; a compound that can chelate iron and make the bound iron available to the plants (Burd et al., 1998; Dimpka et al., 2008).
Rice seeds treated with cell suspension of MBRL 10 enhanced vigor index, germination percentages and growth of rice seedlings. Challenged with fungal pathogen reduced the rice seedlings vigor index. However, treatment with the cell suspension enhanced vigor index, germination percentage and growth of rice seedlings. Rice seeds soaked in Streptomyces sp. suspension have been reported to show enhanced germination rate and increased root and shoot lengths of the rice seedlings (Gopalakrishnan et al., 2012). MBRL 10 strain could also significantly enhance the growth of rice plants under nethouse conditions. Streptomyces sp. has been reported to enhance the growth of rice plants (Rungin et al., 2012). Acidophilic Streptomyces sp. MBRL 10 can be regarded as a potential agent for fungal disease protection and plant growth promotion in rice plants. Further investigation is needed in order to develop a formulation for application in agricultural crops especially rice, where soil pH can drop below 5.0 due to prolonged and excessive use of agrochemicals such as ammonia-based fertilizers or environmental factors such as acid rain.
5 Conclusions
Acidotolerant Streptomyces sp. MBRL 10 showed significant antagonism against important rice fungal pathogens by diffusible and volatile compound(s) production. Culture filtrates also exhibited significant inhibition zone. The strain could produce fungal cell wall degrading enzymes such as chitinase, β-1,3-glucanase, lipase and protease. It also showed positive results for IAA and siderophore production, and solubilize inorganic P. MBRL 10 treated rice seeds showed higher germination percentage and significantly enhance the growth of seedlings even under pathogen challenged conditions. Rice plants treated with the strain significantly promote the growth under nethouse conditions. Strain MBRL 10 can be regarded as potential for biocontrol and plant growth promoting agent especially for rice plant.
Acknowledgements
K.T. wishes to thank Council of Scientific and Industrial Research (CSIR), India for conferring him the CSIR-SRF. S.N. wishes to thank the University Grants Commission (UGC), Government of India, for offering him the Rajiv Gandhi National Fellowship. Thanks are due to Department of Biotechnology (DBT), Government of India for funding the project (BT/PR11469/AGR/21/275/2008 of 2008) which enabled the launch of rice biocontrol agent work at MBRL, Manipur University. We wish to acknowledge the facilities of SBTHub, DBT, Government of India that partly facilitated this work.
References
- Production and characterisation of cellulase by Bacillus pumilus EB3. Int. J. Eng. Technol.. 2006;3:47-53.
- [Google Scholar]
- Colorimetric determination of the components of 3,4-dihydroxphenylalaninetyrosine mixtures. J. Biol. Chem.. 1937;118:531-537.
- [Google Scholar]
- Vigor determination in soybean seed by multiple criteria. Crop Sci.. 1973;13:630-633.
- [Google Scholar]
- Characterization of a new Pseudomonas aeruginosa strain NJ-15 as a potential biocontrol agent. Curr. Microbiol.. 2003;46:324-328.
- [Google Scholar]
- A plant growth-promoting bacterium that decreases nickel toxicity in seedlings. Appl. Environ. Microbiol.. 1998;64:3663-3668.
- [Google Scholar]
- Microbiology: A Laboratory Manual. 1992. New York
- Genetics of differentiation in Streptomyces. Annu. Rev. Microbiol.. 1993;47:685-713.
- [Google Scholar]
- Comparative studies of actinomycete populations in acid podzolic and neutral mull forest soil. Proc. Soil Sci. Am.. 1964;28:68-69.
- [Google Scholar]
- Hydroxamate siderophore produced by Streptomyces acidiscbies E13 bind nickel and promote growth in cowpea (Vigna unguiculata L.) under nickel stress. Can. J. Microbiol.. 2008;54:163-172.
- [Google Scholar]
- Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol.. 1958;75:592-601.
- [Google Scholar]
- Promotion of tomato (Lycopersicon esculentum Mill.) plant growth by rhizosphere competent 1-aminocyclopropane-1-carboxylic acid deaminase-producing streptomycete actinomycetes. Plant Soil. 2008;308:161-174.
- [Google Scholar]
- F.A.O., 2000. Bridging the gap in rice production. http://www.fao.org/News/2000/001203-e.htm.
- Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783-791.
- [Google Scholar]
- Search and Discovery of Industrially Significant Actinomycetes. In Microbial Products, New Approaches: Cambridge University Press; 1989.
- Plant growth-promoting traits of Streptomyces with biocontrol potential isolated from herbal vermicompost. Biocontrol Sci. Technol.. 2012;22:1199-1210.
- [Google Scholar]
- Influence of soil acidity on Streptomyces population inhabiting forest soils. Appl. Environ. Microbiol.. 1976;32:368-375.
- [Google Scholar]
- Effect of glyphosate on soil microbial activity and biomass. Weed Sci.. 2000;48:89-93.
- [Google Scholar]
- Reproduction of ammonia by Enterobacter cloacae and its possible role in the biological control of Pythium preemergence damping-off by the bacterium. Phytopathology. 1988;78:1075-1078.
- [Google Scholar]
- Powdered chitin agar as a selective medium for enumeration of Actinomycetes in water and soil. Appl. Microbiol.. 1975;29:422-426.
- [Google Scholar]
- I.R.R.I., 2006. Bringing Hope, Improving Lives: Strategic Plan, 2007–2015. Manila (Philippines), p. 61.
- I.R.R.I., 2008. International Rice Research Institute: IRRI World Rice Statistics (WRS), Facts and Figures: 1960–2010.
- Phosphate solubilisation potential and phosphatase activity of rhizospheric Trichoderma spp. Braz. J. Microbiol.. 2010;41:787-795.
- [Google Scholar]
- Actinomycetes isolated from medicinal plant rhizospheric soils: diversity and screening of antifungal compounds, indole-3-acetic acid and siderophore production. World J. Microbiol. Biotechnol.. 2009;25:649-655.
- [Google Scholar]
- Indole-3-acetic acid production by Streptomyces sp. isolated from Thai medicinal rhizosphere soils. Eur. Asian J. Biol. Sci.. 2010;4:23-32.
- [Google Scholar]
- Studies on the ecology of actinomycetes in soil: VIII. Distribution and characteristics of acidophilic actinomycetes. Soil Biol. Biochem.. 1975;7:345-348.
- [Google Scholar]
- Introducing EzTaxon-e: a prokaryotic 16S rRNA Gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol.. 2012;62:716-721.
- [Google Scholar]
- The Neutral Theory of Molecular Evolution. Cambridge University Press; 1983.
- Rare genera of actinomycetes as potential producers of new antibiotics. Antonie Van Leeuwenhoek. 2000;78:399-405.
- [Google Scholar]
- Georgenia ruanii sp. nov., a novel actinobacterium isolated from forest soil in Yunnan (China), and emended description of the genus Georgenia. Int. J. Syst. Evol. Microbiol.. 2007;57:1424-1428.
- [Google Scholar]
- An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr. Microbiol.. 2001;43:51-56.
- [Google Scholar]
- Ornibactin production and transport properties in strains of Burkholderia vietnamiensis and Burkholderia cepacia (formerly Pseudomonas cepacia) Biometals. 1995;8:309-317.
- [Google Scholar]
- Streptomyces manipurensis sp. nov., a novel actinomycete isolated from a limestone deposit site in Manipur, India. Antonie van Leeuwenhoek. 2012;102:133-139.
- [Google Scholar]
- Streptomyces hundungensis sp. nov., a novel actinomycete with antifungal activity and plant growth promoting traits. J. Antibiot.. 2013;66:205-209.
- [Google Scholar]
- Antagonistic activities of local actinomycete isolates against rice fungal pathogens. Afr. J. Microbiol. Res.. 2009;3:737-742.
- [Google Scholar]
- Detection, isolation and characterization of siderophores. Methods Enzymol.. 1994;235:329-344.
- [Google Scholar]
- Acidophillic actinomycetes from rhizospheric soil: diversity and properties beneficial to plants. J. Antibiot.. 2015;68:106-114.
- [Google Scholar]
- Antifungal potential of extracellular metabolites produced by Streptomyces hygroscopicus against phytopathogenic fungi. Int. J. Biol. Sci.. 2008;4:330-337.
- [Google Scholar]
- Rice biotechnology for developing countries in Asia. NABC Report 16: agriculture. Biotechnology 2004:201-232.
- [Google Scholar]
- Chitinase-overproducing mutant of Serratia marcescens. Appl. Environ. Microbiol.. 1981;41:664-669.
- [Google Scholar]
- Gluconic acid production and phosphate solubilization by the plant growth-promoting bacterium Azospirillum spp. Naturwissenschaften. 2004;91:552-555.
- [Google Scholar]
- A plant growth enhancing effects by a siderophore-producing endophytic streptomycete isolated from a Thai jasmine rice plant (Oryza sativa L. cv. KDML105) Antonie Van Leeuwenhoek. 2012;102:463-472.
- [Google Scholar]
- The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol.. 1987;4:406-425.
- [Google Scholar]
- Production of microbial iron chelators (siderophores) by fluorescent Pseudomonads. Ind. J. Biotechnol.. 2005;4:484-490.
- [Google Scholar]
- Streptomyces sp. 9p as effective biocontrol against chilli soilborne fungal pathogens. Eur. J. Exp. Biol.. 2012;2:163-173.
- [Google Scholar]
- MEGA 5: molecular evolutionary genetic analysis using maximum likelihood evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol.. 2011;28:2731-2739.
- [Google Scholar]
- In vitro evaluation of antagonistic properties of Pseudomonas corrugate. Microbiol. Res.. 2008;163:329-336.
- [Google Scholar]
- Ventura, L.A., 2000. The Effect of Soil pH on Plant Growth. Science Experiments on File. Revised. Facts on the File, Inc. 4, pp. 1–5.
- The role of Streptomycetes in decomposition of chitin in acidic soils. J. Gen. Microbiol.. 1981;127:55-63.
- [Google Scholar]
- Isolation and characterization of actinomycetes antagonistic to pathogenic Vibrio spp. from nearshore marine sediments. World J. Microbiol. Biotechnol.. 2005;21:679-682.
- [Google Scholar]
- Antagonistic activity of soil acidophilic actinomycetes. Biol. Bull.. 2007;34:329-332.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jksus.2016.10.003.
Appendix A
Supplementary data







