Translate this page into:
A study on the role of aedes mosquitoes in arboviruses and SARS-CoV-2 infection: A new challenge
⁎Corresponding authors. shahidmahboob60@hotmail.com (Shahid Mahboob)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Chikungunya, Zika, Dengue Viruses, and now Novel Coronavirus are global health challenges that cause human diseases ranging from febrile illnesses to death. Most of these viruses are mainly vectored by Aedes mosquitoes worldwide. Molecular detection of arboviruses was made in female Aedes mosquito pools caught from all the seven districts by using a reliable molecular technique, “RT-PCR.” From 216 collections of Aedes species, arboviruses were detected in 27, including only Alphavirus genus to determine mosquito abundance and evaluate the potential role of Aedes aegypti and Ae. albopictus mosquitoes in arboviruses and nvel Coronavirus transmission. 5322 mosquitoes were collected using aspirators; 35.31% (n = 2049) were identified as female Aedes using morphological keys, pooled into 216 pools, and tested for arboviruses and coronaviruses by using RT-PCR with the help of specific primers. Novel Coronavirus was not detected in this study. Only the Flavivirus genus was detected in twenty-seven pools giving an infection rate of 62.96% (n = 17) for DENV2, while DENV3 was 37.03% (n = 10). Furthermore, our results indicated no role of mosquitoes in the spread of Covid-19. Results showed a higher infection rate in urban sites than in rural ones. The detection of arboviruses indicates possible human health risk due to active role of these mosquitoes in spreading of arbovirus in the study area.
Keywords
Aedes aegypti
Aedes albopictus
Arboviruses
Dengue
Mosquito
Novel coronavirus
RT-PCR
1 Introduction
Old and evolving mosquito-borne pathogens posed a significant challenge to public health authorities and the human population. These pathogens cause significant worldwide disease pressure. Half of the world is at risk of infection by vector-borne pathogens (Anonymous, 2007). The Zika virus, rift valley fever virus (RVFV), yellow fever virus (YFV), dengue virus (DENV), and Chikungunya virus (CHIKV) are the arthropod-borne viruses. They are considered the most common emerging pathogens vectored by Aedes mosquitoes in tropical and subtropical countries worldwide (World Health Organization, 2011). The increase in human activities in forested areas around the globe, especially tropical areas, having hot and humid conditions is an essential factor for the spreading of these arboviruses. (Appawu et al., 2006). A recent increase in the distribution of the urban mosquito vector, mainly Aedes aegypti, even in non-endemic regions, is due to a rise in man's traveling (Alan-Barrett, 2010). Infectious diseases caused by arboviral infections transmitted by arthropods are common in several districts of Pakistan; they cause a high morbidity and mortality rate in human beings and animals because they are often misdiagnosed and treated as malaria (Fig. 1).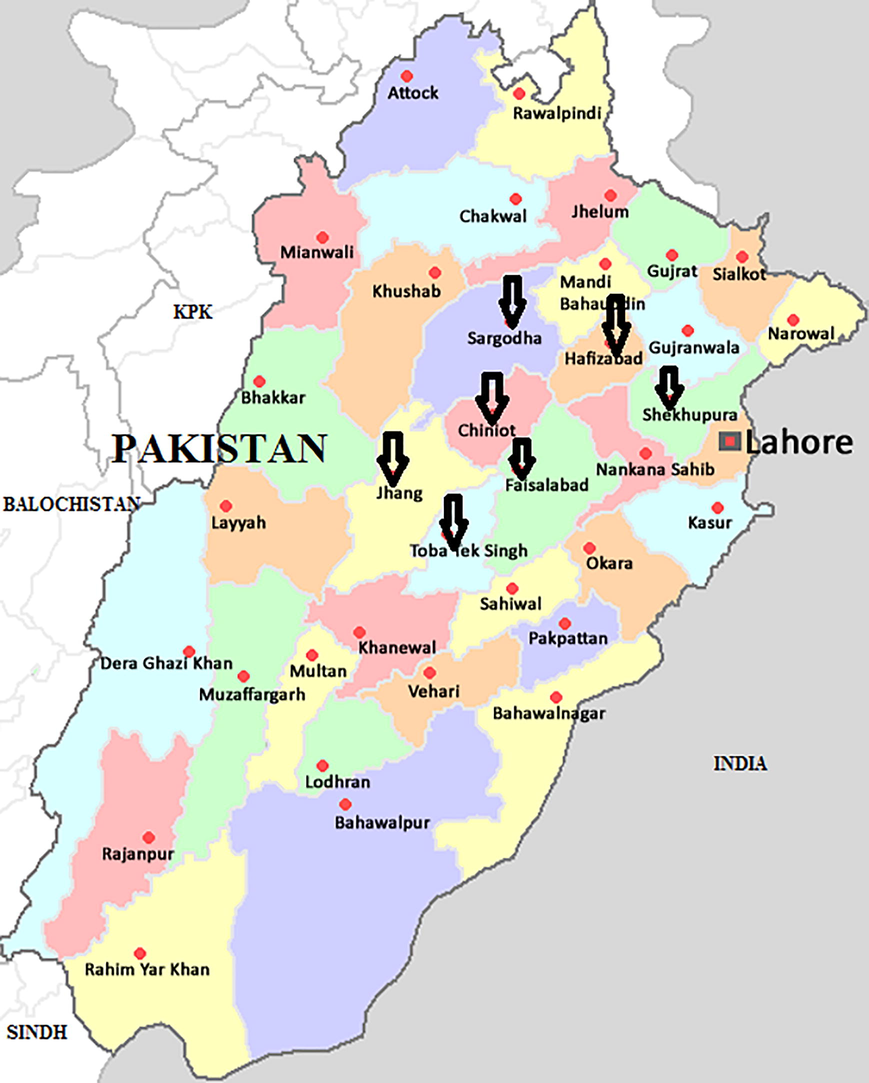
Map of the Punjab, Pakistan, showing the districts selected for collection of mosquitoes.
Dengue fever is caused by the Aedes-borne virus and transmitted by Ae. aegypti and, to a lesser degree Ae. albopictus in urban and peri-urban areas. Dengue virus has four distinct serotypes, which are DENV1, DENV2, DENV3, and DENV4 (Munoz et al., 2009). In Pakistan, serotypes 2 and 3 are prevalent in different cities in Punjab. Due to unsafe water, packed cities, insufficient sanitation, overprovision of shelters, and squat vaccination exposure, dengue is endemic in Pakistan, and the situation usually attains peaks during the rainy season (Jahan, 2011).
Novel Coronavirus belongs to the family Coronoviridae and genera Betacoronavirus cause pneumonia-like symptoms, first appeared in Wuhan, China, in December 2019, and has rapidly increased in pandemic scale. This was named coronavirus disease (Covid-19) on the 11th of February 2020 by WHO (Zhou et al., 2020). The outbreak of this disease has quickly spread worldwide, caused uncountable human deaths, and paralyzed the economy, health, trade, and all other systems of the planet earth in the short period of six months. The genome of SARS-SoV-2 was 85% homologous to bat SARS-like virus and possessed 79% homology with SARS-CoV. In addition to this, pangolins have also been reported to be an intermediate host of SARS-CoV-2 (Lake, 2020).
Aedes mosquitoes play an active role in transmitting the Chikungunya virus (CHIKV) (Borgherini et al., 2007). Chikungunya is now spreading rapidly in Karachi, Pakistan. Chikungunya virus was found circulating in rodents in Pakistan as early as 1983 (Darwish et al., 1983). More than 4000 cases have been confirmed through qualitative RT-PCR by the “National Institutes of Health and Armed Forces Institute of Pathology” in Pakistan (Rauf and Fatima-tuz-Zahra, 2107). Last year, 4868 Chikungunya cases were reported from Sindh, and in Karachi, 73 patients were reported in December 2017.
Arboviruses were detected by using the molecular techniques in the field caught Aedes mosquitoes from Al-Madinah Al-Munawwarah, Saudi Arabia (Ayman et al., 2012), from five sites in Kyela district, Kenya (Bisimwa et al., 2016), from Swat, Khyber Pakhtunkhwa, Pakistan (Khan et al., 2018) by using the molecular technique of real-time RT-PCR.
Pakistan has had multiple arbovirus outbreaks resulting in economic and public health distress. Pakistan, along with other Asian countries, is facing climate change. Winters are getting shorter every year while the summers are becoming longer and harsher. Due to the warmer climate, many arboviral illnesses have occurred in this region, such as dengue, malaria, and Chikungunya. Most Asian countries have bad sanitary conditions that favor the arthropod vectors by providing excellent breeding sites. Rapidly increasing dengue, Chikungunya, and covid-19 cases in Pakistan have created an alarming situation. Due to the lack of efficient measures and fragmentation of natural ecosystems, diseases caused by arboviruses and Coronavirus will become a severe public health problem both in endemic developing countries and many non-endemic countries (Rauf and Fatima-tuz-Zahra, 2107). Thus, detecting viruses in their hosts (mosquitoes) is necessary to determine the viral activities. The objectives of the present study to detect the role of Aedes mosquitoes in the transmission of arboviruses and Coronavirus by using RT-PCR, collected from seven districts of central Punjab, Pakistan.
2 Materials and methods
2.1 Sampling of mosquitoes
Adult mosquitoes were collected in the mornings (7–10 am) and evenings (5–7 pm) using a battery-operated aspirator (Florencio et al., 2014) amongst the vegetation and from both indoor and outdoor resting sites. Field samplings were carried out four times a year from May 2018 to May 2020 during four seasons, viz. spring (February to April), early summer (May to July), rainy season (August to October), and Winter (November to January) from the seven districts (Faisalabad, Sargodha, Hafizabad, Shaikhupura, Toba Tek Singh, Jhang and Chiniot) of the province Punjab, Pakistan. Samples were collected separately from urban and rural areas, including city parks, industrial sites, residential areas, from and near water storage containers, old used tyres, grounded vehicles, and trains. Commercial ports (dry ports) and airports were also surveyed to collect the invasive mosquito species (IMS). In addition, sampling of mosquitoes was done from urban and suburban areas, including bushes and shrubs, crops, parks, construction sites, marshes, ponds, forests, and all types of vegetation found in the study area.
2.2 Mosquito analysis
After collection, specimens were brought back to the Zoology Laboratory, Department of Zoology, Government College University, Faisalabad, inside vials closed with muslin cloth after providing a specific field number, date, and time collection, type of habitat, and location. After bringing in the laboratory, adult Aedes mosquitoes were sorted out after identification using a microscope (Olympus) and appropriate taxonomic key, according to Becker et al. (2010). Female Aedes mosquitoes were pooled (up to 15 mosquitoes per pool) based on collection sites, and season and RNA were extracted on the same day.
2.3 Extraction and analysis of viral RNA
According to the manufacturer's recommendations, a pure Link™ RNA Mini Kit extracted the RNA from pooled mosquito samples.
2.3.1 Lysis and Homogenization:
Sample (2.5 mg mosquito tissues) was put in the RNase free tube and made centrifuge at 2,000 rpm for 5 min at the temperature of 4 °C to form the pellets, then mixed 0.6 ml of lysis buffer with 2-mercapto ethanol to the sample. Vortex was made to continue with high speed up to the lysis of cells. Further homogenization was processed by transferring the lysate to a homogenizer and centrifuging at 12,000 rpm for 2 min.
2.3.2 Binding, washing, and elution
We added 03 ml of 70% ethanol in homogenate and mixed thoroughly by vortexing until the disappearance of a precipitate. After centrifuging, we purged it by removing the supernatant, putting it in the spin cartridge, and then in the same collection tube. Centrifuged the sample at 12,000 rpm for 20 s at room temperature after adding the 700 μl wash buffer I into the spin cartridge and again made purification by discarding the supernatant. After that, centrifuged and dried the membrane of a spin cartridge to which attached RNA. Centrifuged was made again to elute the RNA from the membrane into the recovery tube.
2.3.3 Spectrophotometric analysis
1 µl of RNA was diluted with 39 µl of DEPC treated water. We used a 10ul micro cuvette to take OD at 260 nm and 280 nm to determine the purity and sample concentration. Using the formula that 1 OD at 260 is equal to 40 µg/ml RNA, the A260/A280 ratio is maintained above 1.6.
2.3.4 cDNA synthesis
4 μl of extracted RNA was mixed with 16 μl of a master mix, 2 μl of 10x enzyme mix, and 10 μl nuclease-free water, and the whole volume was brought up to 20 μl. The tube contents were mixed by vortexing for 15 s and then incubated to synthesize cDNA.
2.3.5 Detection of arbovirus by RT-PCR
To detect the arboviruses and Coronavirus in mosquitoes, primers were used from Invitrogen USA. The sequences of these primers were taken from an already published article. Positive samples were further tested to detect the presence of specific viruses by using conserved genes targeting primers, as shown in Tables 1 and 2. The type-specific primers anneal specifically to each of their respective genotype (Lanciotti, 2003; Chan et al., 2020). All the primers are given according to their individual published sequence.
Virus
Target gene or protein
Primer
Sequence (5′→3′)
Position
PCR product size (bp)
Reference
Alphavirus
NSP4
VIR2052F
VIR2052RTGG CGC TAT GAT GAA ATC TGG AAT GTT
TAC GAT GTT GTC GTC GCC GAT GAA6971–6997
7086–7109150
Eshoo et al. (2007)
Bunyavirus
N Protein
BCS82C
BCS332VATC ACT GAG TTG GAG TTT CAT GAT GTC
GCTGT TCC TGT TGC CAG GAA AAT86–114
309–329251
Bryant et al., 2007
Flavivirus
NS5
FU1
CFD2TAC AAC ATG ATG GGA AAG AGA GAG AA
GTG TCC CAG CCG GCG GTG TCA TCA GC9007–9032
9308–9283220
Kuno et al., 1996
Coronavirus
COVID −19 – S
Spike/Forward
Spike/Reverse
ProbeCCTACTAAATTAAATGATCTCTGCTTTACT
CAAGCTATAACGCAGCCTGTA
HEX -CGCTCCAGGGCAAACTGGAAAG–IABkFQ
30
21
22Chan et al., 2020
COVID −19 – N
Nucleocapsid/Forward
Nucleocapsid/reverse
ProbeGCGTTCTTCGGAATGTCG
TTGGATCTTTGTCATCCAATTTG
FAM -AACGTGGTTGACCTACACAGST- lABkFQ
18
23
22Chan et al., 2020
Virus
Primers
Sequence (5′-3′)
Region, position
Reference
DENV
D1 F
TCA ATA TGC TGA AAC GCG CGA GAA ACC G
3′UTR, 10520–10541
Lanciotti, 2003
D2F
TTG CAC CAA CAG TCA ATG TCT TCA GGT TC
3′UTR, 10674–10694
D2R
CGTCTCAGTGATCCGGGGG
568–586, Size 482
TS-1
CGCCACAAGGGCCATGAACAG
232–252, Size: 119
TS-2TAACATCATCATGAGACAGAGC
400-421Size: 290
TS-3
CTCTGTTGTCTTAAACAAGAGA
506–527 Size: 392
ZIKV 1086
TS-4
CCGCTG CCC AAC ACA AG
Lanciotti et al., 2017
The ordered primers were delivered in the lyophilized form. Each primer was reconstituted to a 1000 µM concentration by adding 100 µl nuclease-free water. In PCR, denaturation of DNA was made by heating at 94 °C for 15 min, then annealing at 57 °C for 60 s, and extension was made at 72 °C for 10 min. Last, 15 µl from PCR solution was analyzed by electrophoresis in 2% agarose gel stained with ethidium bromide at 80 V for one hr. Then the product was observed under ultraviolet light, and different amplified bands were observed and captured with a camera.
3 Results
3.1 Collection of mosquitoes
During this study, 5802 adult mosquitoes were caught from 140 selected sites (10 from urban areas while 10 from rural areas of each district) from May 2018 to May 2020 from seven selected districts, viz. Faisalabad, Sargodha, Shaikhupura, Toba Tek Singh, Hafizabad, Jhang, and Chiniot districts, Punjab, Pakistan. Female Aedes were separated from other mosquitoes and pooled into 216 pools, each containing up to 15 mosquitoes on the bases of collection sites and season consisting of 41, 37, 34, 33, 27, 26, and 18 pools from Faisalabad, Sargodha, Shaikhupura, Hafizabad, Toba Tek Singh, Jhang and Chiniot districts respectively.
3.2 Seasons distribution of Aedes mosquitoes
Physical factors (temperature, rainfall, and relative humidity) prevailing in the selected areas had a significant influence on the prevalence of the mosquito population. More rain falls during seasons; summer 18, rainy 18, and rainy 19 resulted in more relative humidity and suitable temperature (30–35 °C), favoring the mosquito population. Hence, the highest mosquito population was observed in these seasons. The Aedes population recorded the highest population when the mean temperature was below 30 °C, as shown in Fig. 2. So, the maximum Aedes population was noted during springs 19 & 20.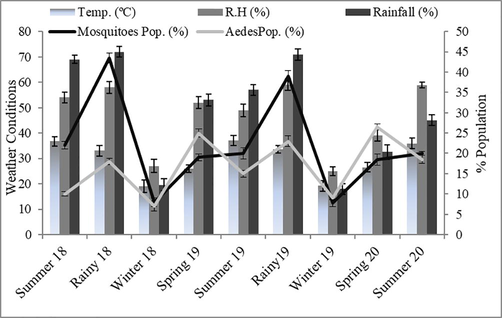
Relationship of different physical factors with mosquito population as a whole and Aedes mosquitoes.
3.3 Molecular detection of arboviruses in female Aedes mosquitoes
RNA was extracted from pooled mosquito samples using the Pure Link™ RNA Mini Kit according to the manufacturer's recommendations. Molecular detection was performed in two steps; firstly, screening was made to detect different viruses (Bunyavirus, Alphavirus, Flavivirus, and Coronavirus). From 216 Aedes pools screened, arboviruses were detected in 27 pools belonging to Flavivirus. No sample was found positive for Alphavirus, Bunyaviruses and Coronavirus. The positive mosquito samples (actual pools 4, 9, 12, 15,17, 20, 24, 36, 40, 48, 55, 61, 67, 78, 88, 90, 110, 135, 150, 168, 179, 180, 186, 196, 200, 204, 215) were then further tested by using specific primers (Figs. 3 and 4).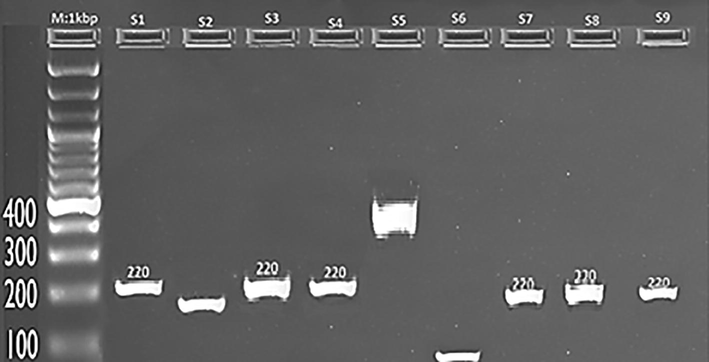
Visualization of RT-PCR product of the arbovivirus and Coronavirus M: Marker(1000 bp), The expected size of amplicon was 220 bp. Samples 1,3,4, 7, 8, and 9 were positive for Flavivirus, while samples 2, 5, and 6 were negative for any arbovivirus and Coronavirus.
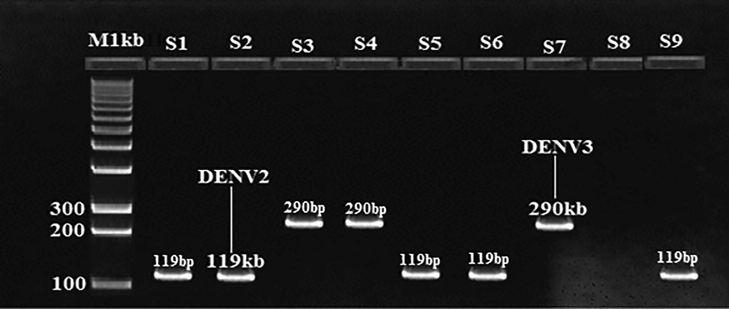
Visualization of RT-PCR products for Dengue virus. The expected PCR product size was 119 bp for DENV2 and 290 bp for DENV3.M: DNA marker (1000 bp). Samples 1, 2, 5,6, and 9 were positive for DENV2, while samples 3, 4, and 7 were positive for DENV3.
Over all infection rate of DENV2 62.96% (n = 17) while DENV 3 was 37.03% (n = 10). The highest number of DENV2 was detected in mosquitoes sampled from Sargodha 10.81% (n = 5) followed by Faisalabad 09.75% (n = 4), Shaikhupura 08.82% (n = 3), Hafizabad 06.06% (n = 2), Chiniot 05.55% (n = 1) Jhang 03.84% (n = 1), and then Toba Tek Singh 03.70% (n = 1). DENV3 was found to be more positive in Sargodha 10.81% (n = 4) then in Faisalabad 07.31% (n = 3), Toba Tek Singh 03.70% (n = 1), Hafizabad 03.03% (n = 1), Shaikhupura 02.94% (n = 1), Jhang (nil) and Chiniot (nil) as shown in Fig. 4. Results showed that, higher rate of infection was found in the urban sites than rural ones.
4 Discussion
Mosquitoes play a vital role in spreading arboviral disease throughout the world. To determine high-risk areas where the emergence and circulation of arboviral diseases might occur, the study of arboviral vectors is an initial aspect. The present study investigated Aedes mosquito-borne viruses in seven districts (Faisalabad, Sargodha, Shaikhupura, Toba Tek Singh, Hafizabad, Jhang, and Chiniot) of central Punjab, Pakistan. During this study, 5802 adult mosquitoes were collected using battery-operated aspirators from May 2018 to May 2020. Female Aedes mosquitoes were separated and selected for this study. The results showed that out of 5802 collected mosquitoes, Female Aedes were 35.31% (n = 2049), while other than female Aedes, mosquitoes were 64.68% (n = 3753).
According to our results, female Aedes mosquitoes were the most prevalent from February to April (spring season) 2019 and 2020, while the least number in the winter season (November to January) 2018 & 19. This season-wise fluctuation in the population indicated the effect of climate on the reproduction of these mosquitoes. They begin to increase their population size on hot temperatures and humid air availability. Mosquito abundance differed dramatically among the ecologically distinct districts where the mosquitoes were collected due to large livestock, uncontrolled human population, urbanization, and increasing breeding sites in these areas for several species, particularly Aedes mosquitos. These results were similar to the findings of Matthew et al. (2008). These observations are also supported by studies (Rasheed, 2012). Our results are also very close to Akram et al. (2009), who reported the abundance of mosquitoes from March to September. Dhimal et al. (2014) collected vectors (mosquitoes) of malaria, Chikungunya and dengue virus, lymphatic filariasis, and Japanese encephalitis in the same season. Mori and Wada (2007) also reported the most minor population of Aedes mosquitoes during the winter (December to January. These results are at par with our findings. Our results also showed that populated areas like Faisalabad and Sargodha provided more suitable habitats for Aedes mosquitoes. These results agree with previous surveillance studies conducted in Pakistan, such as in Murree Hills (Qasim et al., 2014), Kasur and Sheikhupura (Oneeb et al., 2016). They recorded species composition and then analyzed these results with temperature, relative humidity, and rainfall for different species of mosquitoes. They also collected the least mosquitoes in winter (December to January), but they found the maximum population in July due to more rains and breeding sites. These results differ from our findings because we observed the entire people in March to April due to more showers and humidity than in July and August.
Molecular detection of arboviruses was made in female Aedes mosquito pools caught from all the seven districts by using a reliable molecular technique, “RT-PCR.” From 216 collections of Aedes species, arboviruses were detected in 27, including only Alphavirus genus. All samples showed a negative result for Flavivirus and Bunya viruses. The positive mosquito samples were then further tested using specific primers. This result indicates that Aedes mosquitoes are involved in arbovirus transmission in these districts. Most infected pools were detected from Faisalabad, Sargodha, and Shaikhupura. These three regions are classified as semi-arid with a high level of human population and industrialization, ideal for reproducing arboviral vectors. Mosquitoes collected in these areas may feed on infected people and become infected with arboviruses. These findings are supported by the study of Anyambaet al. (2001). These results are different from our findings because we found peak dengue infection in March and April.
This study detected dengue virus (DENV2 and DENV3) in the collected mosquitoes. These results are supported by the findings of Jahan (2011), who also observed that serotypes 2 and 3 were prevalent in different cities of Punjab, Pakistan. Khan et al. (2018) conducted field sampling of mosquitoes in Swat, Khyber Pakhtunkhwa, Pakistan, and analyzed field data for epidemiological trends of dengue. They used the reverse transcriptase PCR technique to detect all types of dengue viruses. They found that dengue was at its peak (56%) in September 2013 and 24% in October 2014.
Studies carried out in New California by Dupont et al. (2012) and in Argentina (Domingo et al., 2005) confirmed the involvement of Aedes aegypti in Chikungunya and dengue transmission during epidemics. The findings of Cumberland (2009) demonstrated that Filariasis, Japanese encephalitis, Yellow fever, Plague, Dengue fever, Malaria, Zika, and Chikungunya are transmitted among vertebrate animals, including human beings, and create great concern for public health. Indeed, detection of infection of arboviruses in this area indicates that Aedes mosquitoes can vector arboviruses, including dengue fever virus. The low positivity obtained for genera-specific could be due to the mosquito preservation method used and the time between sample collection and laboratory analysis. In this investigation, a new Aedes mosquito was used for RNA extraction. The findings of Mbanzulu et al. (2017), Bisimwa et al. (2016), Monika et al. (2016), and Ayman et al. (2012) also supported the results of this study. They also detected arboviruses in the Aedes mosquitoes responsible for causing Chikungunya and dengue. Our results indicated no involvement of Aedes mosquitoes in the spread of Novel Coronavirus (SARS-CoV-2) involved in COVID-19. This finding is in line with the findings of (Huang et al., 2020; Xia et al., 2020), who also found similar results, but their methodology is different from our studies. They did not use mosquitoes collected from the fields. Instead, they tried to replicate the Coronavirus in mosquitoes under lab conditions. We performed our analysis on field-collected mosquitoes.
5 Conclusions
It has been concluded that there is no involvement of Aedes mosquitoes in the spread of Novel Coronavirus (SARS-CoV-2) involved in COVID-19. The low positivity obtained for genera-specific could be due to the mosquito preservation method used and the time between sample collection and laboratory analysis. The detection of arboviruses indicates possible human health risk due to active role of these mosquitoes in spreading of arbovirus in the study area.
Acknowledgements
The analytical facilities are provided by Hi-Tech Lab, Government College University, Faisalabad, Punjab, Pakistan. The authors express their sincere appreciation to the Researchers Supporting Project Number (RSP2022R466) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Seasonal distribution and species composition of daytime biting mosquitoes. Entomological Research. 2009;39(2):107-113.
- [Google Scholar]
- Climate-disease connections: Rift Valley fever in Kenya. Cadernos de Saude. Publica. 2001;17:133-140.
- [Google Scholar]
- Surveillance of Viral Haemorrhagic Fevers in Ghana: Entomological Assessment of the Risk of Transmission in the Northern Regions. Ghana. Ghana Medical Journal. 2006;40(4):137-141.
- [Google Scholar]
- Molecular study on the dengue virus in Aedes aegypti from Al-Madinah, Saudi Arabia using one step real time RT-PCR. Science Parasitology. 2012;13(4):133-138.
- [Google Scholar]
- Molecular detection of arboviruses in Aedes mosquitoes collected from Kyela district, Tanzania. Revue de Medecine Veterinaire. 2016;167:138-144.
- [Google Scholar]
- Outbreak of Chikungunya on Reunion Island: early clinical and laboratory features in 157 adult patients. Clinical Infectious Diseases. 2007;44(11):1401-1407.
- [Google Scholar]
- Isolation of arboviruses from mosquitoes collected in northern Vietnam. American Journal of Tropical Medicine and Hygiene. 2007;73:470-473.
- [Google Scholar]
- Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-polymerase chain reaction assay validated in vitro and with clinical specimens. Journal of Clinical Microbiology. 2020;58(5):e00310. e00320
- [Google Scholar]
- Sero-epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1983;77:442-445.
- [Google Scholar]
- Species composition, seasonal occurrence, habitat preference and altitudinal distribution of malaria and other disease vectors in estern Nepal. Parasites & Vectors. 2014;9(4):1-22.
- [Google Scholar]
- Molecular detection of dengue viruses in field caught Aedes aegypti mosquitoes from northeastern Argentina. Revista Latinoamericana de Microbiologia. 2005;47(3–4):82-87.
- [Google Scholar]
- Chikungunya Virus and the Mosquito Vector Aedes aegypti in New Caledonia (South Pacific Region) Vector Borne and Zoonotic Diseases. 2012;12(12):1036-1041.
- [Google Scholar]
- Direct broad-range detection of alphaviruses in mosquito extracts. Journal of Virology. 2007;368(2):286-295.
- [Google Scholar]
- Biodiversity patterns in a macro invertebrate community of a temporary pond network. Insect Conservation and Diversity. 2014;7(1):4-21.
- [Google Scholar]
- SARS-CoV-2 and mosquitoes: an extreme challenge. Scientific Reports. 2020;10:11915.
- [Google Scholar]
- Mosquito-borne viruses circulating in Kinshasa, Democratic Republic of the Congo. International Journal of Infectious Diseases. 2017;57:32-37.
- [Google Scholar]
- The changing epidemiological pattern of Dengue in Swat. Khyber Pakhtunkhwa. PLOS One. 2018;13(4):e0195706.
- [Google Scholar]
- Detecting Bunyaviruses of the Bunyamwera and California serogroups by a PCR technique. Journal of Clinical Microbiology. 1996;34(5):1184-1188.
- [Google Scholar]
- What we know so far: COVID-19 current clinical knowledge and research. Clinical Medicines. 2020;20(2):124-127.
- [Google Scholar]
- Molecular amplification assays for the detection of flaviviruses. Advances in Virus Research. 2003;61:67-99.
- [Google Scholar]
- Genetic and serologic properties of Zika Virus associated with an epidemic, Yap state. Micronesia. Emerging Infectious Diseases. 2017;14(8):1232-1239.
- [Google Scholar]
- Risk factors for house-entry by Culicine mosquitoes in a rural town and satellite villages in The Gambia. Parasites & Vectors. 2008;1:141-142.
- [Google Scholar]
- Phylogeography of Aedes aegypti(L.) and Aedes albopictus (Skuse) Based on mitochondrial DNA variation. Genetics Research. 2016;86(1):1-11.
- [Google Scholar]
- The seasonal abundance of Aedes albopictus in Nagasaki. Tropical Medicines. 2007;20:29-37.
- [Google Scholar]
- Seasonal distribution of Anopheline species and their association with meteorological factors in Punjab, Pakistan. Journal of Animal and Plant Sciences. 2016;26(5):1255-1261.
- [Google Scholar]
- Mosquito (Diptera: Culicidae) of Murree Hills. Punjab. Pakistan. Pakistan Journal of Zoology. 2014;46:523-529.
- [Google Scholar]
- Dengue vector dynamics in Pakistan, a thesis submitted for the degree of PhD at the. Pakistan: University of Sheffield; 2012. p. :pp241..
- Rauf, M., Fatima-tuz-Zahra, Manzoor, S., Mehmood, A. & Bhatti, S. (2107) Outbreak of Chikungunya in Pakistan. Lancet Infectious Diseases, 17(3):258.
- World Health Organization The top 10 causes of death: The 10 leading causes of death in the world 2011 2000 and 2011. WHO.
- SARS-CoV-2 does not replicate in Aedes mosquito cells nor present in field-caught mosquitoes from Wuhan. Virologica Sinica. 2020;35:355-358.
- [Google Scholar]
- A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270-273.
- [Google Scholar]







