A novel approach to assess the heavy metal content in the feathers of shorebirds: A perspective of environmental research
⁎Corresponding authors at: Department of Zoology and Wildlife Biology, AVC College (Autonomous), Mannampandal 609 305, Mayiladuthurai, Tamilnadu, India. (J. Pandiyan); Unit of Vector Control, Phytochemistry and Nanotechnology, Department of Zoology, Annamalai University, Annamalainagar 608 002, Tamil Nadu, India (M. Govindarajan). dunlinpandiyan@gmail.com (Jeganathan Pandiyan), drgovind1979@gmail.com (Marimuthu Govindarajan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Metals are major toxic elements and cause various diseases and damage shorebirds. The study envisaged the heavy metals content in the feathers of shorebirds, prey, water, and sediment from Pichavaram Mangrove Forest (PMF). Primary feathers of shorebirds species such as Curlew Sandpiper, Eurasian Curlew, and Painted Stork were collected and analyzed the following metals viz., Cd, Cu, Cr, Co, Pb, Hg, Ni, and Zn. The levels of Hg, Cr, Pb and Ni varied significantly among the metals studied (p < 0.05). The metal contamination pattern of shorebirds followed in the following order: Painted stork > Eurasian Curlew > Curlew sandpiper. However, in the habitat, Cd, Co, Pb, Hg, Ni and Zi varied significantly between water and sediment (p < 0.05) and Cd, Cu, Hg, Pb, Ni, and Zi differed significantly among the prey species (p < 0.05). Overall, except for Cd, whereas, other seven metals showed significant differences between the feathers of shorebirds and the environment such as water, sediment, polychaetes, mollusc, crabs, prawns and fishes (p < 0.05). Nevertheless, the resident bird (Painted stork) had a higher level of metal accumulation than migratory species, showing that PMF is under threat and requires proper monitoring, management and conservation strategies to sustain organisms that depend on it.
Keywords
Pollution
Heavy metals
Wintering grounds
Shorebirds
Habitats
1 Introduction
Heavy metals are major toxic elements and cause various diseases and damage wildlife when their load exceeds normal levels. Higher content of Cd in feathers of birds can wreck their flying mechanism and lead to poor development of bones (Spahn and Sherry, 1999). Pb mainly deposits in the tissues of feathers of birds (Jayakumar and Muralidharan, 2011), and a higher content of it could destroy their thermoregulation, growth of nestlings, and recognition of their siblings (Burger and Gochfeld, 2000). The toxicity of Cr had several impacts on birds, such as the development of embryo and hatching success of eggs in Mallard (Kertész and Fáncsi, 2003). Even in lower concentrations, Ni can affect pigment colouring of feathers during moulting (Honda et al., 1990). Zn at higher concentrations can affect reproduction and increase kidney toxicity (Carpenter et al., 2004). Rising Hg levels in birds can affect their breeding success (Gochfeld, 1997), and above 5 ppm adversely affects reproduction (Evers et al., 2007). Co is considered as a significant element necessary for metabolism but can negatively affect it in excessive concentrations (Roginski and Mertz, 1977). The role of metals in benthic organisms is also significant since they are involved in littoral trophic mechanisms and benthic organisms, including fishes, are essential prey for the majority of shorebirds (Wilson, 1989).
The Pichavaram Mangrove Forest (PMF) is a significant wetland (latitude 11°23′ to 11°30′N, longitude 79°45′ to 79°50′E), Cuddalore District, Tamil Nadu, Southern India, which supports several shorebirds seasonally. The density and diversity of the shorebird population have declined at PMF, probably due to pollution (Jagadheesan and Pandiyan, 2015). Toxic pollution threatens the migratory shorebirds due to severe damage to it, including the burgeoning number of tanneries, aquaculture industries, and construction of a port adjacent to the mangrove forest (Agoramoorthy and Pandiyan, 2016).
In the present study, we analyze the various metals in the feathers of three different species of shorebirds such as Curlew sandpiper, Eurasian Curlew and Painted stork. This research also assessed the habitat, including water, sediment and prey species of shorebirds to understand the current levels of pollution in the wetland as the PMF supports several species of migratory and resident shorebirds seasonally.
2 Materials and methods
2.1 Study area
The Pichavaram Mangrove Forest (PMF) is located between latitude at 11°23′ to 11°30′N, longitude 79°45′ to 79°50′E, Tamil Nadu, India (Fig. 1). The Cauvery River provides water to the Pichavaram Mangrove Forest through its tributary Coleroon River which runs through highly populated and fertile farmland areas and carries with it fertilizers, insecticides, weedicides and polluting metals (Ramanathan et al., 1999). The PMF covers an area of 11 km2, 50% of which is tidally dominated, 40% has urban waterways and 10% is covered by mud and sand flats, and its annual temperatures range between 18 and 36 °C (Ramanathan et al., 1999).
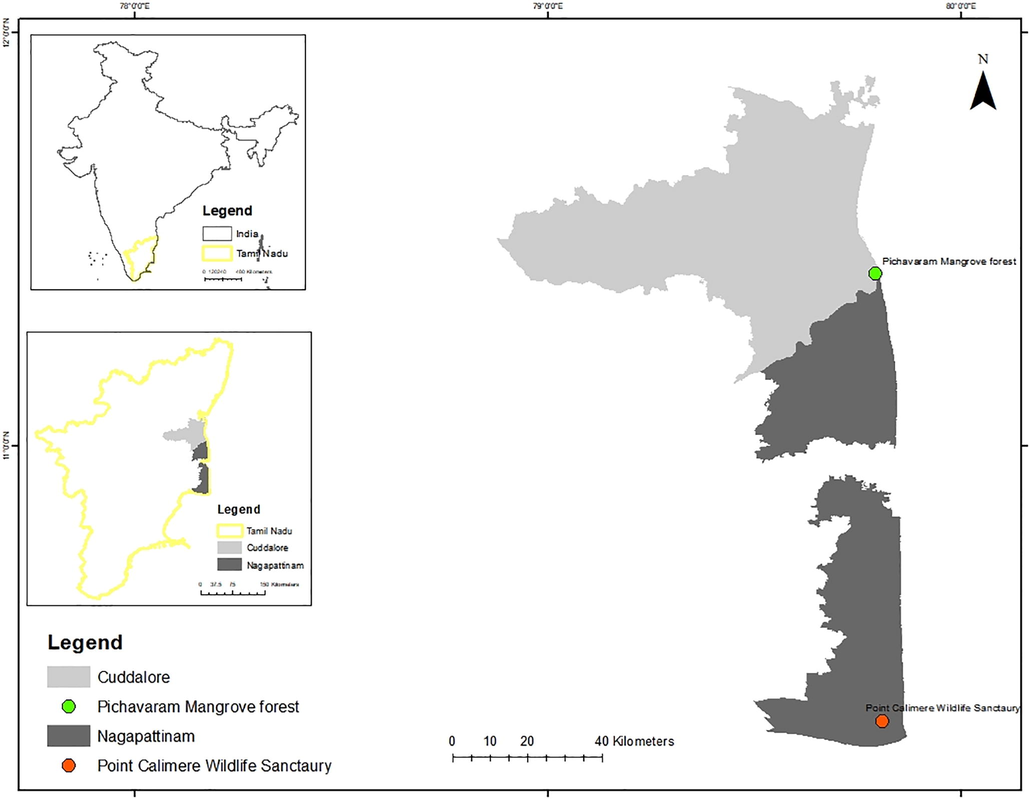
- Map showing the Pichavaram Mangrove Forest, Cuddalore District, Tamil Nadu, India.The map is generated by using ArcGIS 10.0; ENVI + IDL License No.239509; The software is purchased by the Department of Zoology and Wildlife Biology, AVC College (Autonomous), Mannampandal, Mayiladuthurai, Tamil Nadu, India; The ArcGIS webpage: https://www.arcgis.com/index.html.
2.2 Collection and processing of samples
2.2.1 Water samples
Water samples (1000 ml each) were collected from three sampling sites in clean polypropylene bottles at about 2–3 feet below the surface at PMF and filtered. The filtered water samples were preserved in 5 ml of HNO3 (55%)/1000 ml of water to prevent contamination and kept in the lab at 4 °C till analysis. To 100 ml of water from the sample, 5 ml of concentrated H2SO4 was taken in a conical flask and then heated on the hot plate for two hours at 105 °C to 25 ml and later transferred to a 100 ml volumetric flask. Distilled H2O was then added to the volumetric flask until 100 ml upper meniscus mark in the container and it was used for the metal analysis (Adebayo, 2017).
2.2.2 Soil sediment samples
Soil sediment samples (500 g) were collected from three different sites using a core sampler at a depth of 20 cm from the surface of the PMF (Boncompagni et al., 2003). The samples were preserved in Ziploc bags at − 20 °C in the lab. The preserved sample was dried and it was ground to a fine powder and sieved with a 2 mm sieve. In a beaker, 2 g of the sample, along with 5 ml of nitric acid was added together with 2 ml of HClO4. HF (5 ml) and the sample solution was heated for 1 h on a water heater at 160 °C; it was allowed to cool and then filtered. The filtered sample was transferred into a 100 ml volumetric flask and made up to 100 ml with distilled H2O. The sample solutions were subjected to metal analysis (Adebayo, 2017).
2.2.3 Benthic prey species
The mud samples (2 kg) were collected from three different sites at a depth of 10-cm diameter (78.5 cm2), and the samples were sieved by using 0.5, 1.0 and 1.5 mm sieves and polychaetes, molluscs and crustaceans (crabs) were collected from mud samples and preserved at − 20 °C in the lab. 25 g of pooled tissue samples of each prey item was taken into a polystyrene tube, dried at 50 °C and cooled at 25 °C. Subsequently, 1 ml of HNO3 was added to each polystyrene tube, and the samples were kept for 24 h at room temperature and again for 4 h at 50 °C for proper digestion. 100 ml of hydrogen peroxide (H2O2) was added to each tube and the samples were heated at 50 °C for 1 h duration to complete the digestion as described by Newman and McIntosh (1983). The samples were diluted with deionized water and made into 7 ml as the final volume of each tube and it was used for the metal analysis.
2.2.4 Fishes and prawns
Fishes and prawns were captured using a small gill net from three different sites and the samples were carried to the lab in an icebox and kept at −30 °C until metal analysis. The tissue samples of fishes and prawns were washed with deionized water; the samples were dehydrated for about 24 h at 105 °C in a hot air oven. Sample tissues weighing 0.5–1.0 g were transferred into a 100 ml beaker, and 10 ml of concentrated HNO3 was added to the sample. The sample was placed on the hot plate for 1 h at 40 °C, then heated at 140 °C for about 3 more hours. Later, the samples were cooled at room temperature. 40 ml of ultra-purified distilled H2O was added to the sample and the samples were filtered using filter papers. The filtrates were used for metal analysis (Raja et al., 2009).
2.2.5 Bird feather samples
The primary feathers from the dead carcasses of Curlew Sandpiper (N = 3), Eurasian Curlew (N = 4) and Painted Stork (N = 4) were collected from the PMF during fieldwork. The feather samples were washed in deionized water and then dried for 24 h in an oven at 60 °C. Indeed, the samples were digested in a 4:1 mixture of 65% nitrogenous acid and 70% perchloric acid. Subsequently, the samples were diluted by adding double-distilled water up to 10 ml and stored in metal-free polypropylene vials at 20 °C until further analysis (Jayakumar and Muralidharan, 2011).
2.3 Quality control and analytical procedures
Assessment and quality of the instrument’s stability a quality control (QC) sample were injected for every fifteen samples. Besides, for better accuracy blank, standard and sample were run in a set of three (triplicate) for each analytical course. For each metal, separate calibration curves were prepared at 05, 1.0, 2.0, 5.0 and 10 ppm. For every set of samples, the instrument was set to zero concentration by using a blank solution. The results of each metal arrived from (triplicate) samples. Analyses were performed by using Double Beam Atomic Absorption Spectrophotometer (AAS). The results are expressed as ppm (Adebayo, 2017).
2.4 Statistical analysis
The results of the data are expressed as Mean ± SE. One-Way ANOVA was used to understand the level of variations of different metals among the feathers of shorebirds, water, sediment and benthic prey organisms. The inter-correlational analysis was performed to understand the relationship of metals in the feathers of three different species of shorebird. The hierarchical cluster method was performed using the Pearson correlation matrix as the distance measure to understand the patterns of metals in the bird’s feathers. Statistical analyses were done using SPSS 16.0 and the results were interpreted using standard statistical procedures by adapting the method prescribed by Sokal and Rohlf (2012).
3 Results
3.1 Heavy metals in feathers of shorebirds
Heavy metals such as Cd, Cr, Co, Cu, Pb, Hg, Ni, and Zn were analysed from the feathers of shorebirds such as Curlew Sandpiper (Calidris ferruginea), Eurasian Curlew (Numenius arquata) and Painted Stork (Mycteria leucocephala) and water, sediment, prey items at PMF during 2015–16. The Curlew Sandpiper and Eurasian Curlew are migrant species and the Painted Stork is a resident species and belongs to the Near-Threatened (NT) category as per IUCN, Red Data Book (2020). Pb was the highest in Curlew sandpiper (12.05 ± 0.92 ppm), but in the Eurasian Curlew and Painted Stork Hg content was the highest at 7.7 ± 0.46 and 8.6 ± 0.60 ppm, respectively. The concentration of Hg, Cr, Pb and Ni varied significantly (P < 0.05) (Table 1 and Fig. 2). Pb and Ni showed negative correlation with Hg (r = –0.815, r = –0.815) and Cr (r = –0.719, –0.837), respectively, but Zn correlated positively with Cu (r = 0.696) (Table 2). The pattern of metal contamination among the three shorebirds followed the order Painted stork > Eurasian curlew > Curlew sandpiper. The pattern of metals in their feathers is as follows: Pb > Hg > Zi > Cr > Ni > Co > Cu > Cd. The metals in feathers of shorebirds belonged to two different groups based on Ward’s distance method, in which Hg and Pb belonged to one group and the other six metals such as Zi, Cr, Ni, Co, Cu and Cd to the second group. However, within-group 2, two subgroups were identified, i.e. Cr and Zi one group and the Cd, Co, Cu and Ni were assembled as another group (Fig. 2).
| Metals | Name of the shorebirds | |||
|---|---|---|---|---|
| Curlew Sandpiper | Eurasian Curlew | Painted Stork | Overall differences | |
| Mean ± SE (ppm) | ||||
| Hg | 5.6 ± 0.44 | 7.7 ± 0.46 | 8.6 ± 0.60 | 0.013a |
| Cd | 0.2 ± 0.10 | 0.4 ± 0.14 | 0.4 ± 0.11 | 0.735 |
| Cu | 0.5 ± 0.22 | 0.2 ± 0.05 | 0.6 ± 0.18 | 0.641 |
| Cr | 0.2 ± 0.13 | 2.5 ± 0.34 | 1.4 ± 0.26 | 0.002 a |
| Co | 0.5 ± 0.14 | 0.5 ± 0.05 | 0.7 ± 0.07 | 0.273 |
| pb | 12.0 ± 0.92 | 6.3 ± 1.04 | 8.1 ± 0.85 | 0.014 a |
| Ni | 2.5 ± 0.31 | 0.3 ± 0.16 | 0.6 ± 0.11 | 0.001 a |
| Zn | 1.6 ± 0.47 | 1.5 ± 0.17 | 1.6 ± 0.17 | 0.962 |
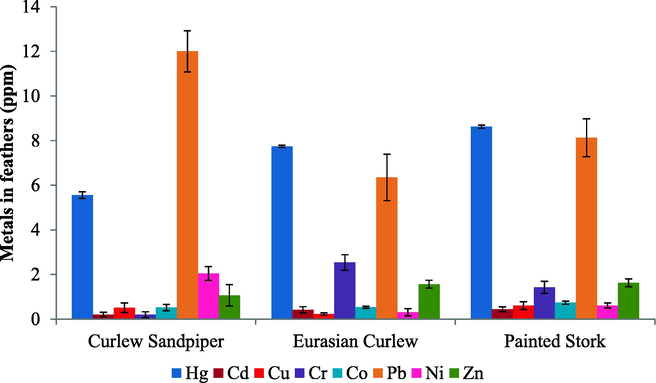
- The concentration of Cd, Cr, Co. Cu, Pb, Hg, Ni and Zn evaluated from the feathers in the Curlew Sandpiper, Eurasian Curlew and Painted stork, Pichavaram Mangrove Forest, Cuddalore District, Tamil Nadu, India.
| Metals | Hg | Cd | Cu | Cr | Co | pb | Ni | Zi |
|---|---|---|---|---|---|---|---|---|
| Hg | 1 | |||||||
| Cd | 0.061 | 1 | ||||||
| Cu | −0.447 | 0.532 | 1 | |||||
| Cr | 0.488 | 0.207 | −0.305 | 1 | ||||
| Co | 0.553 | −0.037 | −0.378 | −0.015 | 1 | |||
| pb | −0.671a | −0.331 | 0.257 | −0.719a | −0.330 | 1 | ||
| Ni | −0.815a | −0.146 | 0.332 | −0.837a | −0.265 | 0.777 | 1 | |
| Zn | −0.267 | 0.351 | 0.696 a | 0.112 | −0.541 | 0.332 | 0.061 | 1 |
3.2 Heavy metals in water, sediment and prey species
Cr was the highest in water (1.8 ± 0.25 ppm) than the other metals (Table 2). In sediment, polychaetes and molluscs, Hg content was the highest at 34.3 ± 9.17, 5.1 ± 0.30 and 21.7 ± 4.43 (ppm), respectively. Pb was the highest in crabs, fishes and prawns at 8.2 ± 0.98, 8.8 ± 0.63 and 8.4 ± 0.75 ppm, respectively. The Cd, Co, Pb, Hg, Ni and Zi varied significantly between water and sediment (p < 0.05). Metals such as Cd, Cu, Hg, Pb, Ni, and Zi showed significant differences (p < 0.05) among the prey items (Table 3). Overall, except for Cd, the other seven metals showed significant differences between the feathers of shorebirds and in water, sediment, polychaetes, mollusc, crabs, prawns and fishes (p < 0.05).
| Metals | Prey species | Over all differences | ||||||
|---|---|---|---|---|---|---|---|---|
| water | Sediment | Polychaetes | Molluscs | Crab | Fish | Prawn | ||
| Mean ± SE (ppm) | ||||||||
| Hg | 0.9 ± 0.25 | 34.3 ± 9.17 | 5.1 ± 0.30 | 21.7 ± 4.43 | 7.6 ± 0.58 | 6.7 ± 2.08 | 8.1 ± 1.77 | 0.001a |
| Cd | 0.1 ± 0.02 | 0.4 ± 0.07 | 0.5 ± 0.10 | 0.7 ± 0.03 | 0.6 ± 0.18 | 0.7 ± 0.22 | 0.4 ± 0.17 | 0.002 a |
| Cu | 0.2 ± 0.04 | 0.30.03 | 0.5 ± 0.15 | 2.0 ± 0.28 | 1.9 ± 0.28 | 0.5 ± 0.15 | 1.6 ± 0.20 | 0.023 a |
| Cr | 1.8 ± 0.25 | 1.5 ± 0.64 | 1.7 ± 0.18 | 2.1 ± 0.38 | 1.6 ± 0.07 | 1.5 ± 0.13 | 0.9 ± 0.18 | 0.468 |
| Co | 0.1 ± 0.05 | 2.1 ± 0.52 | 0.5 ± 0.16 | 1.1 ± 0.22 | 0.6 ± 0.07 | 0.7 ± 0.23 | 0.5 ± 0.16 | 0.455 |
| pb | 0.3 ± 0.16 | 2.9 ± 0.86 | 4.5 ± 0.23 | 6.0 ± 2.38 | 8.2 ± 0.98 | 8.8 ± 0.63 | 8.4 ± 0.75 | 0.000 a |
| Ni | 0.4 ± 0.03 | 2.1 ± 0.02 | 0.7 ± 0.16 | 3.0 ± 0.89 | 1.4 ± 0.29 | 1.5 ± 0.19 | 1.8 ± 0.11 | 0.002 a |
| Zn | 0.06 ± 0.01 | 0.2 ± 0.02 | 0.58 ± 0.25 | 2.0 ± 0.30 | 1.8 ± 0.10 | 1.6 ± 0.06 | 1.1 ± 0.17 | 0.000 a |
4 Discussion
PMF is a crucial stopover site for migratory birds as it is in the path of the Central Asian Flyway. It needs assessment concerning its quality as it attracts many species of migratory and resident migratory shorebirds annually, including near-threatened and endangered ones (Jagadheesan and Pandiyan, 2015). The present investigation has significance as it is the first of its kind at PMF and shows the level of metals in its water system as seen by its heavy presence in the feathers of shorebirds, water, sediment and their prey species. The metal accumulation showed significant differences among the feathers of shorebirds, water, sediment, and prey species, except for Cd, perhaps due to the uniqueness of the bird species, their metal-detoxifying mechanisms, diet selection, foraging style.
4.1 Lead (Pb)
The present study showed that Pb showed significant differences among the shorebirds, and water, sediment and prey species (p < 0.05). Several species of shorebirds showed higher ranges of Pb, particularly for Red knot (484 ppm), Sanderling (367 ppm) and Semipalmated sandpiper (411 ppm) than the present study (Burger et al., 2015). However, Kim and Koo (2008) reported a low level of Pb in the feathers of different species of birds compared with the present study. Studies found higher Pb in benthic invertebrates in coastal mudflats since they are filter feeders (Mado-Filho et al., 2008) and the same was found in the current study. Pb is higher in Painted stork as it predominantly feeds on fishes than the other species of prey items, and studies have shown that Pb is higher in fishes as they are secondary predator and are a rich magnification of a trophic structure of an aquatic ecosystem (Kumar et al., 2012). The impact of Pb level in the feathers (4 lg/g) is on thermoregulation impairment, daily movement pattern, affecting the survival of gull nestlings, and delayed recognition of siblings (Burger and Gochfeld, 2000).
4.2 Mercury (Hg)
Mercury (Hg) was the highest in Painted stork and Eurasian Curlew than Curlew Sandpiper and it was also higher in prey species (Tables 1 and 3). The painted stork mainly feeds on fishes, but it could also feed on molluscs, insects and crustaceans (Prabhakar and Dudhmal, 2016). The present study showed that Hg was the highest in molluscs, followed by crab, prawn and fishes. Studies have shown that it is higher in fishes, molluscs and crustaceans in coastal and mangrove wetlands (Kumar et al., 2012). Therefore, the higher residue of Hg in Painted stork suggests that the bird might have consumed not only fishes but also could be feeding on other prey in the foraging ground. Higher-level Hg recorded from various species such as Red knot, Sanderling, Semipalmated Sandpiper and Canada Goose than the current study (Burger et al. 2015). Benthic or mud-dwelling organisms are exposed more to metals and these metals directly settle in the benthic organisms through their diet in sediment and water (Wyn et al., 2009). This study shows that the higher level of Hg (5 ppm) at PMF in the feathers of shorebirds and the higher Hg levels in birds can affect their behaviour, physiology and reproductive rates (Wolfe et al., 1998).
4.3 Chromium (Cr)
The Cr differed significantly among the shorebirds studied (p < 0.05). Eurasian Curlew had higher level of Cr than the other two species of shorebirds. However, it is lower than the previous reports (Abdullah et al., 2015) in the feathers of various species of water birds. On the other hand, a comparatively higher level of Cr was reported in different species of shorebirds (Burger et al., 2015) compared to the present study. Studies show that Cr is rich in sediment and benthic organisms and fishes and prawns (Kumar et al., 2012) the same results also found in the current study. The Eurasian curlew mainly feeds on molluscs and other benthic organisms, and hence it might be a reason for its more significant level of Cr than the other two species (Jagadheesan and Pandiyan, 2015). Cr at and above 4 ppm level is harmful and the other biological reductants will damage their DNA and protein (Stohs and Bagchi, 1995). However, the current study not showed any harmful level of Cr.
4.4 Nickel (Ni)
The Ni varied significantly among the three shorebirds studied (p < 0.05). The present study found comparatively higher levels of Ni in the feathers Little egret and Cattle egret (Abdullah et al., 2015) compared to the current study. The study also found a more considerable amount of Ni in the prey species, primarily molluscs and sediment, suggesting that it might be the reason behind the higher level of Ni in Curlew Sandpiper since the bird predominantly forages on molluscs. Painted stork showed a higher level of Ni than the Eurasian Curlew. The present study showed that a higher level of Ni in its prey items be a source of Ni in Pained stork. Studies show that fishes and prawns in the coastal ecosystem have a higher level of Ni (Kumar et al., 2012). Ni is not a vital trace element in organisms, but at high levels, it can have serious health issues. If nickel accumulation is beyond a specific limit in the environment causes severe damage in feather pigmentation and other physiological defects in birds (Honda et al., 1990).
4.5 Zinc (Zn)
The levels of Zn did not vary significantly (p > 0.05) among the three species of shorebirds studied (Table 1 and Fig. 2), but comparatively higher than the feathers of several species of shorebirds (Kim and Koo, 2008). Similarly, the feathers of great tit and greenfinch from China showed lower concentrations compared with those from the present study (Deng et al., 2007). Studies show that zinc is one of the metals with the highest concentrations in benthic invertebrates. The present study also showed that the level of Zn is higher in their prey species, and explains its accumulation in the feathers of the three species of shorebirds through their prey species. Beyond the normal range, zinc could affect the biota (Abdullah et al., 2015; Solgi et al., 2020).
4.6 Cobalt (Co)
The present study did not show significant differences among the species of shorebirds and also water, sediment and prey species (p > 0.05). Studies indicate that benthic invertebrates, particularly molluscan forms, have a relatively higher range of Co in their body since they feed on detritus matters (Youssef et al., 2017). Molluscan forms are densely available at PMF and studies also show that molluscans and fishes have a larger amount of Co (Kumar et al., 2012). This could also be another reason for the accumulation of Co in Painted stork and the other two species of shorebirds studied. Nevertheless, comparatively higher levels of Co were reported from Chukar partridge (1.2 ppm), See-see partridge (5.4 ppm) and Common pigeon (1.3 ppm) (Norouzi et al., 2012) than the present study. Co is also an essential element necessary for metabolism, but in excess harms the organism, including certain enzymes in animal physiology (Roginski and Mertz, 1977).
4.7 Copper (Cu)
The Cu did not vary significantly among the shorebirds studied (p > 0.05). Painted stork showed a higher level of Cu than the other two species. Molluscs, crabs and prawns showed a higher level of Cu than water, sediment and other prey species (Table 2). Cu level could be higher in Painted stork as it predominantly feeds on molluscs, crab and prawn if fishes are not in sufficient number in their foraging ground (Urfi, 2011). However, globally, Buff-breasted sandpiper (Scherer et al., 2015) and Semipalmated Sandpiper (Burger, 2008) showed a higher level of Cu than the present study. A higher concentration of copper and its impact can damage the kidney and impair reproduction (Carpenter et al., 2004).
4.8 Cadmium (Cd)
The Cd in the shorebirds did not vary significantly (p > 0.05), but there were significant differences among water, sediment and prey species of shorebirds (p < 0.05). Painted stork and Eurasian Curlew showed a similar level of Cd than the Curlew Sandpiper (Table 1). Cd in fishes and molluscs showed a higher level than in the other prey species and that could be a reason for the Eurasian Curlew and the Painted stork to have Cd in their feathers. Studies on benthic organisms and fishes show a significant level of Cd in coastal ecosystems (Everaarts et al., 1989). Comparatively, the Terek Sandpiper and the Great tits (Kim and Koo, 2008) showed a greater level of Cd than the present study. Studies reveal that Cd accumulation can cause toxicity of kidney, thinning of eggshells, nutritional deficiencies, disruption of calcium metabolism, and intestinal tissue damage in birds (Burger, 2008).
4.9 Metals in feathers: Inter-correlational and assemblage patterns of metals
The inter-correlational analysis of metals in feathers shown that Pb and Ni had negative correlation with Hg (r = -0.815, r = -0.815) and Cr (r = -0.719, −0.837), respectively. On the other hand, Zn correlated positively with Cu (r = 0.696). Pb and Ni have correlation but negative relationships, which indicates that if Pb and Ni level is increased, the level of Hg and Cr could be decreased. However, Zn correlated positively with Cu (r = 0.696). The other metals, particularly Cd and Co, did not show correlation with any other metals studied from the feathers of the three species of shorebirds at PMF. Cluster analysis (dendrogram) demonstrated that metals are assembled into two different major groups, in which Hg and Pb belong to one group and the other six metals to another group (Fig. 3). This kind of unique variation of metals in the shorebird species might be based on the uniqueness of the birds about their physiology, detoxification mechanism, foraging strategy, abundance and distribution of their prey matters, selection of diets, the consumption rate of their diets, size and weight of the prey species, quality of the habitat. Studies show that the removals of toxic substances, including metals from their body by various means such as through excrement, deposition in preen or oil glands and salt glands, or elimination into growing feathers or eggshells (Costa and Vonesh, 2013).
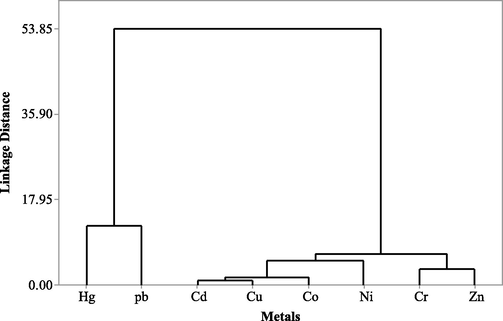
- Dendrogram of metals in feathers of three species of shorebirds obtained using Ward’s method as a linkage method and linkage distance matrix (distances reflect the degree of association between different metals).
5 Conclusion
Overall, the results of the present study revealed that PMF is highly contaminated and polluted. Of the three shorebirds studied, Painted Stork is a resident bird, and the other two species, such as Curlew Sandpiper and Eurasian Curlew, are migratory. There is a higher load of metals in the resident species than those of the other two migratory species. Scientists working in avian ecology and conservation argue that bird populations are drastically declining globally due to climate change, habitat degradation, inter- and intra-species competition, predation, various pollutions. Therefore, an intensive study of the birds with comprehensive knowledge on various elements of their environment, food and prey items, different organs and tissues, excreta and reproductive toxicology could provide clues to lacunae in toxicological studies for better management and conservation of shorebirds.
Acknowledgments
The authors express their sincere appreciation to the Researchers Supporting Project No. RSP-2020-24 the King Saud University, Riyadh, Saudi Arabia. We thank the DST-SERB, Government of India, for funding this project (Ref No. SERB/LS-512/2013 dated.20.09.2013). The authors express their sincere thanks to the Tamil Nadu Forest Department for permits to carry out the study at Pichavaram Mangrove Forest, Cuddalore District, Tamil Nadu (Ref No. D/6139/2014 dt.12.2014). The authors, thanks to the Management of AVC College and Dept of Zoology and Wildlife Biology, for providing necessary facilities for the said project.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Avian feathers as a non-destructive bio-monitoring tool of trace metals signatures: a case study from severely contaminated areas. Chemosphere. 2015;119:553-561.
- [Google Scholar]
- Determination of Heavy Metals in Water, Fish and Sediment from Ureje Water Reservoir. J. Environ. Anal. Toxicol.. 2017;7:1-4.
- [Google Scholar]
- Toxic pollution threatens migratory shorebirds in India. Environ. Sci. Pollut. Res.. 2016;23:15771-15772.
- [Google Scholar]
- Egrets as monitors of trace metal contamination in wetland of Pakistan. Arch. Environ. Contam. Toxicol.. 2003;45:399-406.
- [Google Scholar]
- Assessment and management of risk to wildlife from cadmium. Sci. Total Environ.. 2008;389:37-45.
- [Google Scholar]
- Metals in laysan albatrosses from midway atoll. Arch. Environ. Contam. Toxicol.. 2000;38:254-259.
- [Google Scholar]
- Mercury, Lead, Cadmium, Arsenic, Chromium and selenium in feathers of shorebirds during migrating through delaware bay, New Jersey: comparing the 1990s and 2011/2012. Toxics. 2015;3:63-74.
- [Google Scholar]
- Zinc toxicosis in a free-flying Trumpeter Swan (Cygnus buccinator) J. Wildlife Dis.. 2004;40:769-774.
- [Google Scholar]
- Prey subsidy or predator cue? Direct and indirect effects of caged predators on aquatic consumers and resources. Oecologia. 2013;173:1481-1490.
- [Google Scholar]
- Trace metal concentration in great tit (Parus major) and greenfinch (Carduelissinica) at the western mountains of Beijing, China. Environ. Pollut.. 2007;148:620-626.
- [Google Scholar]
- Copper, zinc and cadmium in benthic organisms from the Java Sea and estuarine and coastal areas around East Java. Netherland J. Sea Res.. 1989;23:415-426.
- [Google Scholar]
- Evers, D.C., Han, Y.J., Driscoll, C.T., Kamman, N.C., Goodale, M.W., Lambert, K,F., Holsen, T.M,, Chen, C.Y., Clair, T.A., Butler, T., 2007. Identification and evaluation of biological hotspots of mercury in the Northeastern U.S. and Eastern Canada. BioScience 57, 29–43.
- Factors influencing susceptibility to metals. Environ. Health Perspect.. 1997;105:817-822.
- [Google Scholar]
- Population characteristics of migratory shorebirds in the Point Calimere Wildlife Sanctuary, Tamil Nadu. India. J. Sci. Trans. Environ. Technol.. 2015;10:31-36.
- [Google Scholar]
- Metal contamination in select species of birds in Nilgiris District, Tamil Nadu, India. Bull Environ. Contam. Toxicol.. 2011;87:166-170.
- [Google Scholar]
- Adverse effects of (surface water pollutants) Cd, Cr and Pb on the embryogenesis of the mallard. Aquat. Toxicol.. 2003;65:425-433.
- [Google Scholar]
- Heavy metal concentrations in feathers of Korean shorebirds. Arch. Environ. Contam. Toxicol.. 2008;55:122-128.
- [Google Scholar]
- Distribution of heavy metals in valuable coastal fishes from North East Coast of India. Turkish J. Fish. Aquat. Sci.. 2012;12:81-88.
- [Google Scholar]
- Mado-Filho, G.M., Salgado, L.T., Rebelo, M.F., Rezende, C.E., Karez, C.S., Pfeiffer, W.C., 2008. Heavy metals in benthic organisms from Todosos Santos Bay, Brazili. Brazilian J. Biol. 68, 95-100.
- Lead elimination and size effects on accumulation by two freshwater gastropods. Arch. Environ. Contam. Toxicol.. 1983;12:25-29.
- [Google Scholar]
- Comparison of the metal concentrations in the feathers of three bird species from southern Iran. Bull. Environ. Contam. Toxicol.. 2012;89:1082-1086.
- [Google Scholar]
- Painted Stork (Mycteria leucocephala): population status, shift in food and behavioural ecology from isolated ponds of godavari river basin in nanded district. India. Sci. Res. Rep.. 2016;6:50-57.
- [Google Scholar]
- Heavy metal concentration in four commercially valuable marine edible fish species from Parangipettai coast, south east coast of India. J. Anim. Vet. Adv.. 2009;1:10-14.
- [Google Scholar]
- Environmental geochemistry of the Pichavaram mangrove ecosystem (Tropical), southeast coast of India. Environ. Geol.. 1999;37:223-233.
- [Google Scholar]
- Trace elements concentrations in buff-breasted sandpiper sampled in Lagoa do Peixe National Park, southern Brazil. Brazilian J. Bio.. 2015;75I:932-935.
- [Google Scholar]
- Biometry: The Principles and Practice of Statistics in Biological Research (fourth ed.). New York: W. H. Freeman and Company; 2012.
- Comparison of accumulation of some heavy metals in different tissues of Liza auratus from the coasts of nowshahr and its consumption risk assessment in 2016. J. Environ. Health Eng. 2020:1-14.
- [Google Scholar]
- Cadmium and lead exposure associated with reduced growth rates, poorer fledging success of little blue heron chicks (Egretta caerulea) in south Louisiana wetlands. Arch. Environ. Contam. Toxicol.. 1999;37:377-384.
- [Google Scholar]
- Oxidative mechanisms in the toxicity of metal ions. Free Rad. Bio. Med.. 1995;18:321-336.
- [Google Scholar]
- Urfi, A.J., 2011. The Painted Stork. Ecology and Conservation, (Ed. Urfi 2011), Springer, New York.
- Effects of Mercuryon wildlife: a comprehensive review. Toxicol. Chem.. 1998;17:146-160.
- [Google Scholar]
- Mercury bio-magnification in the food webs of acidic lakes in Kejimkujik National Park and National Historic Site, Nova Scotia. Can. J. Fish Aquat. Sci.. 2009;66:1532-1545.
- [Google Scholar]
- Invertebrate shells (mollusca, foraminifera) as pollution indicators, Red Sea Coast, Egypt. J. African Earth Sci.. 2017;133:74-85.
- [Google Scholar]







