Translate this page into:
A new sulphur containing heterocycles having azo linkage: Synthesis, structural characterization and biological evaluation
⁎Corresponding author. jathikeshavayya1959@gmail.com (Keshavayya Jathi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present study aims the development of new S-heterocyclic azo dyes synthesized from 1, 3-benzothiazole-2-thiol with various amines by diazo-coupling method and their structures are established by physico-chemical techniques. The target molecules were screened for antimicrobial, antitubercular, anticancer, and molecular docking studies. These compounds have shown appreciable inhibitory effect against studied microbial strains. The in silico molecular docking study exposed the significant interaction properties of the azo compounds against target receptor RpsA and showed an appreciable binding affinity of −4.3 to −5.5 kcal/mol.
Keywords
Benzothiazole
Diazotization
Azo dyes
Biological activity
1 Introduction
The development in the field of colour chemistry has become more advanced due to the outstanding contributions from the number of researchers across the world. Particularly the synthesis of azo dyes and their useful applications ruled out the whole colour industries about 60–70% from the overall dyes and pigments domain. Several applications were found on the design of azo dyes but the use of azo dyes as an analytical reagent in colorimetric, spectrophotometric, and electroanalytical techniques is common (Moylan et al, 2004; Hao et al, 2008; He et al., 2009. This is because of their chief availability, cost-effectiveness, easy synthetic procedure, and purity. Therefore, large number of azo molecules can be prepared by changing the aromatic substitution of the amine as well as the coupling component and change in the aromatic rings can be useful in tuning the properties of the whole molecule as compared to the simple aniline derivatives and thus the area of greater interest in various fields. Incorporation of heterocyclic systems into the azo dyes exhibits better applications related to electrochemical, biological, optical, and thermal properties than compared to the compounds containing simple benzene analogues (Wade, 1995; Cliffe, 1958).
From the recent investigations, it is proved that the versatility of azo dyes made them as the most studied class of organic compounds due to their applicability in textile, paint, food, electronic and pharmaceutical industries. The presence of heterocyclic skeleton in these molecules makes them still better candidates for the advanced applications in the above-said areas (Yang et al, 2009; Tsai and Wang, 2005; Kumar et al, 2005; Sung et al, 2011; Szabó et al, 2007; Chimichi et al, 2006). In the previous work, we have investigated the bio-potency of a novel azo dyes containing S-heterocycle and it has been observed that the presence of an heteroatom in conjugation with the azo chromophore enhance the biological properties of the dyes. In this study, we have tried to check the pharmacological properties of the newly synthesized azo dyes by the incorporation of the heterocycles (Mallikarjuna and Keshavayya, 2020). Therefore, we have synthesized azo dyes containing 1,3-benzothiazole-2-thiol as the main core. The sulphur-containing azo dye exhibits excellent pharmaceutical applications like antimicrobial, anti-inflammatory, anticancer, antitubercular, and antiviral activities. Furthermore, also found extensive applicability in optical storage devices, optoelectronics, nonlinear optical materials, corrosion inhibition, sensors, solar energy devices, etc. (Erişkin et al., 2014; Atwal et al, 1991; Kappe, 1993; Karcı and Demirçalı, 2006).
Thus, by considering the above issues and from our earlier studies (Mallikarjuna et al., 2018a, 2018b; Mallikarjuna and Keshavayya, 2020; Matada and Jathi, 2019; Maliyappa et al, 2019; 2020), in the present paper we have reported the synthesis, structural confirmation and biological studies on bioactive azo dyes containing 1, 3-benzothiazole-2-thiol nucleus. The antimicrobial, antitubercular, anticancer, and in silico molecular docking studies are included in this work.
2 Experimental
The starting materials used for the synthesis of azo dyes were purchased from Sigma Aldrich and were used without purification. The melting points were recorded on the electro thermal melting point apparatus. The analytical data of the compounds were obtained by recording their elemental composition on a Vario EL III CHN analyser. The electronic spectra of the compounds were recorded on an Elico-SL 164 double beam spectrometer in different solvents in a range of 200–800 nm. The FT-IR spectra were measured in KBr pellets in the wavelength range of 4000–400 cm−1. The NMR spectra were recorded with TMS as an internal standard reference on a 400 MHz Avance III instrument. The LC-mass spectra of the compounds were obtained from LCMS 2010, SHIMADZU mass analyser.
2.1 General procedure for the synthesis of 1, 3-benzothiazole-2-thiol azo dyes (D1-D4)
The azo molecules are obtained by the simple diazo-coupling reaction between the aromatic amine and the coupling components in the presence of NaNO2 in an acidic medium at 0–5 °C (VinodKumar et al., 2018) as represented in the Scheme 1. In this case, the aromatic amines (a-d) having different heterocycles have been used to get diazonium salts and these were coupled with the 1, 3-benzothiazole-2-thiol (1) at 0–5 °C. The formed azo compounds are obtained in good yield and they were recrystallized from ethanol.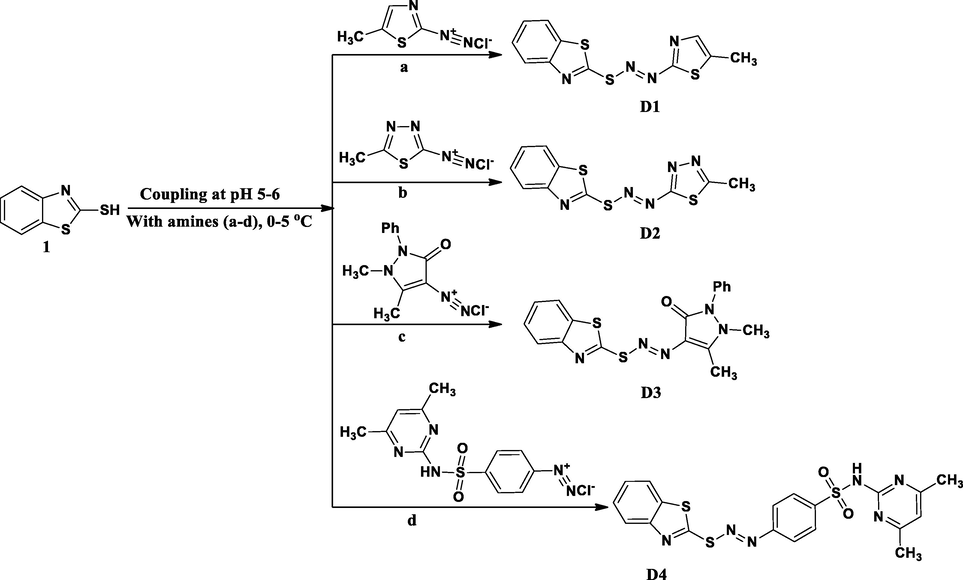
Schematic route for the synthesis of azo dyes (D1-D4).
2.1.1 2-{[(E)-(5-methyl-1, 3-thiazol-2-yl) diazenyl] sulfonyl}-1, 3-benzothiazole (D1)
A Pale yellow coloured compound was obtained by the reaction between 5-methyl-1, 3-thiazol-2-amine (a) and 1, 3-benzothiazole-2-thiol (1) with 71% yield. m. p. 215–217 °C. IR (KBr, cm−1): 3063 (ν(C—H) aromatic), 2918 (ν(C—H) aliphatic), 1626 (C⚌N), 1467 (N⚌N), 1316 (C—N). 1H NMR (DMSO‑d6, δppm): δ 7.96–7.94 (d, 3H, Ar-H, J = 8 Hz), 7.82 (s, 1H, Ar-H of thiazole ring), 7.70–7.68 (d, 1H, Ar-H, J = 8 Hz), 2.55 (s, 3H, CH3). LC–MS: m/z (%) = 293 [M + 1] +. Anal. Calcd. (%) For C11H8N4S3: C, 45.18; H, 2.76; N, 19.16. Found (%): C, 45.13; H, 2.72; N, 19.10.
2.1.2 2-{[(E)-(5-methyl-1, 3, 4-thiadiazol-2-yl) diazenyl] sulfonyl}-1, 3-benzothiazole (D2)
A Yellow coloured azo dye was obtained by the reaction between5-methyl-1, 3, 4-thiadiazol-2-amine (b) and 1, 3-benzothiazole-2-thiol (1) with 78% yield. m. p. 218–220 °C. IR (KBr, cm−1): 3435 (ν(C—H) aromatic), 1631 (C⚌N), 1469 (N⚌N), 1313 (C—N). 1H NMR (CDCl3, δppm): δ 8.00–7.98 (d, 1H, Ar-H), 7.87–7.85 (d, 1H, Ar-H, J = 8 Hz), 7.48–7.46 (d, 1H, Ar-H, J = 8 Hz), 7.40–7.38 (d, 1H, Ar-H, J = 8 Hz), 2.78 (s, 3H, CH3). LC–MS: m/z (%) = 294 [M + 1]+. Anal. Calcd. (%) For C10H7N5S3: C, 40.94; H, 2.40; N, 23.87. Found (%): C, 40.88; H, 2.36; N, 23.82.
2.1.3 4-[(E)-(1, 3-benzothiazol-2-ylsulfanyl) diazenyl]-1, 5-dimethyl-2-phenyl-1, 2-dihydro-3H-pyrazol-3-one (D3)
A Light brown coloured molecule was got by the reaction of4-amino-1, 5-dimethyl-2-phenyl-1, 2-dihydro-3H-pyrazol-3-one (c) with 1, 3-benzothiazole-2-thiol (1) and got the yield of 73%. m. p. 214–216 °C. IR (KBr, cm−1): 3115 (ν(C—H) aromatic), 2841 (ν(C—H) aliphatic), 1642 (C⚌O), 1597 (C⚌N), 1497 (N⚌N), 1321 (C—N). 1H NMR (DMSO‑d6, δppm): 7.56–7.26 (m, 9H, Ar-H), 2.68 (s, 6H, CH3). LC–MS: m/z (%) = 382 [M + 1]+. Anal. Calcd. (%) For C18H15N5OS2: C, 56.67; H, 3.96; N, 18.36. Found (%): C, 56.62; H, 3.90; N, 18.31.
2.1.4 4-[(E)-(1, 3-benzothiazol-2-ylsulfanyl) diazenyl]-N-(4, 6-dimethylpyrimidin-2-yl) benzenesulfonamide (D4)
A creamy yellow coloured dye was obtained by the reaction between 4-amino-N-(4, 6-dimethylpyrimidin-2-yl) benzenesulfonamide (d) and 1, 3-benzothiazole-2-thiol (1) with 81% yield. m. p. 262–264 °C. IR (KBr, cm−1): 3455 (NH), 3115 (ν(C—H) aromatic), 2840 (ν(C—H) aliphatic), 1624 (C⚌N), 1499 (N⚌N), 1320 (C—N). 1H NMR (DMSO‑d6, δppm): 13.64 (s, 1H, NH), 7.59–7.57 (d, 2H, Ar-H, J = 8 Hz), 7.32–7.31 (d, 4H, Ar-H, J = 4 Hz), 7.27–7.26 (d, 2H, Ar-H, J = 4 Hz), 6.68 (s, 1H, Ar-H), 2.28 (s, 6H, CH3). LC–MS: m/z (%) = 457 [M + 1]+. Anal. Calcd. (%) For C19H16N6O2S3: C, 49.98; H, 3.53; N, 18.41. Found (%): C, 49.93; H, 3.49; N, 18.38.
2.2 Biological investigations
2.2.1 Antimicrobial activity
The antimicrobial efficiency of the azo dyes (D1-D4) was measured by tube dilution assay as mentioned in the literature (Schwalve et al., 2007). In the present study, two bacterial strains E. coli, E. faecalis and two fungal strains C. albicans and A. flavus were selected. Ciprofloxacin and Fluconazole were used as a standard drug to compare with the potency of the synthesized dyes.
2.2.2 Antimycobacterial activity
The antitubercular efficacy of the prepared azo dyes (D1-D4) was explored against M. tuberculosis by microplate blue Almar assay as reported in the literature (Mandewale et al, 2018). The results of the activity were analysed by defining the minimum inhibition concentration (MIC) which is the lowest concentration of the drug by changing the colour of the samples by pink to blue. Further, the experimental results of the tested samples were matched with the standard drugs.
2.2.3 Anticancer activity
The anticancer property of the newly azo dyes (D1-D4) was checked by 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) assay as followed from the literature (Canner et al., 2009) against three cancerous cell lines human mammary tumour cell line (MDA-MB-231), human lung carcinoma cell line (A549) and human chronic myeloid leukaemia cell line (K562). The results of the study were obtained in terms of IC50 values and are interpreted in the discussion section.
2.2.4 In silico molecular docking studies
The in silico molecular docking studies of our target compounds (D1-D4) carried out against biological receptor protein RpsA. The RpsA, a ribosomal protein S1 of M. tuberculosis and it was newly recognized protein as a target of pyrazinamide based on it binding interaction with its active form known as pyrazinoic acid. The active from of the pyrazinamide mainly inhibits the trans-translation of RpsA protein. Therefore, we chosen this as a target receptor for the in silico molecular docking studies (Yang et al., 2015). The structures of the azo dyes were improved by using the Chem Bio Draw tool (Chem Bio Office Ultra 14.0 suite) with 2D-orientation and further transformed into 3D-format with the minimization of energy by Schrodinger Maestro. The 2XCT-Protein Data Bank (PDB) was used to get the 3D-coordinates of the target receptor and the best docked conformation of the tested structures was got on the basis of glide energy, docking score, active hydrogen bonding sites and hydrophobic interactions (El-Sonbati et al., 2015).
3 Results and discussion
The chemistry behind the synthesis of these novel disperse azo dyes (D1-D4) is that the primary amines containing heterocyclic nucleus (a-d) were used to get the diazonium salts under the acidic condition followed by coupling with the 1, 3-benzothiazole-2-thiol (1) at 0–5 °C. The obtained coloured azo dyes were purified by recrystallization in ethanol and they were characterized by different spectroscopic methods. The analytical data of the compounds were found to be in good correlation with the proposed structures and are summarized in Table 1.
Compounds
Mol. Formula
M.P. (°C)
Mol.wt.
Colour
Elemental analysis (%) Calcd. (Found)
C
H
N
D1
C11H8N4S3
215–217
292.40
Pale yellow
45.18 (45.13)
2.76 (2.72)
19.16 (19.10)
D2
C10H7N5S3
218–220
293.30
Yellow
40.94 (40.88)
2.40 (2.36)
23.87 (23.82)
D3
C18H15N5OS2
214–216
381.47
Light brown
56.67 (56.62)
3.96 (3.90)
18.36 (18.31)
D4
C19H16N6O2S3
262–264
456.56
Creamy yellow
49.98 (49.93)
3.53 (3.49)
18.41 (18.38)
3.1 IR spectral data
The chemical structures of the azo dyes (D1-D4) were examined by FT-IR spectroscopy in KBr pellets at a range of 4000–400 cm−1 and the results were presented in Table 2. In the FT-IR spectra of all the compounds, a broad peak appeared in the region 3435–3063 and 2918–2840 cm−1 due to aromatic and aliphatic CH vibrations respectively. The azo group of all the dyes displayed medium intensity peak in all the spectra at a wavelength range of 1499–1467 cm−1. Further, the peaks appeared in the regions 1631–1624 and 1321–1313 cm−1 assigned to the ʋC⚌N and ʋC—N stretching vibrations respectively.
Compounds
ʋAr-CH
ʋAliphatic-CH
ʋNH/ʋC⚌O
ʋN⚌N
ʋC⚌N
ʋC—N
D1
3063
2918
-
-
1467
1626
1316
D2
3435
–
–
–
1469
1631
1313
D3
3115
2841
–
1642
1497
1597
1321
D4
3115
2840
3455
–
1499
1624
1320
3.2 Electronic absorption spectral data
The electronic spectra (Figs. S1-S3 of supplementary material) of the azo molecules (D1-D4) were obtained in chloroform, DMF, and DMSO with varying polarity at a concentration of 10−6 M in the wavelength range of 200–800 nm and the results were summarized in Table 3. The compounds exhibited the prominent peaks in the regions of 330–388, 332–374, and 326–386 nm in DMSO, DMF, and chloroform due to n → π* transition of the N-atom of the azo group due to the interaction with the solvent molecules. Electronic substitution also influences on the shifting of the absorption maxima towards a longer wavelength. In the present case, the absorption maxima shifted towards longer wavelengths in DMSO as compared to the DMF and chloroform and this is again the influence of polarity of the solvent molecules. Therefore, from these spectral results, it is inferred that the polarity, conjugation, and electronic substitutions are the key tool in understanding the properties of the azo dyes (Rau, 1990; Kim et al, 1999).
Compounds
λmax (nm)
Logε
DMSO
DMF
Chloroform
DMSO
DMF
Chloroform
D1
388
373
386
5.39
6.16
5.86
D2
376
374
380
5.85
5.95
5.69
D3
330
332
330
6.01
6.09
6.07
D4
374
334
326
6.15
6.29
5.62
3.3 NMR spectral data
The azo dyes (D1-D4) were structurally confirmed by the 1H NMR spectral studies at room temperature. The compounds D1, D3, and D4 are recorded in the DMSO‑d6 solvent and that of D2 was recorded in CDCl3 in the presence of tetra methyl silane (TMS). In the spectrum of the compound D4, a broad peak appeared at 13.64 ppm and it is due to the presence of NH proton which is attached to the pyrimidine ring of the sulfamethazine. The observed high δ value for NH proton because of its high acidic character as it is flanked in between the acidic SO2 group and the pyrimidine ring. The aromatic protons present in all the dyes were resonated in the region 7.96–7.68, 8.00–7.38, 7.56–7.26 and 7.59–6.68 ppm as multiplets. Further, the methyl groups of all the azo dyes were displayed signals in the region 2.55, 2.78, 2.68, and 2.28 ppm as singlets respectively. Therefore, it is apparent that the NMR data was found to be in agreement with the proposed structures of the azo dyes.
3.4 Mass spectral studies
The LC–MS spectra of the azo dyes (D1-D4) were recorded and their corresponding molecular ion peaks were identified. The mass spectra of the compounds indicated the molecular ion peaks which are recorded at m/z 293, 294, 382, and 457 consistent to the formula weights 292.40, 293.30, 381.47, and 456.56 respectively. Further, the probable fragmentation mode for all the compounds was proposed and are presented in the following Schemes 2–5 and they are in agreement with the structures of the synthesized compounds.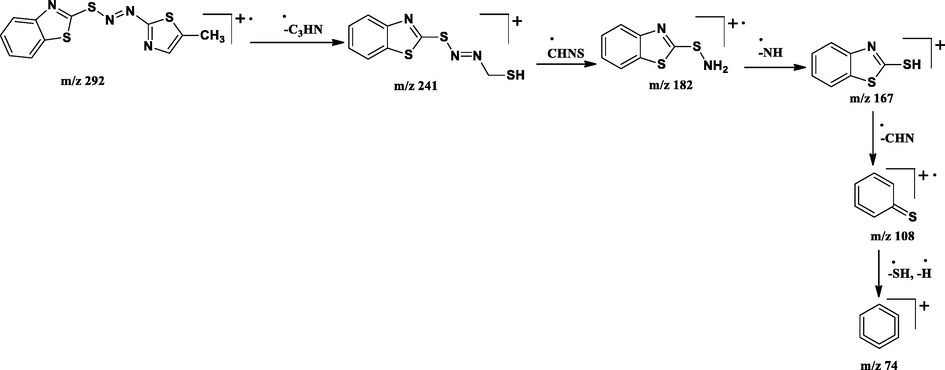
Tentative mass spectral fragmentation of azo dye D1.
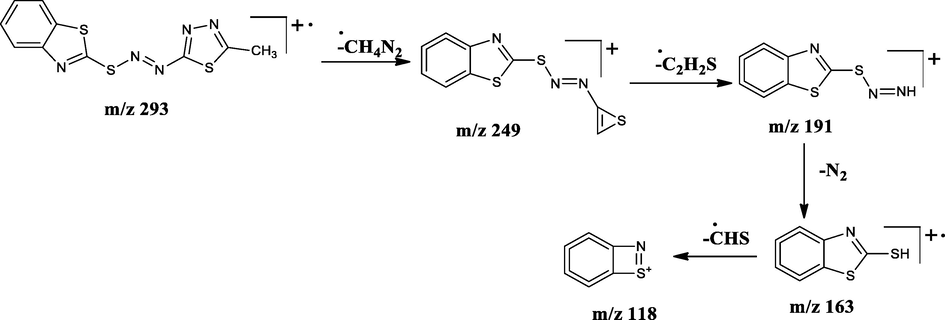
Tentative mass spectral fragmentation of azo dye D2.
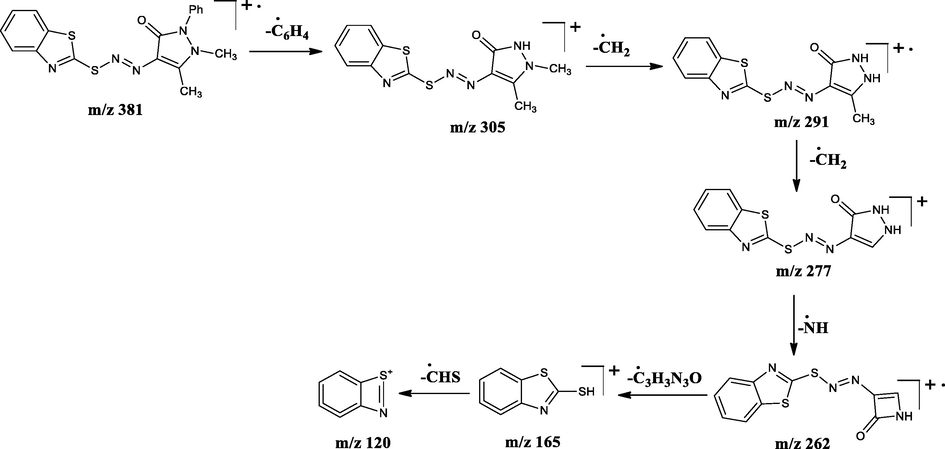
Tentative mass spectral fragmentation of azo dye D3.
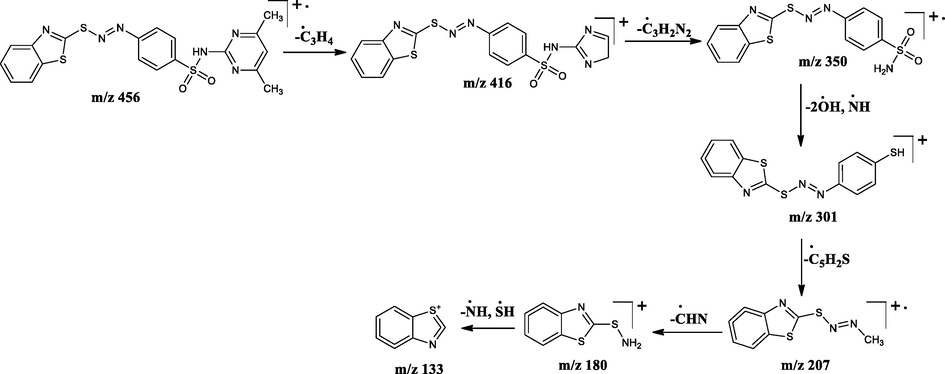
Tentative mass spectral fragmentation of azo dye D4.
3.5 Biological studies on mercapto benzothiazole incorporated azo dyes (D1-D4)
3.5.1 Antimicrobial activity
The azo molecules derived from mercapto benzothiazole (D1-D4) were screened for their microbial inhibition by modified tube dilution assay against two bacterial strains E. coli, E. faecalis and two fungal strains C. albicans, and A. flavus and the results were correlated with the ciprofloxacin and fluconazole (Table 4). From the study, the compounds D2 and D3 showed the highest antibacterial activity with MIC equal to 12.5 mg/mL against E. coli, whereas the moderate activity shown against E. faecalis by the compound D3 with MIC value of 25 mg/mL. From the above discussion, it is obvious that among the studied dyes, D3 showed more inhibitory effect compared to rest of the molecules. This may attributed to the presence of electron releasing phenyl and methyl groups extensively increase the conjugation and this will results in the higher antimicrobial activity of the studied compounds and this can be supported by the earlier reports (Zhang et al., 2006). From the literature review, it is come to know that the molecules bearing a heterocyclic system enhance the pharmacological behaviour and it was true in our case (Kumar et al., 2014). Where, S – Sensitive, R – Resistant
Compounds (mg/mL)
100
50
25
12.5
6.25
3.12
1.6
0.8
0.4
0.2
E. coli
D1
S
S
R
R
R
R
R
R
R
R
D2
S
S
S
S
R
R
R
R
R
R
D3
S
S
S
S
R
R
R
R
R
R
D4
S
S
S
R
R
R
R
R
R
R
E. faecalis
D1
S
R
R
R
R
R
R
R
R
R
D2
S
S
R
R
R
R
R
R
R
R
D3
S
S
S
R
R
R
R
R
R
R
D4
S
S
R
R
R
R
R
R
R
R
Ciprofloxacin
S
S
S
S
S
S
S
S
S
S
C. albicans
D1
S
S
S
R
R
R
R
R
R
R
D2
S
S
R
R
R
R
R
R
R
R
D3
S
S
S
R
R
R
R
R
R
R
D4
S
S
S
R
R
R
R
R
R
R
A. flavus
D1
S
S
R
R
R
R
R
R
R
R
D2
S
S
S
R
R
R
R
R
R
R
D3
S
S
R
R
R
R
R
R
R
R
D4
S
S
S
R
R
R
R
R
R
R
Fluconazole
S
S
S
S
S
S
S
S
S
R
With respect to the antifungal activity, the tested compounds exhibited promising antifungal results (MIC = 25 mg/mL), except the compound D1 which showed moderate activity (MIC = 50 mg/mL) against both the fungal strains C. albicans and A. flavus. Therefore, the prepared compounds are having some potential antimicrobial activity due to the existence of heterocyclic ring in their structure (Raman et al., 2014; Barros et al, 2018; Ravi et al., 2020a).
3.5.2 Antituberculosis activity
The antitubercular assay was done for the synthesized molecules against M. tuberculosis to check the antimycobacterial efficiency. The results of the activity were recorded as MIC and are represented in Table 5 and Fig. S4 (supplementary material). The azo dye D2 exhibited significant activity with MIC equal to 3.12 µg/mL, the other compounds D3 and D4 shown moderate activity and D1 don’t show any activity against M. tuberculosis (Vinod Kumar et al, 2020). Thus, this study suggested that the compounds having nitrogen in the heterocyclic system are better pharmacological agents than the compounds of simple benzene analogous (Maria et al, 2007; Kumar and Jathi, 2019). Where, S – Sensitive, R – Resistant
Compounds
100 µg/mL
50 µg/mL
25 µg/mL
12.5 µg/mL
6.25 µg/mL
3.12 µg/mL
1.6 µg/mL
0.8 µg/mL
D1
R
R
R
R
R
R
R
R
D2
S
S
S
S
S
S
R
R
D3
S
S
R
R
R
R
R
R
D4
S
R
R
R
R
R
R
R
Pyrazinamide
S
S
S
S
S
S
R
R
Ciprofloxacin
S
S
S
S
S
S
R
R
Streptomycin
S
S
S
S
S
R
R
R
3.5.3 Anti-cancer activity results
The azo dyes containing 1, 3-benzothiazole-2-thiol (D1-D4) were studied for their anticancer activity against K562, A549, and MDA-MB-231 cell lines by MTT assay. The results of the activity were provided in the following Table 6 and it is found to have some potent anticancer property against tested cell lines. Among the studies compounds, D1 showed a maximum inhibitory effect against A549 with IC50 value 16.96 µM. The good to moderate activity was shown by the rest of the compounds with IC50 values in the range 38–50 µM against all the cell lines (Ravi et al., 2020a; b).
K562
A549
MDA-MB-231
Sample
IC50 µM
Sample
IC50 µM
Sample
IC50 µM
D1
>50
D1
16.96
D1
>50
D2
>50
D2
48.34
D2
>50
D3
43.27
D3
>50
D3
44.50
D4
>50
D4
>50
D4
38.79
3.5.4 In silico molecular docking results
The possible interaction between the target proteins and the title compounds of drugs can be studied by in silico molecular docking. Therefore, we applied the in silico docking of the title compounds with respect to the target protein RpsA, and the obtained results were compared with the pyrazinamide and they were summarized in Table 7. Fig. S5 (Supplementary material) represents the 3D-images of the interaction between the protein and the compounds. From the results of the docking, a significant interaction between the drugs (D1-D4) and the receptor was achieved. The target compounds showed effective bonding with the amino acids of the protein active pockets. All the dyes displayed favourable binding affinity towards the receptor with binding energy ranging from −4.3 to −5.5 kcal/mol which is nearly equivalent to the energy standard drug (Koohshekan et al, 2016; Diab et al, 2016). Thus, form the above experiments we can conclude that the above compounds may be the effective for the improvement of antibiotics in the future.
LIGAND
Affinity (kcal/mol)
H-bonds
H-bond length (Å)
H-bond with
Hydrophobic interactions
D1
−4.3
–
–
–
Phe310, Glu318, Arg355, Arg357
D2
−5.5
2
2.86
4NNI:Arg357::D2:N4
Tyr280, Phe310, Glu318
2.99
4NNI:Arg35::D2:N5
D3
−5.3
1
3.06
4NNI:Arg357::D3:O
Lys303, Phe310, Glu318, Arg355
D4
−4.9
–
–
–
Lys303, Phe310, Glu318, Arg355
Pyrazinamide
−6.1
3
3.16
4NNI:Arg357::PYZ:O
Met284, Phe310, Glu318, Gly319, Arg356
3.19
4NNI:Tyr280::PYZ:N3
3.24
4NNI:Ile358::PYZ:O
4 Conclusion
This paper explores the synthetic, structural, and biological studies of the sulphur containing heterocycles bearing azo linkage. The structural features of the azo dyes were accomplished by various physical and spectroscopic techniques and they are consistent with the proposed structures of the dyes. The biological activities like antimicrobial, antitubercular, anticancer, and docking studies were able to prove that the sulphur containing azo molecules can be used in the design and development of medications to treat the diseases caused by pathogens.
Acknowledgments
The authors are grateful to the Department of Chemistry of Kuvempu University for providing laboratory facility. Authors are also acknowledge the SAIF, CIL-Panjab University for help in getting spectral data and Maratha Mandal’s Central Research Laboratory, Belagavi for providing biological data.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1, 2, 3, 4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid esters as orally effective antihypertensive agents. J. Med. Chem.. 1991;34:806-811.
- [Google Scholar]
- Anti-Mycobacterium tuberculosis activity of naphthoimidazoles combined with isoniazid and rifampicin. Tuberculosis. 2018;111:198-201.
- [Google Scholar]
- Br. J. Cancer. 2009;101:774-781.
- Synthesis, structural determination and photo-antiproliferative activity of new 3-pyrazolyl or-isoxazolyl substituted 4-hydroxy-2 (1H)-quinolinones. Tetrahedron. 2006;62:90-96.
- [Google Scholar]
- Chem. Ind. London 1958:1248.
- Geometrical structures, molecular docking, spectroscopic characterization of mixed ligand and Schiff base metal complexes. J. Mol. Liq.. 2016;218:571-585.
- [Google Scholar]
- Supramolecular structure, molecular docking and thermal properties of azo dye complexes. J. Mol. Liq.. 2015;212:487-502.
- [Google Scholar]
- Synthesis, characterization, and biological activities of 4-imino-3-arylazo-4H-pyrimido [2, 1-b][1, 3] benzothiazole-2-oles. Med. Chem. Res.. 2014;23:3733-3743.
- [Google Scholar]
- A novel NLO azothiophene-based chromophore: synthesis, characterization, thermal stability and optical nonlinearity. Mater. Lett.. 2008;62(6–7):973-976.
- [Google Scholar]
- Novel nonlinearity–transparency–thermal stability trade-off of thiazolylazopyrimidine chromophores for nonlinear optical application. Dyes Pigm.. 2009;80(1):6-10.
- [Google Scholar]
- 100 years of the Biginelli dihydropyrimidine synthesis. Tetrahedron. 1993;49:6937-6963.
- [Google Scholar]
- Synthesis of 4-amino-1H-benzo [4, 5] imidazo [1, 2-a] pyrimidin-2-one and its disperse azo dyes. Part 2: Hetarylazo derivatives. Dyes Pigm.. 2006;71:97-102.
- [Google Scholar]
- Synthesis and characterization of novel polyimide-based NLO materials from poly (hydroxy-imide) s containing alicyclic units (II) Polymer. 1999;40:6157-6167.
- [Google Scholar]
- Protective effects of aspirin on the function of bovine liver catalase: a spectroscopy and molecular docking study. J. Mol. Liq.. 2016;218:8-15.
- [Google Scholar]
- Synthesis, structural investigations and in vitro biological evaluation of N, N-dimethyl aniline derivatives based azo dyes as potential pharmacological agents. J. Mol. Str.. 2019;1186:404-412.
- [Google Scholar]
- Synthesis and antibacterial activity of some new 1-heteroaryl-5-amino-4-phenyl-3-trifluoro methyl pyrazoles. Eur. J. Med. Chem.. 2005;40(2005):922-927.
- [Google Scholar]
- Synthesis, characterization and electrochemical investigations of azo dyes derived from 2-amino-6-ethoxybenzothiazole. Chem. Data Collections. 2018;17:13-29.
- [Google Scholar]
- Synthesis, characterization and biological potency of butylpyridone based azo dyes. Chem. Select. 2020;5:5460-5464.
- [Google Scholar]
- Synthesis, antimicrobial, anticancer, antiviral evaluation and QSAR studies of 4-(1-aryl-2-oxo-1, 2-dihydro-indol-3-ylideneamino)-N substituted benzenesulfonamides. Arab. J. Chem.. 2014;7(4):396-408.
- [Google Scholar]
- Synthesis, characterization, pharmacological and computational studies of 4, 5, 6, 7-tetrahydro-1, 3-benzothiazole incorporated azo dyes. J. Mol. Str.. 2019;1179:630-641.
- [Google Scholar]
- 6-Substituted benzothiazole based dispersed azo dyes having pyrazole moiety: synthesis, characterization, electrochemical and DFT studies. J. Mol. Str.. 2020;1199:126959.
- [CrossRef] [Google Scholar]
- Synthesis, spectroscopic characterization and pharmacological studies on novel sulfamethaxazole based azo dyes. JKSUS. 2020;32:251-259.
- [Google Scholar]
- Synthesis, characterization, thermal and biological evaluation of Cu (II), Co (II) and Ni (II) complexes of azo dye ligand containing sulfamethaxazole moiety. J. Mol. Str.. 2018;1165:28-36.
- [Google Scholar]
- Synthesis, spectroscopic characterization, antimicrobial, antitubercular and DNA cleavage studies of 2-(1H-indol-3-yldiazenyl)-4, 5, 6, 7-tetrahydro-1, 3-benzothiazole and its metal complexes. J. Mol. Str.. 2018;1173:557-566.
- [Google Scholar]
- Synthesis, structural studies and antituberculosis evaluation of new hydrazone derivatives of quinoline and their Zn (II) complexes. J. Saud. Chem. Soc.. 2018;22:218-228.
- [Google Scholar]
- Maria, C.S., Lourenco Marcus V.N., DeSouza Alessandra C., Pinheiro Marcelle de L. Ferreira Rasnisb, B., Goncalves Thais Cristina M., Nogneira Monica A., Peralta, 2007. Evaluation of anti-Tubercular activity of nicotinic and isoniazid analogues. ARKIVOC. 176 181-191.
- A novel azo metal complexes of 5, 5, 7-trimethyl-4, 5, 6, 7-tetrahydro-1, 3-benzothiazol as potential pharmacological agents: Synthesis and spectroscopic characterization. J. Mol. Str.. 2019;1180:196-208.
- [Google Scholar]
- Challenging the auxiliary donor effect on molecular hyper polarizability in thiophene-containing nonlinear chromophores: X-ray crystallographic and optical measurements on two new isomeric chromophores. J. Org. Chem.. 2004;69(24):8239-8243.
- [Google Scholar]
- Exploring DNA binding and nucleolytic activity of few4-aminoantipyrine based amino acid Schiff base complexes: a comparative approach. Spectrochim. Acta A.. 2014;125:404-413.
- [Google Scholar]
- Photochemistry and Photo physics. Boca Raton: CRC Press; 1990.
- Synthesis, characterization and pharmacological evaluation of 2-aminothiazole incorporated azo dyes. J. Mol. Str.. 2020;1204:127493
- [Google Scholar]
- Synthesis, characterization, cyclic voltammetric and cytotoxic studies of azo dyes containing thiazole moiety. CDC. 2020;25:100334
- [Google Scholar]
- Antimicrobial Susceptibility Testing Protocols. Crc Press; 2007.
- Synthesis and structure activity relationship studies of urea-containing pyrazoles as dual inhibitors of cyclooxygenase-2and soluble epoxide hydrolase. J. Med. Chem.. 2011;54:3037-3050.
- [Google Scholar]
- New celecoxib derivatives as anti-inflammatory agents. J. Med. Chem.. 2007;51:142-147.
- [Google Scholar]
- Synthesis and solvatochromic properties of some disazo dyes derived from pyrazolo [1, 5-a] pyrimidine derivatives. Dyes Pigm.. 2005;64(3):259-264.
- [Google Scholar]
- Wade, L.G., J. Organic Chemistry, 3rd edition, Prentice Hall, Inc., Upper Saddle River, New Jersey (1995) 906.
- Structural basis for targeting the ribosomal protein S1 of Mycobacterium tuberculosis by pyrazinamide. Mol. Microb.. 2015;95:791-803.
- [Google Scholar]
- The synthesis of 5-amino-4-arylazo-3-methyl-1H-pyrazoles and 5-aryl-3-methylpyrazole [3, 4-e] [1, 2, 3, 4] tetrazines. Dyes Pigm.. 2009;83(2):144-147.
- [Google Scholar]
- Inhibitory study of some novel Schiff base derivatives on Staphylococcus aureus by micro calorimetry. Thermochim. Acta. 2006;440(1):51-56.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.09.016.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







